Abstract
We studied onchocerciasis transmission and impact on ocular morbidity in three health districts in North Region, Cameroon, where annual mass ivermectin treatment has been provided for 12–17 years. The studies, which took place from 2008 to 2010, consisted of skin snips for microfilariae (mf), palpation examinations for nodules, slit lamp examinations for mf in the eye, and Simulium vector dissections for larval infection rates. Adults had mf and nodule rates of 4.8% and 13.5%, respectively, and 5.5% had mf in the anterior chamber of the eye. Strong evidence of ongoing transmission was found in one health district, where despite 17 years of annual treatments, the annual transmission potential was 543 L3/person per year; additionally, children under 10 years of age had a 2.6% mf prevalence. Halting ivermectin treatments in North Cameroon now might risk recrudescence of transmission and ocular disease.
Introduction
Onchocerciasis, a leading cause of blindness, is caused by human infection with Onchocerca volvulus, a parasitic worm that forms nodules under the skin. The female worms produce microfilariae (mf) that live in the nodules and inflame the skin; mf may enter the eyes, giving rise to inflammatory lesions there as well. The mf are picked up by species of Simulium that serve as vectors, where they develop through three distinct larval stages (L1–L3). The L3 (infective) larvae may be passed on to others on subsequent bites, completing the life cycle. Black flies breed in fast flowing rivers and streams, lending the name river blindness to the condition. Ivermectin is a safe and effective microfilaricidal drug that has been donated by Merck & Co, Whitehouse Station, NJ (Mectizan®) since 1987 for mass treatment of onchocerciasis. The medicine kills the mf and reduces the risk of developing eye and skin diseases associated with the infection. Ivermectin also reduces the fecundity of adult worms and apparently, also shortens their life span, but treatment still must be given for an undetermined length of time to effect cure.1,2
Since 1996, The Carter Center has provided ongoing assistance to Cameroon Ministry of Health (MOH) for distribution of ivermectin for onchocerciasis control in endemic areas of North Region, taking over a project that was launched by the MOH and the River Blindness Foundation in 1992. The African Program for Onchocerciasis Control (APOC) joined The Carter Center in supporting North Cameroon from 1998 to 2003. APOC was designed to substantially financially support delivery of an annual dose of ivermectin through community-directed treatment with ivermectin (CDTI) for 5 years. The objective was to establish a mechanism for sustained delivery of an annual dose of ivermectin, thereby achieving reduction of prevalence and transmission to a point where onchocerciasis would no longer be of public health or socioeconomic concern.
In a recent report, APOC indicated that, since its launching, the prevalence of onchocerciasis infection in the endemic countries under its mandate has been reduced from the 1995 pre-control level of 46.5% to 28.5% in 2008.3 However, the APOC goal of eliminating onchocerciasis as a public health problem (EPHP) is not quantitatively defined. The EPHP goal was logically defined as when prevalence is driven below the original baseline threshold required to launch the mass ivermectin treatment program, which is an onchocercal nodule rate ≥ 20% or an mf rate ≥ 40%.4 Katabarwa and others4 noted that achieving EPHP defined at these levels did not necessarily indicate that transmission has been interrupted. In the case that it has not, halting mass treatment could result in a return of transmission to pretreatment levels and disease recrudescence.4–9
An important study in hyperendemic foci in Mali and Senegal showed that 15–17 years of annual ivermectin treatment had eliminated onchocerciasis transmission in the north and center of the Rio Faleme focus and that mass treatment could be safely stopped.10 The two criteria used by Diawara and others10 to make this determination were entomological (< 0.5 infected flies/1,000 flies) and epidemiological (< 5% mf prevalence in all communities examined and < 1% in 90% examined). It is important to note that, in the south of the Rio Falema focus, there were seven villages with mf prevalence between 1% and 13% after 15–17 years of annual treatment. Diawara and others10 called for additional studies in areas with similar durations of treatment to determine if other successes (or failures) could be documented across various onchocerciasis ecological transmission zones in Africa. Accordingly, the objective of the present paper is to determine if up to 17 years of annual mass treatment in North Region, Cameroon, has resulted in achieving interruption of transmission of onchocerciasis using the Diawara and others10 entomological and epidemiological criteria. We also evaluated a sample of children for infection as well as assessed the morbidity component of the elimination question by examining a sample of residents for recent ocular lesions of onchocerciasis.
Materials and Methods
Study sites.
The study was carried out in North Region (Figure 1), Cameroon. North Region is about 66,460 km2, and the hyper- and mesoendemic onchocerciasis areas under mass ivermectin treatment cover 46,260 km2, about 69.6% of the whole region. This part of Cameroon is known as an area where onchocerciasis is associated with severe ocular complications.11–13 We selected 12 communities in three health districts under ivermectin treatment: Tchollire district (four communities), Rey-Bouba district (two communities), and Touboro (six communities). Touboro had 17 years of annual ivermectin treatment compared with 12 years of annual ivermectin treatment for Tchollire and Rey-Bouba. Accordingly, Touboro results were analyzed separately from Tchollire and Rey-Bouba.
Figure 1.
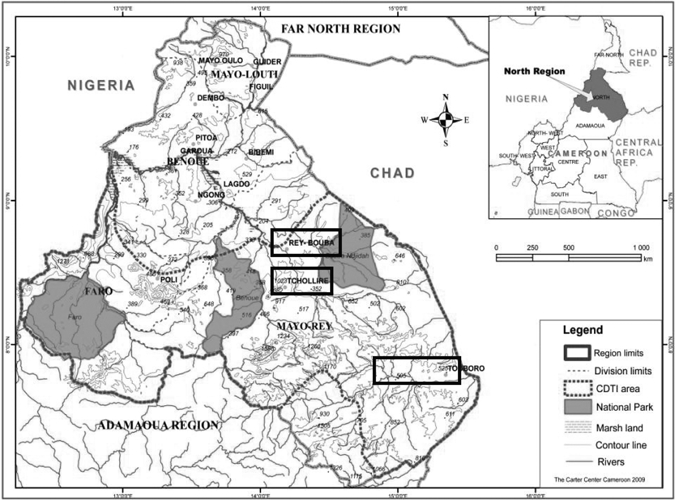
Rapid epidemiological map of North Region, Cameroon, showing CDTI areas where the study was done.
Baseline data.
Communities were selected in the first two districts (Tchollire and Rey-Bouba) based on data provided by A. Renz (unpublished data) that included mf and nodule prevalence and vector dissections from surveys that he conducted as part of the research for his 1985 doctorate thesis.14 The Renz dataset was based on examination of a total of 1,409 adults for mf in Tchollire and Rey-Bouba and 1,279 adults for nodules in Tchollire. Unpublished baseline mf data for children in four communities of Tchollire (Douffin and Mayo-Galke in 1991) and Rey-Bouba (Larki and Rey-Manga in 1991) from Renz were also available (unpublished data). There was no baseline data available for Touboro.
History of mass treatment with ivermectin.
Annual mass ivermectin treatment has been provided for 12 years in Tchollire and Rey-Bouba and 17 years in Touboro. Monthly treatment coverage reports were available since inception of the program, and from these reports, annual ivermectin treatment coverage of the combined eligible population for the three health districts was obtained. Annual treatment records starting in 1993 for Touboro health district showed population coverage of at least 85% of the eligible population and 97–100% geographical coverage of affected communities. Treatments in Tchollire and Rey-Bouba health districts reached over 90% of the eligible population and full geographical coverage in 1998. Annual treatment coverage surveys have been conducted to validate reported coverage by sampling households by multistage random sampling done every year from 2003 to 2009.15 The samples sizes for the household interview coverage surveys varied through the years based on availability of resources; however, all samples were sufficient to provide 95% confidence with an error of ±5%.16 Results confirmed high reported coverage by the program, with surveyed treatment coverage from 2003 to 2009 ranging from 85.4% to 94.1% of eligible population (Table 1).
Table 1.
Ivermectin treatment coverage determined by household questionnaire surveys conducted in North Region from 2003 to 2009
| Period | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|---|
| Number interviewed | 1,482 | 1,487 | 1,457 | 1,484 | 1,454 | 353 | 354 |
| Number treated | 1,297 | 1,362 | 1,312 | 1,347 | 1,241 | 324 | 333 |
| Percent treated | 87.5 | 91.6 | 90.0 | 90.8 | 85.4 | 91.8 | 94.1 |
2008–2009 surveys.
Multiple follow-up surveys undertaken in the three health areas include (1) a parasitological (nodule and mf prevalence) survey in March of 2009 (timed to be 10–11 months after the 2008 mass treatment), (2) an ocular morbidity survey in December 2008, and (3) a 1-year-long entomological survey from November 2008 to October 2009 in Touboro and from November 2009 to October 2010 in Tchollire. The methodology of each survey is described below.
Nodule survey.
After obtaining consent, an individual's name, age, and gender were recorded on an individual registration form. Every participant was examined in a well-lit, private room. Trained health workers performed a palpation examination on the partially undressed participant, paying attention to bony prominences of the torso, iliac crests, and upper trocanter of the femurs. Onchocercal nodules were identified clinically as being firm, painless, and mobile.17–19 Results were recorded on the form as positive or negative. Nodule prevalence was expressed as a percent (number of persons positive for nodules divided by number examined × 100). Our examination procedure for nodules focused on the same anatomical areas as reported in the Renz baseline survey in Tchollire and Rey-Bouba (Renz A, unpublished data).
Mf survey.
After obtaining consent, two skin snip samples were taken from over the posterior iliac crests. After cleaning the site with an antiseptic, a 2- to 3-mg sample of skin was taken with the help of a disposable sterile dermal hook and razor blade. The skin samples were placed immediately in wells of microtitration plates containing a sterile normal saline solution and held at room temperature for 12–24 hours.20–22 The corresponding well numbers were reflected on the patient form. When the plate was full, it was sealed with a transparent adhesive tape. After 12 hours, the snips were removed, and the fluid from each well was examined separately on a slide for microfilaria under high-power (40×) magnification. The results were expressed for each individual as positive or negative. The results were recorded on the original (field) registration form. Mf prevalence was expressed as a percent (number positive divided by number examined × 100).23
Our examination procedure for mf differed from the procedures of the 1976 and 1991 Renz baseline surveys in Tchollire and Rey-Bouba. In those surveys, two snips were taken, one snip at the outer canthus and another snip from the buttock, using a corneoscleral punch. Each snip was placed in three drops of normal saline in separate microtiter wells and examined microscopically 4 hours later. The baseline mean mf rate for Tchollire in 1971 was 38.8% (N = 1,279), and the baseline mean mf rate for Rey Bouba in 1991 was 57.7% (N = 130).
Ophthalmological survey.
We assessed ocular morbidity in eight communities in the follow-up surveys (i.e., two communities [Douffin and Mayo-Galke] in Tchollire and six communities in Touboro). The participants were asked to lean forward for about 5 minutes to facilitate the descent of mf in the anterior chamber to a point where mf observation was possible. Afterward, the participants' anterior segments of the eyes was examined by an experienced ophthalmologist (S.Y.) using a slit lamp under 25× magnification. The pupil was dilated with a mydriaticum collyre, and the posterior segment of the eye was examined (results not reported here). Ocular lesions related with onchocerciasis were recorded including iritis, mf in the anterior chamber, corneal stromal opacities (punctuate keratitis), and sclerosing keratitis. Only corneal stromal opacities where mf or mf fragments could be observed were counted as punctate keratitis.24 These strict criteria requiring presence of visible mf in the corneal inflammation may have reduced the rates for punctate keratitis compared with other studies. Iritis and sclerosing keratitis were considered chronic lesions that would not necessarily respond to ivermectin treatment, whereas mf in the anterior chamber or cornea were considered acute lesions incident within the last 2 years. Patients with conditions that required immediate treatment were referred to the nearest hospital.
Entomological surveys.
Surveys were conducted in Tchollire and Touboro health districts, where baseline entomological data were available (Tchollire, 1977–1978; Touboro, 1976).14
In Touboro (the district with 17 years of annual ivermectin treatment), vectors were collected from November 2008 to October 2009 in three fly collection sites: (1) Mbailara (near Koumane community), (2) Vina Bridge (near Kuobao community), and (3) Babidan community. In Tchollire (the district with 12 years of annual ivermectin treatment), vectors were collected from November 2009 to October 2010 at two fly collection sites: (1) Douffin and (2) Mayo-Galké. Each collection team consisted of two members. The team members were at least 20 years of age and provided written informed consent after they were informed of the nature of the work and their ability to opt out of the study if they so wished at any time without any repercussions. The collectors sat at the five sites near the river bank and exposed their legs between 0600 and 1200 hours and between 1200 and 1800 hours for 5 days/month.22 As female S. damnosum s.l. seeking a blood meal settled on the exposed legs, suction tubes were used to catch them before they bit. The flies were preserved in 70% ethanol in batches of up to 50 for later dissection. The tubes in which the vector flies were placed were labeled by collection site, date, and time. The tubes were routinely collected and returned to the laboratory in Yaounde, Cameroon. In the laboratory, the flies were stained with Mayer's hematoxylin, dissected, and analyzed for onchocercal larval stages in the head, thorax, and abdomen.25 Mayer's hematoxylin ensures progressive nuclei stain and improves visibility of all forms of O. volvulus in flies stored in alcohol. Using a dissecting microscope (40× magnification), an experienced dissector opened the vector flies' abdomens, thoraxes, and heads. Dissected flies where then examined under a light microscope for identifying the presence of infection and counting the number of L1, L2, and L3 when present. Infective flies were defined as flies with L3, and only L3 was considered in the calculations of annual transmission potential (ATP).
Monthly biting rates (MBR) were calculated as per the standard method as
The annual biting rate (ABR) was calculated as the sum of the individual MBR. Monthly transmission potentials (MTPs) were obtained from dissection data:
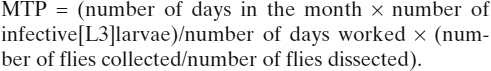 |
The ATP was calculated as sum of the individual MTPs over the course of the year.26
Ethical review.
The study was approved by the MOH in Cameroon, and Emory University Institutional Review Board classified the study as a period program performance assessment (non-research). Consent was obtained from all participants, and assent was obtained from parents of young children. All participating communities were educated about the importance of the study, and participants were assured that there would be no repercussions for refusing to participate.
Results
Mf and nodule prevalence.
Table 2 shows the 2009 mf and nodule prevalence in adults by health district. The mf prevalence was 0.8% in Tchollire (range = 0–3.3%) and 2.5% in Rey-Bouba (range = 0–5%); these two health districts were not significantly different (P = 0.1813). In contrast and despite a longer treatment period, Touboro health district had a 2009 mf prevalence of 7.7% (range = 0–17.1%), which was significantly higher than rates in Tchollire (P < 0.001) and Rey-Bouba (P < 0.039), and it was above the threshold of 5% allowed by Diawara and others10 in 10% of communities. Mf prevalence was uncomfortably high (≥ 8%) in three of six communities of Touboro, with prevalence being highest in Koubao and Babidan at 12.9% and 17.1%, respectively. There was a significant drop from baseline mean mf rates in Tchollire (38.8%; N = 1,279) and Rey Bouba (57.7%; N = 130) and follow-up results (0.8%; N = 250 and 2.5%; N = 119), respectively (P < 0.001) (Figure 2). There were no baseline data on mf rates in Touboro.
Table 2.
2009 assessment of adults for mf prevalence (N = 775) and nodule prevalence (N = 1,015) in three health districts (Touboro, Tchollire, and Rey-Bouba) of North Region, Cameroon
| Community name | Assessment (2009) | 1993–2009 treatments | Years under treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mf prevalence | Nodule prevalence | |||||||||
| Number assessed | Mean age | Number of persons + ve | Percent of persons with mf | Number assessed for nodules | Number of persons + ve | Percent of persons with nodules | Percent eligible population treated range | Mean treatment coverage (%) | ||
| Touboro | ||||||||||
| Sora-Mbourn | 67 | 22.0 | 3 | 4.5 | 113 | 19 | 16.8 | 72.7–100.0 | 97.2 | 17 |
| Reh | 59 | 27.5 | 2 | 3.4 | 57 | 8 | 14.0 | 81.0–100.0 | 87.0 | |
| Ribao | 70 | 29.9 | 0 | 0.0 | 175 | 25 | 14.3 | 71.0–100.0 | 85.0 | |
| Koumane | 70 | 29.2 | 6 | 8.6 | 144 | 27 | 18.8 | 81.0–98.0 | 85.6 | |
| Koubao | 70 | 47.5 | 9 | 12.9 | 36 | 19.5 | 22.8 | 84.0–99.0 | 88.7 | |
| Babidan | 70 | 30.2 | 12 | 17.1 | 121 | 28 | 23.1 | 73.3–97.0 | 87.2 | |
| Subtotal | 406 | 31.0 | 32 | 7.7 | 646 | 123 | 23.3 | |||
| Tchollire | ||||||||||
| Yokout | 70 | 34.6 | 0 | 0.0 | 70 | 1 | 1.4 | 76.8–96.6 | 81.4 | 12 |
| Douffin | 60 | 31.3 | 0 | 0.0 | 60 | 0 | 0.0 | 81.0–100.0 | 94.0 | |
| Laboun | 60 | 27.0 | 0 | 0.0 | 60 | 2 | 3.1 | 85.0–100.0 | 96.5 | |
| Mayo-Galké | 60 | 28.6 | 2 | 3.3 | 60 | 5 | 8.3 | 94.0–100.0 | 94.1 | |
| Subtotal | 250 | 30.4 | 2 | 0.8 | 250 | 8 | 3.3 | |||
| Ray-Bouba | ||||||||||
| Larki | 59 | 28.2 | 0 | 0.0 | 59 | 2 | 3.4 | 94.2–100.0 | 94.4 | 12 |
| Rey-Manga | 60 | 37.1 | 3 | 5.0 | 60 | 1 | 1.7 | 95.0–98.1 | 96.3 | |
| Subtotal | 119 | 32.6 | 3 | 2.5 | 119 | 3 | 2.5 | |||
| Grand total | 775 | 38.9 | 37 | 4.8 | 1,015 | 137 | 13.5 | 71.0–100.0 | 90.6 | 12–17 |
Reported mean ivermectin treatment coverage from 1993 to 2009 is also shown.
Figure 2.
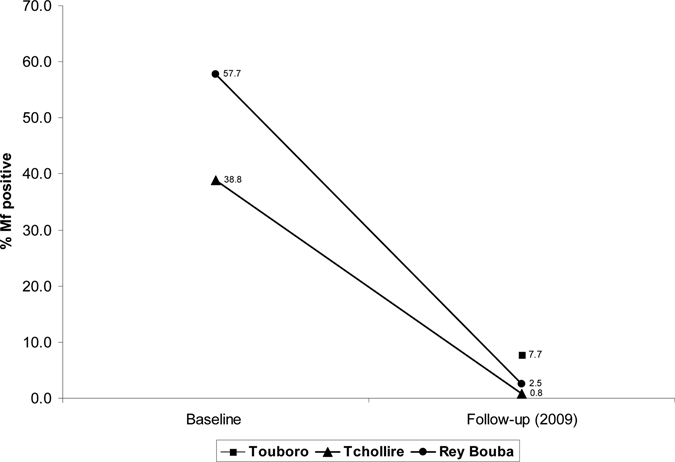
North Region, Cameroon, mf prevalence in adults. Baseline (Rey Bouba and Tchollire) and 2009 follow-up (Rey-Bouba, Tchollire, and Touboro) prevalence.
Nodule prevalence among adults in Tchollire was 3.3% (range = 0–8.3%), and nodule prevalence in Rey-Bouba was 2.5% (range = 1.7–3.4%). In the same manner as mf rates, nodule rates in 2009 were higher in Touboro (23.3%; range = 14–52.8%), a district with 17 years of annual ivermectin distribution compared with Tchollire or Rey-Bouba districts with 12 years (P < 0.001). Nodule rates were uncomfortably high (≥ 14%) in all six surveyed communities of Touboro (Table 2). Despite belonging to the district having the longest ivermectin treatment history, two Touboro communities (Koubao and Babidan) had high nodule rates greater than the 20% EPHP threshold. The most unusual was Koubao community, with 52.8% after 17 years of annual mass treatment with ivermectin.
Results from studies in children under 10 years of age show that 0 of 51 children in Rey Bouba and Tchollire health districts were positive for mf in 2009. This finding is in contrast to baseline mf data available from 1991 that showed 18.7% of 107 children were mf-positive in four communities—Tchollire (Douffin and Mayo-Galke) and Rey-Bouba (Larki and Rey-Manga). The difference between the 1991 baseline and the 2009 survey was statistically significant (P < 0.001). In 2009, 2 (2.6%) of 106 children examined were positive in Touboro (Table 3). All positive children came from two communities (Koumane and Babidan).
Table 3.
2009 assessment of mf and nodule prevalence (N = 157) in children under 10 years of age in three health districts (Touboro, Tchollire, and Rey-Bouba) of North Region, Cameroon
| Community name | Number assessed | Mean age | Number of children + ve for mf | Percent children with mf | Number of children + ve for nodules | Percent of persons with nodules |
|---|---|---|---|---|---|---|
| Touboro | ||||||
| Sora-Mboun | 26 | 5.4 | 0 | 0.00 | 0 | 0 |
| Reh | 20 | 5.8 | 0 | 0.00 | 0 | 0 |
| Ribao | 14 | 5.1 | 0 | 0.00 | 0 | 0 |
| Koumane | 14 | 5.5 | 1 | 7.14 | 0 | 0 |
| Koubao | 20 | 5.9 | 0 | 0.00 | 0 | 0 |
| Babidan | 12 | 4.9 | 1 | 8.33 | 0 | 0 |
| Subtotal | 106 | 5.4 | 2 | 2.58 | 0 | 0 |
| Tchollire | ||||||
| Yokout | 8 | 6.4 | 0 | 0.00 | 0 | 0 |
| Douffin | 9 | 7.1 | 0 | 0.00 | 0 | 0 |
| Laboun | 4 | 6.3 | 0 | 0.00 | 0 | 0 |
| Mayo-Galké | 10 | 6.4 | 0 | 0.00 | 0 | 0 |
| Subtotal | 31 | 6.5 | 0 | 0.00 | 0 | 0 |
| Rey-Bouba | ||||||
| Larki | 11 | 5.6 | 0 | 0.00 | 0 | 0 |
| Rey-Manga | 9 | 6.1 | 0 | 0.00 | 0 | 0 |
| Subtotal | 20 | 5.9 | 0 | 0.00 | 0 | 0 |
| Grand total | 157 | 5.87 | 2 | 1.50 | 0 | 0 |
Ocular morbidity.
Four hundred seventy-two persons had slit lamp examinations. Acute lesions observed included mf in the anterior chamber in 5.5% and punctuate keratitis in 3.6%. These acute onchocercal ocular lesions were largely observed in Touboro health district (Table 4). However, in Douffin (Tchollire), one person had punctuate keratitis, and another person in May-Galke (Rey Bouba) had mf in the anterior chamber. The chronic lesions of sclerosing keratitis and iritis were found in 6.4% and 6.8% of the 472 persons examined, respectively.
Table 4.
Ocular morbidity results of 2009 (N = 472) in Rey-Bouba, Tchollire, and Touboro health districts, North Region, Cameroon
| Sentinel communities | Number assessed | Mean age | Iritis cases | Percent iritis cases | Number with mf in anterior chamber | Percent mf in anterior chamber | Punctate keratitis cases | Percent punctate keratitis | Sclerosing keratitis cases | Percent sclerosing keratitis |
|---|---|---|---|---|---|---|---|---|---|---|
| Tchollire | ||||||||||
| Douffin | 56 | 33.2 | 3 | 5.4 | 0 | 0.0 | 1 | 1.8 | 0 | 0.0 |
| Rey-Bouba | ||||||||||
| Mayo-Galké | 67 | 32.2 | 9 | 13.4 | 1 | 1.5 | 0 | 0.0 | 7 | 10.4 |
| Touboro | ||||||||||
| Koubao | 58 | 27.5 | 3 | 5.2 | 6 | 10.3 | 2 | 3.4 | 9 | 15.5 |
| Babidan | 60 | 33.8 | 0 | 0.0 | 7 | 11.7 | 4 | 6.7 | 3 | 5.0 |
| Koumane | 58 | 41.2 | 2 | 3.4 | 4 | 6.9 | 4 | 6.9 | 3 | 5.2 |
| Ribao | 60 | 35.8 | 3 | 5.0 | 4 | 6.7 | 1 | 1.7 | 4 | 6.7 |
| Reh | 53 | 25.6 | 7 | 13.2 | 2 | 3.8 | 0 | 0.0 | 4 | 7.5 |
| Sora-Mbourn | 60 | 27.4 | 5 | 8.3 | 2 | 3.3 | 5 | 8.3 | 0 | 0.0 |
| Total | 472 | 32.2 | 32 | 6.7 | 56 | 5.5 | 17 | 3.6 | 30 | 6.3 |
Entomology.
Table 5 and Figure 3 show a comparison of entomological indicators in the baseline and follow-up for Touboro and Tchollire. The infection rate (all larval stages) dropped in Touboro from 8.6% to 2.1% (P < 0.0001). In Tchollire, the infection rate in Douffin dropped from 3.7% to 0.4% (P < 0.0001), and the infection rate in Mayo Galke dropped from 5.1% to 0.8% (P < 0.0001). The infective rate (L3 larval stages) dropped in Touboro from 2.7% to 1.4% (P < 0.0001), whereas in Tchollire, it dropped in Douffin from 0.6% to 0.1% (P < 0.318) and in Mayo Galke from 0.7% to 0.4% (P < 0.0078). All infective rates measured in the follow-up surveys were above the threshold of 0.5/1,000 (0.05%) used by Diawara and others10 as a criterion for transmission interruption. In Toubouro, the 2008–2009 ATP was 543.4, which is considerably lower that the value of 2,767 calculated in 1976, representing an 80.4% reduction after 17 years of annual mass treatment with ivermectin. Biting rates were similar at baseline and follow-up, and therefore, this change reflects the fact that the proportion of infective flies was considerably lower in 2008–2009 compared with 1976. In Tchollire, where ivermectin mass treatment had been administered for 12 years, the ATP at Douffin (20.7) showed an 86.8% reduction from 157, and the ATP at Mayo-Galke showed an 89.4% reduction from 377 to 40. This drop occurred despite the fact that biting rates at baseline were lower in Douffin compared with follow-up surveys, which would push the ATP baseline down.
Table 5.
Comparison of entomological indicators in the baseline and follow-up results in Touboro and Tchollire health districts of North Region, Cameroon
| Touboro health district | Tchollire health district | |||||
|---|---|---|---|---|---|---|
| Touboro | Douffin (tributary) | Mayo-Gallé | ||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Year of black fly collection | 1976* | 2008–2009 | 1977* | 2009–2010 | 1978* | 2009–2010 |
| Number of Simulium vector flies caught and dissected | 5,239 | 12,107 | 541 | 1,549 | 4,733 | 1,064 |
| Number of Simulium vector flies infected | 450 | 261 | 20 | 6 | 239 | 8 |
| Infection rate (%) | 8.6 | 2.1 | 3.7 | 0.4 | 5.1 | 0.8 |
| Infective rate (%) | 2.7 | 1.4 | 0.6 | 0.1 | 0.7 | 0.4 |
| Annual biting rate per person | 26,100 | 25,109 | 2,300 | 15,770 | 27,700 | 10,843 |
| Annual transmission potential (M.N.K.) | 2,767.0 | 543.4 | 157.0 | 20.7 | 377.0 | 40.0 |
Renz A, unpublished data.
Figure 3.
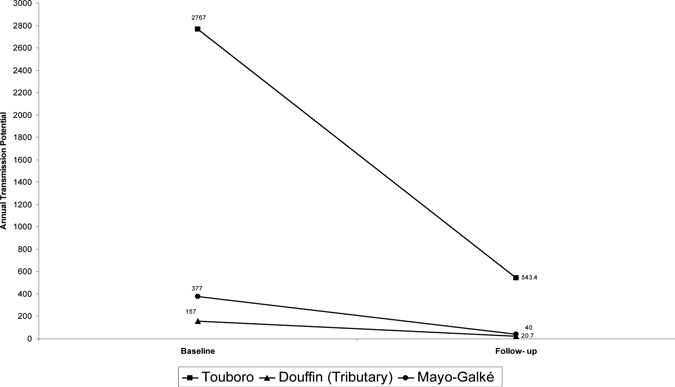
Comparison of ATP of the baseline and follow-up assessment in 2008–2009 and 2009–2010, respectively, determined by dissection of S. damnosum sl. Baseline data from Renz's14 thesis for Touboro was from 1976. Data from Douffin (Tchollire) was from 1977 to 1978, and data from Mayo-Galke (Tchollire) was from 1977 to 1978; follow-up data for Touboro (2008–2009) and Douffin and May-Galke (2009–2010) were also collected.
Discussion
The impact of an annual dose of ivermectin on reducing mf infection prevalence was obvious in this study. Mf prevalence overall in 2009 was only 4.8% (range = 0–17.1%) compared with a baseline mean of > 40%. Although the metrics of EPHP are not defined, compared with the threshold required to launch mass treatment programs (onchocercal nodule rates > 20% or mf rate > 40%), all communities and all health districts studied are highly successful. In contrast, nodule rates in two Touboro communities (Koubao, > 50%; Babidan, 23.1%) remained unacceptably high and above the 20% threshold. The wide range of nodule rates (0–53.8%) in the communities surveyed compared with the lower mf rates (0–17%) highlights that ivermectin treatment effects are primarily to reduce mf in skin, and they will not necessarily impact the adult worms that reside in the nodules.27 It also highlights the difficulties in comparing these two indices in mature ivermectin treatment zones. Despite low mf prevalence, if the nodules that remain in North Region contain viable O. volvulus adult worms, then a major concern should be that transmission will recover (given that competent vectors remain abundant) when ivermectin treatments stop.
We were able to compare our 2009 community surveys with baseline mf and nodule data for the same communities visited only for Tchollire and Rey-Bouba districts (Figure 2). However, previous work in Toubouro showed mf rates of > 90% in two of six villages (Babidan and Koubao) that we reexamined in 2009.28 This result is higher than the baseline recorded by Renz for Tchollire (39%) or Rey-Bouba (58%; unpublished data). Baseline ATP (Figure 3) in Toubouro was over seven times the baseline ATP in Tchollire. The force of infection is a combination of high vector density and human hyperendemicity, and these observations suggest that the initial force of transmission may have been highest in Toubouro.8,28 However, the standard measure of intensity of infection (which is related to force of infection) is the community mf load (CMFL); this measure could not be calculated in the present study, because mf counts were not done and weights of the snips were not known. Corneoscleral punches, which have traditionally been used to provide a consistent skin snip sample (mf/snip) from which CMFL can also be calculated, were not used in this study. These instruments are expensive and carry a risk for spreading human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDs) and hepatitis if they are not properly sterilized between patients, which is difficult to do under remote field conditions. We elected to use disposable sterile dermal hooks and razor blades for each individual patient. Calculation of CMFL in this case would have required a sensitive balance for weighing the snips, which would add to the cost and complications of the study. Ultimately, the salient intention of the study was to mentor personnel from a poorly resourced MOH to apply a simple and cheap but practical and effective technology for routine onchocerciasis program evaluations. The 2009 evaluations showed that transmission is ongoing in Touboro despite 17 years of annual ivermectin treatment with good reported and surveyed coverage. Mf prevalence among adults was over 5% in three of six villages. Two communities (Koubao and Babidan) had mf prevalence above 10%, and two other communities (Babidan and Koumane) had infection rates of > 5% among children less than 8 years of age. Entomologic studies show 1.4% L3 rates and an ATP value of 543.
Tchollire and Rey-Bouba, where baseline mf prevalences were < 60% (e.g., below the threshold that defines hyperendemicity), were different in their 2009 evaluations compared with Touboro. Only two of six communities were positive for mf among adults at follow-up study, although one of these communities had an mf prevalence of 5% (the upper limit acceptable for elimination).10 No mf positive children were observed. However, the entomological findings determined for Tchollire were above the threshold used by Diawara and others,10 with infective (L3) rates > 0.5/1,000 flies (0.05%). The ATPs ranged from 20 to 40, which if assessed within the OEPA, would be interpreted as evidence of low-level transmission, although threshold transmission potentials under which O. volvulus cannot maintain itself have not been defined.29
One would have to conclude that North Cameroon would not satisfy the criteria by Diawara and others10 for transmission interruption. The World Health Organization (WHO) Guidelines for onchocerciasis elimination use the same entomological criteria as Diawara and others10 but (rather than focusing on adults) call for evaluation of infections in young children as indicators of recent transmission events (< 0.1% in children to declare transmission interruption).30 Based on the finding of an overall infection rate of 1.5% in an evaluation of 157 children, one would have to conclude that North Cameroon would also not satisfy the WHO 2001 guidelines for elimination. Based on our surveys, we conclude that mass treatments need to continue in North Cameroon beyond 17 years.
One possibility for the Touboro observations is the report from Ghana that suggests that, in some treatment areas, adult female O. volvulus worms are becoming ivermectin-tolerant, resuming mf reproduction more rapidly after ivermectin treatment than would normally be expected.31 We do not favor that hypothesis and instead, believe that the findings are explained by an initial force of transmission in Touboro that was simply too high to overcome with annual ivermectin treatment. However, it would still be prudent to undertake studies to evaluate the susceptibility of parasites in these communities. Many have questioned whether annual distribution of ivermectin can stop transmission of onchocerciasis in all hyperendemic areas.4–8,32 Modeling studies suggest that, in the same epidemiological settings where annual treatment will fail, a 6-month ivermectin treatment schedule will succeed.7 In field studies in Guatemala, two treatments per year have been shown to both reduce transmission and accelerate the death of adult O. volvulus worms.33 If transmission interruption becomes a goal for Cameroon, then we strongly recommend that the MOH consider a programmatic alteration to two times per year treatment at least for Touboro (if not throughout North Cameroon), including as yet untreated hypoendemic areas adjacent to these CDTI health districts.34
One weakness in our entomological study is that molecular testing was not performed to determine if the L3 larvae were O. volvulus or another (animal) Onchocerca species. A study in this same area during the 1990s showed that 33% of infective larvae in S. damnosum were O. volvulus, whereas 65% were O. ochengi and 2% were O. ramachandrini.35 Based on human mf prevalence in skin and infections in children, we think that at least some of the larvae observed in vectors were O. volvulus. Assuming that 33% of the L3 observed were O. volvulus, then the Touboro ATP reported here would diminish to 179 (0.33 × 543). There is a possibility that the proportion of the infective larvae that is O. volvulus may be even lower than our 179 estimate because of the fact that ivermectin treatment in humans does not affect O. ochengi, and therefore, the ratio would be altered. Again, the presence of mf prevalence in children is indicative of an ATP sufficient to maintain O. volvulus transmission in Touboro.13,36 Of note, the paper by Seidenfaden and others35 concluded that entomological data supported ongoing onchocerciasis transmission in Touboro after 10 years of annual ivermectin treatment. Another weakness in our entomological survey was that we compared our results with the results of Renz,14 who did not standardize collection days for Simulium captures. It is likely that this difference resulted in fewer hours spent on Simulium vector collections in the same time period compared with the collections in our follow-up study. As a result, the abundance of vectors (and calculated vector biting) at baseline is biased down, and baseline ATP is falsely lowered in Table 5 and Figure 3.
Ocular morbidity.
North Region, Cameroon, is part of a large area with a severe, blinding strain of onchocerciasis.12 The presence of mf in the anterior chamber (5.5%) and in the cornea (3.6%) indicated that low rates of recent (acute) onchocercal eye disease were still present after years of ivermectin treatment. From the entomological side, previous studies have shown that ATPs above 100 were associated with a risk of onchocercal-associated eye lesions and blindness.13,36 In particular, Touboro continues to be at risk of onchocercal eye disease based on our estimates that it has an ATP ranging from 179 to 543. Interestingly, Tchollire and Rey-Bouba both have ATP values well below 100, but two ocular cases (one mf in anterior chamber and one punctate keratitis) were discovered where adults and children were skin snip-negative. More studies are required to clarify whether under ATP values below 100 could still carry a risk for new onchocercal-associated eye lesions and blindness.
Sustaining mass treatment in post-APOC financial support era.
Available evidence shows that national governments are often unable to provide adequate financial resources to their onchocerciasis projects.37–39 However, financial responsibility for sustaining mass treatment is increasingly falling on the governments of the endemic countries and their non-governmental organizations (NGO) partners. Given that the APOC program is scheduled to close in 2015, the questions before the onchocerciasis community are (1) has EPHP been achieved in all onchocerciasis-endemic regions?, (2) will affected governments and their NGO partners be able to maintain these achievements if APOC closes in 2015?, and (3) has the CDTI strategy of annual treatment achieved transmission interruption such that mass treatments can be halted? Our answer to each of these questions is no (not everywhere). In that answer emerges the need to encourage national programs to be flexible in their future microplanning. Interventions should now be tailored to epidemiology, addressing both ongoing transmission areas like Touboro with heightened effort and near-elimination successes like Tchollire and Rey-Bouba that could see success threatened by untreated adjacent hypoendemic transmission zones of onchocerciasis.
Conclusion
This study of 12 communities in three onchocerciasis-endemic health districts in North Region concluded that 12 years of single annual dose of ivermectin for onchocerciasis may have resulted in interruption of onchocerciasis transmission in two districts, but it did not interrupt transmission in a third district, where annual treatment was given for 17 years. This district, which had the highest initial force of transmission, showed variable reductions in nodule rates and infections in young children, and an entomological study suggests ongoing transmission and therefore, risk of eye disease reappearing if interventions are stopped. In all districts, there were dramatic reductions in mf prevalence in skin. Although the reports from Senegal and Mali show that, in some areas, onchocerciasis can be eliminated with 15–17 years of annual treatment, we provided at least one example in North Cameroon where it could not. Therefore, it is imperative to focus on new innovative and flexible approaches if transmission interruption becomes the goal throughout Africa.
ACKNOWLEDGMENTS
The authors thank the staff of the Ministry of Health at the national, regional, and health district levels for their field, policy, and administrative support. Dr. Charles Mboka and Dr. Bayoro, District Medical Officers of Touboro and Tchollire health districts, respectively, and the Onchocerciasis Program Coordinator in Touboro, Mr, Mamadou Djaouro, are highly acknowledged for their leadership in smooth implementation of the study. The authors would like to acknowledge local chiefs and community members of Rey-Bouba, Tchollire, and Touboro health districts for their long efforts in sustaining ivermectin treatments for over a decade and supporting and participating in the study. The authors thank the community members who worked as Simulium fly collectors and health workers who supervised them. Baseline information would not have been possible without the thesis of Dr. A. Renz, and his work is gratefully acknowledged. We would also like to thank Dr. Renz for his direct interest in this study and his provision of unpublished mf prevalence data from 1991 for children in two communities in Tchollire. The Lions Clubs International Foundation (LCIF) with Cameroon Lions Clubs through SightFirst initiative, the Government of Cameroon, the African Programme for Onchocerciasis Control (APOC), The Carter Center, and the River Blindness Foundation provided financial support during the 17 years of annual mass treatment with ivermectin in North Region, Cameroon. The follow-up impact assessment conducted during 2008 and 2009 was funded by The Carter Center.
Footnotes
Authors' addresses: Moses N. Katabarwa and Frank O. Richards, The Carter Center, Atlanta, GA, E-mails: mkataba@emory.edu and frich01@emory.edu. Albert Eyamba and Philippe Nwane, The Carter Center, Yaounde, Cameroon, E-mails: eyamba09@hotmail.fr and philino07@yahoo.fr. Peter Enyong, Research Foundation for Tropical Diseases and Environment, Buea, Cameroon, E-mail: enyongap@yahoo.com. Souleymanou Yaya, Jean Baldiagaï, Théodore Kambaba Madi, and Abdoulaye Yougouda, Ministry of Public Health, North Region, Cameroon, E-mails: wadjiri123@yahoo.fr, baldiagaijean@yahoo.fr, theomadi53@yahoo.fr, and drousmanou@yahoo.fr. Gervais Ondobo Andze, Ministry of Public Health, Yaounde, Cameroon, E-mail: andzegervais@yahoo.fr.
References
- 1.Bottomley C, Isham V, Collins RC, Basáñez MG. Rates of microfilarial production by Onchocerca volvulus are not cumulatively reduced by multiple ivermectin treatments. Parasitology. 2008;135:1571–1581. doi: 10.1017/S0031182008000425. [DOI] [PubMed] [Google Scholar]
- 2.Cupp EW, Cupp MS. Short report: impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg. 2005;73:159–161. [PubMed] [Google Scholar]
- 3.Report WHO. African Programme for Onchocerciasis Control—report of the sixth meeting of national task forces, October 2009. Wkly Epidemiol Rec. 2010;4:23–28. [PubMed] [Google Scholar]
- 4.Katabarwa M, Eyamba A, Habomugisha P, Lakwo T, Ekobo S, Kamgno J, Kuete T, Ndyomugyenyi R, Onapa A, Salifou M, Ntep M, Richards FO. After a decade of annual dose mass ivermectin treatment in Cameroon and Uganda, onchocerciasis transmission continues. Trop Med Int Health. 2008;13:1196–1203. doi: 10.1111/j.1365-3156.2008.02126.x. [DOI] [PubMed] [Google Scholar]
- 5.Boatin BA, Hougard JM, Alley ES, Akpoboua LK, Yaméogo L, Dembélé N, Sékétéli A, Dadzie KY. The impact of Mectizan on the transmission of onchocerciasis. Ann Trop Med Parasitol. 1998;92:S46–S60. [PubMed] [Google Scholar]
- 6.Richards F, Hopkins D, Cupp E. Programmatic goals and approaches to onchocerciasis. Lancet. 2000;355:1663–1664. doi: 10.1016/S0140-6736(00)02235-2. [DOI] [PubMed] [Google Scholar]
- 7.Winnen M, Plaisier AP, Alley ES, Nagelkerke NJ, van Oortmarssen G, Boatin BA, Habbema JD. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ. 2002;80:384–391. [PMC free article] [PubMed] [Google Scholar]
- 8.Borsboom GJ, Boatin BA, Nagelkerke NJ, Agoua H, Akpoboua KL, Alley EW, Bissan Y, Renz A, Yameogo L, Remme JH, Habbema JD. Impact of ivermectin on onchocerciasis transmission: assessing the empirical evidence that repeated ivermectin mass treatments may lead to elimination/eradication in West Africa. Filaria J. 2003;2:8. doi: 10.1186/1475-2883-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndyomugyenyi R, Tukesiga E, Büttner DW, Garms R. The impact of ivermectin treatment alone and when in parallel with Simulium neavei elimination on onchocerciasis in Uganda. Trop Med Int Health. 2004;9:882–886. doi: 10.1111/j.1365-3156.2004.01283.x. [DOI] [PubMed] [Google Scholar]
- 10.Diawara L, Traoré MO, Badji A, Bissan Y, Doumbia K, Goita SF, Konaté L, Mounkoro K, Sarr MD, Seck AF, Toé L, Tourée S, Remme JH. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson J, Fuglsang H, Marshall TF, Radolowicz A, Vaughan JP. Studies on onchocerciasis in the United Cameroon Republic. IV. A four-year follow-up of six rain-forest and six savanna villages. The incidence of ocular lesions. Trans R Soc Trop Med Hyg. 1978;72:513–515. doi: 10.1016/0035-9203(78)90172-4. [DOI] [PubMed] [Google Scholar]
- 12.Chippaux JP, Boussinesq M, Fobi G, Lafleur C, Audugé A, Banos MT, Ngosso A, Prod'hon J. Effect of repeated ivermectin treatments on ocular onchocerciasis: evaluation after six to eight doses. Ophthalmic Epidemiol. 1999;6:229–246. doi: 10.1076/opep.6.4.229.4185. [DOI] [PubMed] [Google Scholar]
- 13.Renz A, Wenk P, Anderson J, Fuglsang H. Studies on the dynamics of transmission of onchocerciasis in a Sudan-savanna area of North Cameroon V. What is a tolerable level of annual transmission potential? Ann Trop Med Parasitol. 1987;81:263–274. doi: 10.1080/00034983.1987.11812119. [DOI] [PubMed] [Google Scholar]
- 14.Renz AHJ. Studies on the dynamics of transmission of onchocerciasis in a Sudan–Savanna of North Cameroon areas. Dissertation, University of Tubingen, Tubingen; Germany: 1985. [Google Scholar]
- 15.Katabarwa MN, Habomugisha P, Richards FO., Jr Implementing community-directed treatment with ivermectin for the control of onchocerciasis in Uganda (1997–2000): an evaluation. Ann Trop Med Parasitol. 2002;96:61–73. doi: 10.1179/000349802125000529. [DOI] [PubMed] [Google Scholar]
- 16.Salant P, Dilman DA. How to Conduct Your Own Survey. New York, NY: John Wiley & Sons, Inc; 1994. [Google Scholar]
- 17.Albiez EJ, Buttner DW, Duke BO. Diagnosis and extirpation of nodules in human onchocerciasis. Trop Med Parasitol. 1988;39((Suppl 4):):331–346. [PubMed] [Google Scholar]
- 18.Ngoumou P, Walsh JF, Mace JM. A rapid mapping technique for the prevalence and distribution of onchocerciasis: a Cameroon case study. Ann Trop Med Parasitol. 1994;88:463–474. doi: 10.1080/00034983.1994.11812893. [DOI] [PubMed] [Google Scholar]
- 19.Katabarwa M, Onapa AW, Nakileza B. Rapid epidemiological mapping of onchocerciasis in areas of Uganda where Simulium neavei sl. is the vector. East Afr Med J. 1999;76:440–446. [PubMed] [Google Scholar]
- 20.Prost A, Prod'hon J. Le diagnostique parasitologique de l'onchocercose. Revue critique des methods en usage. Medicine Tropicale. 1978;38:519–532. [PubMed] [Google Scholar]
- 21.Schulz-Key H. A simple technique to assess the total number of Onchocerca volvulus microfilariae in skin snips. Tropenmed Parasitol. 1978;29:51–54. [PubMed] [Google Scholar]
- 22.Report of the World Health Organization . Report of a World Health Organization Expert Committee on Onchocerciasis Control. Technical Report Series 852. Geneva, Switzerland: World Health Organization; 1995. (Onchocerciasis and its control). [PubMed] [Google Scholar]
- 23.Report of the World Health Organization . Strategies for Ivermectin Distribution Through Primary Health Care System. Geneva. Switzerland: World Health Organization; 1991. [Google Scholar]
- 24.Winthrop KL, Proaño R, Oliva O, Arana B, Mendoza C, Dominguez A, Amann J, Punkosdy G, Blanco C, Klein R, Sauerbrey M, Richards F. The reliability of anterior segment lesions as indicators of onchocercal eye disease in Guatemala. Am J Trop Med Hyg. 2006;75:1058–1062. [PubMed] [Google Scholar]
- 25.Davies JB. A rapid staining and clearing technique for detecting filarial larvae in alcohol-preserved vectors. Trans R Soc Trop Med Hyg. 1995;89:280. doi: 10.1016/0035-9203(95)90539-1. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JF, Davies JB, Le Berre R, Grams R. Standardization of criteria for assessing the effect of Simulium control in onchocerciasis control programmes. Trans R Soc Trop Med Hyg. 1978;72:675–676. doi: 10.1016/0035-9203(78)90039-1. [DOI] [PubMed] [Google Scholar]
- 27.Gardon J, Boussinesq M, Kamgno J, Gardon-Wendel N, Demanga-Ngangue, Duke BO. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: a randomised controlled trial. Lancet. 2002;360:203–210. doi: 10.1016/S0140-6736(02)09456-4. [DOI] [PubMed] [Google Scholar]
- 28.Boussinesq M, Prod'hon J, Chippaux JP. Onchocerca volvulus: striking decrease in transmission in the Vina valley (Cameroon) after eight annual large scale ivermectin treatments. Trans R Soc Trop Med Hyg. 1997;91:82–86. doi: 10.1016/s0035-9203(97)90406-5. [DOI] [PubMed] [Google Scholar]
- 29.Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH, Dominguez A, Cruz-Ortiz N, Unnasch TR, Punkosdy GA, Richards J, Sauerbrey M, Castro J, Catú E, Oliva O, Richards FO., Jr Elimination of Onchocercia volvulus transmission in the Santa Rosa focus of Guatemala. Am J Trop Med Hyg. 2007;77:334–341. [PubMed] [Google Scholar]
- 30.Report of the World Healh Organization . Criteria for Certification of Interruption of Transmission/Elimination of Human Onchocerciasis. Geneva, Switzerland: World Health Organization; (2001). (Certification of elimination of human onchocerciasis: criteria and procedures). [Google Scholar]
- 31.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. (2007);369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 32.Ndyomugyenyi R, Lakwo T, Habomugisha P, Male B. Progress towards the elimination of onchocerciasis as a public-health problem in Uganda: opportunities, challenges and the way forward. Ann Trop Med Parasitol. 2007;101:323–333. doi: 10.1179/136485907X176355. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez RJ, Cruz-Ortiz N, Rizzo N, Richards J, Zea-Flores G, Domínguez A, Sauerbrey M, Catú E, Oliva O, Richards FO, Lindblade KA. Successful interruption of transmission of Onchocerca volvulus in the Escuintla-Guatemala focus, Guatemala. PLoS Negl Trop Dis. 2009;3:e404. doi: 10.1371/journal.pntd.0000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katabarwa MN, Eyamba A, Chouaibou M, Enyong P, Kuété T, Yaya S, Yougouda A, Baldiagaï J, Madi K, Andze GO, Richards F. Does onchocerciasis transmission take place in hypoendemic areas? A study from the North Region of Cameroon. Trop Med Int Health. 2010;15:645–652. doi: 10.1111/j.1365-3156.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 35.Seidenfaden R, Fischer A, Bonow I, Ekale D, Tanya V, Renz A. Combined benefits of annual mass treatment with ivermectin and cattle zooprophylaxis on the severity of human onchocerciasis in northern Cameroon. Trop Med Int Health. 2001;9:715–725. doi: 10.1046/j.1365-3156.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 36.Duke BO, Anderson J, Fuglsang H. The Onchocerca volvulus transmission potentials and associated patterns of onchocerciasis at four Cameroon Sudan-savanna villages. Tropenmed Parasitol. 1975;26:143–154. [PubMed] [Google Scholar]
- 37.Amazigo UV, Brieger WR, Katabarwa M, Akogun O, Ntep M, Boatin B, N'doyo J, Noma N, Seketeli A. The challenges of community-directed treatment with ivermectin (CDTI) within the African Programme for Onchocerciasis Control (APOC) Ann Trop Med Parasitol. 2002;96:S75–S92. doi: 10.1179/000349802125000646. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins DR, Richards FO, Katabarwa M. Whither onchocerciasis control in Africa. Am J Trop Med Hyg. 2005;72:1–2. [PubMed] [Google Scholar]
- 39.Rakers LJ, Emukah E, Onyenama J, Amah G, Ukairo N, Enyinnaya U, Miri E, Richards F. Sustainability of ivermectin distribution programmes. Lance. 2009;374:785–786. doi: 10.1016/S0140-6736(09)61590-7. [DOI] [PubMed] [Google Scholar]


