Abstract
The parasite Schistosoma haematobium frequently causes genital lesions in women and could increase the risk of human immunodeficiency virus (HIV) transmission. This study quantifies the HIV target cells in schistosome-infected female genital mucosa. Cervicovaginal biopsies with and without schistosomiasis were immunostained for quantification of CD4+ T lymphocytes (CD3, CD8), macrophages (CD68), and dendritic Langerhans cells (S100 protein). We found significantly higher densities of genital mucosal CD4+ T lymphocytes and macrophages surrounding schistosome ova compared with cervicovaginal mucosa without ova (P = 0.034 and P = 0.018, respectively). We found no increased density of Langerhans cells (P = 0.25). This study indicates that S. haematobium may significantly increase the density of HIV target cells (CD4+ T lymphocytes and macrophages) in the female genitals, creating a beneficial setting for HIV transmission. Further studies are needed to confirm these findings and to evaluate the effect of anti-schistosomal treatment on female genital schistosomiasis.
Introduction
Schistosomiasis is a major cause of morbidity and mortality in sub-Saharan Africa.1 The waterborne parasite is co-endemic with human immunodeficiency virus (HIV) infection and several studies have suggested an association between the two diseases.2–6
The lower female reproductive tract is the main site of HIV-1 transmission, and is also the predilection site for female genital Schistosoma haematobium ova deposition.7–9 Similar to sexually transmitted infections (STIs), it has been suggested that genital lesions caused by S. haematobium ova may provide points of entry for HIV.3,10 The schistosome-infected cervix appears inflamed with abnormal mucosal blood vessels, contact bleeding, and damage to the mucosal surfaces.9
The CD4+ T lymphocytes located in the female genital mucosa are fundamental to sexual transmission of HIV-1 to women.11–13 Cervicovaginal CD68+ macrophages and Langerhans cells are also thought to play a key role.14,15 Studies have shown that CD4+ T lymphocytes and macrophages are present in the local immune response to Schistosoma mansoni ova.16
The aim of this study was to quantify the density of periovular CD4+ T lymphocytes, macrophages, and Langerhans cells in schistosome-infected female genital mucosa.
Material and Methods
Study subjects.
The study populations have previously been described in detail.17,18 In short, following informed consent, a medical history was taken, a gynaecological examination performed, and biopsies from the cervix and/or vagina sampled from 61 sexually active Malawian women 15 to 49 years of age with urinary S. haematobium infection. Female genital schistosomiasis was defined as S. haematobium ova found in cervicovaginal biopsies. Malawian women with urinary schistosomiasis but without genital S. haematobium ova served as endemic negative controls. Serological testing was not performed. Non-endemic control specimens were drawn from the diagnostic tissue biobank at Oslo University Hospital. Biopsies with normal morphology of the ectocervix and biopsies with chronic non-specific cervicitis were included as non-endemic negative and positive controls, respectively.
Histopathology and immunohistochemistry.
The biopsies were fixed in formalin, routinely processed, embedded in paraffin, serial sectioned, and stained. The results of the histological examination of hematoxylin and eosin (HE) stains have been published previously.19
The immunohistochemical stains were performed using Benchmark XT, antibody diluent (251-018), and Detection Kit Ventana ultraView Universal DAB (760-500) (Ventana Medical Systems, Inc., Tucson, AZ). Similar to findings in previous immunohistochemical studies, antibodies to CD4 cross-reacted non-specifically and could not be interpreted.20 Therefore, the density of CD4+ T lymphocytes was quantified by subtracting the density of CD8+ cells (cytotoxic T cells) from the density of CD3+ cells (mature T lymphocytes) on two consecutive 3.5 μm thick sections of each biopsy. The T cells were identified by applying mouse monoclonal antibodies to CD8 (C8/144B, Dako Denmark AS, Glostrup, Denmark) and rabbit monoclonal antibodies to CD3 (clone 2GV6, Ventana), respectively. Macrophages were identified using mouse monoclonal antibodies to CD68 (clone KP1, Dako Denmark) and Langerhans cells were identified using rabbit polyclonal antibodies to S100 protein (Ventana). Negative and positive controls were applied and the immunohistochemical stains were controlled by morphological identification. Unfortunately, the limited quantity of formalin-fixed biopsies in this study proved insufficient for further analyses of receptor expression or activity.
To study the histopathology directly adjacent to the ova, only biopsies with ova present were included in the analyses. Ova negative slides were either included as negative endemic controls or excluded if ova had been found in a previous section of the same biopsy.19 The maximum number of excluded specimens caused by undetectable ova was 18, whereas six endemic cases and 10 endemic controls were excluded because of insufficient tissue.
Microscopy and image analysis.
The sections were examined using a Leica DM3000 microscope (Leica Microsystems GmbH, Wetzlar, Germany) and photographed at 40 times objective magnification, obtaining 2,592 by 1,944 pixels color images with an attached Leica DFC420 digital camera.
The morphological and immunohistochemical analyses were performed in a standardized manner. First, the HE-stained sections were evaluated. Schistosome ova were defined as “viable” if miracidia with eosinophilic glands or germinal cells were seen,21 whereas ova containing dark purple stain identified histologically as calcification were defined as “calcified.” For the quantification of T lymphocytes and macrophages, the HE-stained sections were photographed with the S. haematobium ovum or ova placed centrally. To avoid the influence of adjacent but undetected ova, the biopsies were photographed only in areas containing ova. In biopsies with more than one cluster of ova, the most representative area for the respective biopsy's tissue reaction was included. Likewise, because ova are usually located subepithelially, the control biopsies were also photographed in a subepithelial area representative for the main tissue reaction in the respective biopsy.21,22 Thereafter, the near-exact same areas of the consecutive immunostained sections, identified by histological anatomical structures, were photographed. The investigators were blinded for the immunostains until the HE-stained sections had been chosen and photographed. All sections were examined microscopically and all photographs controlled by an experienced pathologist (BR).21,22
The principles for the computer-assisted image analyses of immunostained target cells are outlined in Figure 1. Stromal cell structures stained with antibodies to CD3, CD8, and CD68 were quantified using ImageJ, a Java image processing application downloaded free of charge from the website of the National Institutes of Health (NIH).23 The computer algorithms for the image analyses were developed according to previously described techniques.24,25 An outline of the measured structures was superimposed on the original photograph to permit manual validation. For the quantification of T lymphocytes, intact lymphocytic cells were counted separately from non-intact cell structures histopathologically identified as apoptotic cell fragments. All photographs and cell counts were controlled by an experienced pathologist and if necessary a second senior pathologist was consulted.
Figure 1.
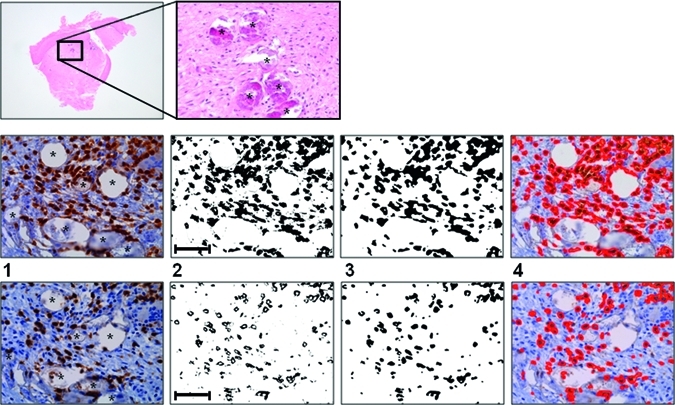
Computer-assisted image analyses of immune cells in genital mucosa. Top row: Low and high magnification of hematoxylin and eosin (HE)-stained female genital mucosa containing Schistosoma haematobium ova. All present ova are calcified (asterisks). Middle row: (1) In a subsequent section of the same biopsy as above, antibodies to CD3 identify mature T lymphocytes adjacent to calcified S. haematobium ova (asterisks). (2) Pixels representing immunohistochemically stained structures are isolated using their color properties. (3) Background noise is then removed to identify structures of interest only. (4) The measured structures are super-imposed on the original image for manual evaluation. The ruler line in picture 2 measures 0.1 mm. Bottom row: As middle row, CD8+ cells are identified by immunohistochemistry on the consecutive 3.5 μm thick section.
For the quantification of Langerhans cells, the epithelium of the immunohistochemically stained sections were photographed at 40× objective magnification and reconstructed using Adobe Illustrator CS5 software (Adobe Systems Inc., San Jose, CA). Langerhans cell structures were counted manually in an area equivalent to one high-power field to each side of the epithelium overlying the original photograph of the HE-stained section (Figure 2). Counted were only immunohistochemically stained epidermal structures with distinct demarcation and immunostained cells with distinct dendritic processes located in the basal and parabasal strata (Ventana). All sections were counted twice by one investigator and every third photograph was counted by an experienced pathologist.
Figure 2.
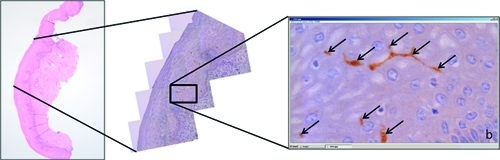
Manual count of epithelial dendritic Langerhans cells. Low and high magnification of stratified squamous epithelium of the ectocervix and S100 protein-detected epithelial dendritic Langerhans cells (arrows); b = basal cell layer.
Statistical analyses.
The statistical analyses and sample size estimation were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL) and PS Power and sample size calculations version 2.1.31. Most variables were not normally distributed and non-parametric tests were therefore used when studying associations. Medians and interquartile ranges (IQR) were used to describe the results. The Mann-Whitney U and Kruskal-Wallis H tests were applied where appropriate. Spearman's rank correlation coefficient was used when studying associations between continuous variables. Intra- and inter-observer reliabilities were determined by calculating the intra-class correlation coefficients after log-transformation of the data. A 5% significance level was used throughout.
Ethical considerations.
Permissions for the histopathological and immunohistochemical investigations of anonymized archival Malawian and Norwegian biopsies, without additional consent from the study subjects, were granted by the National Health Science and Research Committee of Malawi (2009/NHSRC #620) and the Norwegian Regional Ethics Committee (2009/1250a). The permissions were based on the fact that the proposed analyses did not require identifiable information or history, did not have any direct relevance to the physical, mental or social well-being, and did not have any direct diagnostic or therapeutic implications for the study women.
Study information was provided to the original study population in the local languages Yao and Chichewa and free informed oral consent was obtained. Following consent, all women who had urinary schistosomiasis were offered gynaecological examination. Consent was also reascertained by the physician before each step of the investigations and sampling. Treatment and follow-up for schistosomiasis, STIs, cancer, and other conditions were given in collaboration with the physicians in Mangochi District Hospital.18 The participating women were not asked for HIV testing. All women, including those who declined further investigations, were offered treatment with praziquantel. All non-endemic controls were followed up by the referring clinician in Norway.
Results
The median age of the endemic women with genital S. haematobium ova was 20.5 years of age (IQR = 18.0–28.0), of the endemic controls without ova 26.0 years (IQR = 22.0–32.0), and of the non-endemic controls 44.0 years (IQR = 30.5–55.5). Of the endemic cases included in this study, schistosome ova were found in 19 of 42 (45%). The median number of ova per high-power field was 4 (IQR = 2–8), of which 84% were calcified. The majority of ova were localized in the subepithelial tissue. Calcified and viable ova were found together in the same biopsy in one patient only. No worms were seen.
Women with genital schistosomiasis were significantly younger than endemic women without genital schistosomiasis (P = 0.028). However, there was no significant association between age and the density of CD4+ T lymphocytes or macrophages in the two groups (P = 0.51 and P = 0.65, respectively). The median ages were similar in women with viable ova (N = 2) and in women with calcified ova (N = 11); significance testing was not performed because of low sample sizes.
The intra-class correlation between computer-assisted and manual cell counts of CD3, CD8, and CD68 stained sections were 0.998 (95% CI = 0.997–0.999, P < 0.001), 0.980 (95% CI = 0.970–0.990, P < 0.001), and 0.998 (95% CI = 0.997–0.999, P < 0.001), respectively. The inter- and intra-observer reliabilities of the manual counts of Langerhans cells were 0.85 (95% CI = 0.66–0.94, P < 0.001) and 0.92 (95% CI = 0.88–0.95, P < 0.001), respectively.
CD4+ T lymphocytes.
As shown in Figure 3, the density of genital mucosal CD4+ T lymphocytes surrounding calcified schistosome ova was significantly higher compared with endemic and non-endemic controls without ova (P = 0.034 and P < 0.001, respectively). There was no significant difference in the density of CD4+ T lymphocytes between tissue with viable ova (N = 2) and tissue with calcified ova (N = 11) or endemic controls without ova (P = 0.69 and P = 0.89, respectively). As shown in Figure 3, the highest densities of genital mucosal CD4+ T lymphocytes were found in tissue with calcified S. haematobium ova and in non-endemic chronic cervicitis, between which there was no significant difference (P = 0.082). Furthermore, the density of intact CD4+ T lymphocytes was significantly associated with the density of apoptotic CD4+ cells (P = 0.026). There was no significant association between the density of CD8+ T cells and schistosome ova in female genital mucosa (P = 0.23).
Figure 3.
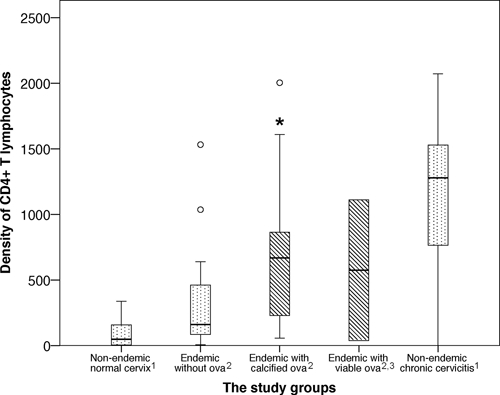
The density of CD4+ T lymphocytes in each study group. The density of CD4+ T lymphocytes/mm2 cervicovaginal stroma was significantly higher surrounding calcified Schistosoma haematobium ova compared with endemic and non-endemic tissue without ova (asterisk, P < 0.05). The whiskers represent the minimum and maximum values < 1.5 times the interquartile range (IQR), whereas the circles represent outliers (values > 1.5 times IQR). The most representative area for each biopsy was carefully selected by an experienced pathologist. In biopsies containing ova, the most representative high-power field surrounding the ova was analyzed. (1) Non-endemic = Norwegian (N = 45). (2) Endemic = Malawian (N = 29). (3) There were no significant differences between specimens with viable schistosome ova (N = 2) and the other study groups.
Macrophages.
The density of CD68+ macrophages was significantly higher in cervicovaginal mucosa with viable S. haematobium ova compared with endemic women with calcified ova (P = 0.044), endemic women without genital schistosomiasis (P = 0.018), and non-endemic women with healthy cervical tissue (P = 0.006). The highest densities of macrophages were found in cervicovaginal mucosa with viable ova and in non-endemic chronic cervicitis, and between them there was no significant difference (P = 0.32). The lowest densities of macrophages were seen in cervicovaginal tissue with calcified ova and endemic and non-endemic controls without ova, and between them there was no significant difference (P = 0.45).
Langerhans cells.
We found no difference in the density of genital mucosal Langerhans cells between women with genital S. haematobium ova and endemic or non-endemic controls without ova (P = 0.25 and P = 0.43, respectively). Furthermore, we found no association between the densities of CD4+ T lymphocytes and epithelial Langerhans cells in the genital mucosa of endemic women with and without ova (P = 0.88).
Medical history.
The density of mucosal CD4+ T lymphocytes was not associated with the different menstrual phases (follicular P = 0.15 and lutheal P = 0.73, respectively). Our sample was too small to perform statistical analyses correcting for the frequency of sexual intercourse, prior treatment of STIs, or prior anti-schistosomal treatment.
Discussion
Schistosoma haematobium ova frequently cause lesions in the female genital mucosa and studies suggest that female genital schistosomiasis may increase the risk of HIV transmission.3,4,26 In this study from Malawi, cervicovaginal mucosa with S. haematobium ova contained more CD4+ T lymphocytes and macrophages than genital mucosa without ova. The CD4+ T lymphocytes were associated with calcified ova and CD4+ cell apoptosis. Calcified ova are typical of chronic S. haematobium infection, although cell apoptosis may indicate an active, ongoing immune reaction in this chronically infected tissue.27 Viable S. haematobium ova on the other hand, were associated with an increased density of macrophages. These findings suggest that both recent and chronic S. haematobium infection stimulate tissue responses in the cervicovaginal mucosa that could influence HIV susceptibility.
In this study, the density of epithelial Langerhans cells in the cervicovaginal mucosa was not associated with schistosome ova in the underlying stroma. A recent study indicates that the function of dendritic cells might be impaired in human S. haematobium infection.28 Results from experimental studies indicate that S. mansoni infection may activate dendritic cells by a modulated pathway.29 It was not possible to explore dendritic cell function or activation in these formalin-fixed specimens because of their limited sizes.
Previous histopathological studies of human S. haematobium infection in the urinary and female genital tracts have shown a range of immune cells surrounding parasite ova.19,21,22,27,30–35 These studies did not include control populations. However, the findings are in line with results from experimental studies on hepato-intestinal schistosomiasis.36–38 To our knowledge, this study from Malawi is the first to quantify the periovular density of HIV target cells in schistosome infected cervicovaginal mucosa.
The sample size is small and the findings are prone to type 1 and type 2 errors. This makes the interpretation of the results related to viable ova difficult, particularly when they are non-significant. Furthermore, in presumed negative cases, schistosome ova might have been located just outside the studied sections. The density of CD4+ T lymphocytes was estimated from the number of CD3+ and CD8+ cells on two consecutive 3.5 μm thick sections. Although the thickness of the sections is less than half the average diameter of a lymphocyte, the reported CD4+ cell number may to some extent differ from its true count.25 Our calculation of CD4+ T lymphocytes may be slightly underestimated because antibodies to CD8 also recognize some γδ T cells and natural killer cells (Dako Denmark).
Some shortcomings, if resolved, would probably increase the analyzed differences between cases and controls. First, it is probable that some endemic controls have a missed diagnosis of genital schistosomiasis.9 Second, the HIV prevalence has subsequently been estimated to be approximately 10% of the population in 1994,39 but individual HIV diagnosis was not a part of the original project and could therefore not be done. The HIV-1 infection would likely reduce the CD4+ cell density.11
In a clinical cross-sectional study in Zimbabwe, the odds for being infected with HIV was nearly three times higher in women with genital schistosomiasis than in women without.4 The results from our study suggest that periovular CD4+ T lymphocytes could play an important role in the interaction between the two infections.40 Clinical data indicate that female schistosomiasis causes chronic damage to the cervicovaginal mucosa and urinary tract if not prevented by anti-schistosomal treatment in childhood.1,41 Mass treatment is deemed necessary to prevent morbidity and reinfection and has been implemented on a large scale in several African countries.42
In conclusion, this study shows that S. haematobium ova in female genital mucosa are surrounded by an increased density of CD4+ T lymphocytes and macrophages. The CD4+ T lymphocytes are concentrated around calcified ova, indicating that S. haematobium ova may sustain an increased long-lasting HIV susceptibility in the cervicovaginal mucosa. Further studies are needed to validate these findings, to further explore the immunopathology, and to evaluate the effect of preventive anti-schistosomal mass treatment on female genital schistosomiasis.
ACKNOWLEDGMENTS
We thank the women who participated in this study, L. Chitsulo, H. Feldmeier, I. Krantz, Helling-Giese, and N. Kumwenda who assisted in the recruitment phase, and J. Richter who took part in clinical examinations. Special thanks to research technician T. Norén for her invaluable experience and stamina, and to B. Myrvang, D. Kvale, and L. Sandvik for excellent support. We also thank I. R. Panadero and C. G. Aurlund for their kind help.
Disclosure: Parts of this study were presented at the South African National AIDS Conference (SAAIDS) in Durban, 7–10 June 2011.
Footnotes
Financial support: This study was supported by the Centre for Imported and Tropical Diseases, Oslo University Hospital Ulleval, the University of Oslo, GlobInf, and Schering-Plough's Research Grant for Young Investigators.
Authors' addresses: Peter Mark Jourdan, Centre for Imported and Tropical Diseases, Department of Infectious Diseases, Oslo University Hospital Ulleval, Oslo, Norway, E-mail: p.m.jourdan@medisin.uio.no. Sigve Dhondup Holmen, Faculty of Medicine, University of Oslo, Oslo, Norway, E-mail: sigve.holmen@gmail.com. Svein Gunnar Gundersen, Research Unit, Sorlandet Hospital HF, Kristiansand, Norway, and Centre for Development Studies, University of Agder, Kristiansand, Norway, E-mail: svein.g.gundersen@sshf.no. Borghild Roald, Centre for Paediatric and Pregnancy Related Pathology, Department of Pathology, Oslo University Hospital Ulleval, Oslo, Norway, and Faculty of Medicine, University of Oslo, Oslo, Norway, E-mail: borghild.roald@medisin.uio.no. Eyrun Floerecke Kjetland, Centre for Imported and Tropical Diseases, Department of Infectious Diseases, Oslo University Hospital Ulleval, Oslo, Norway, E-mail: e.f.kjetland@medisin.uio.no.
Reprint requests: Peter Mark Jourdan, Centre for Imported and Tropical Diseases, Department of Infectious Diseases, Oslo University Hospital Ulleval, Postboks 4956 Nydalen, 0424 Oslo, Norway, Fax: +47 2211 9181, Tel: +47 2211 9101, E-mail: p.m.jourdan@medisin.uio.no.
References
- 1.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chenine AL, Shai-Kobiler E, Steele LN, Ong H, Augostini P, Song R, Lee SJ, Autissier P, Ruprecht RM, Secor WE. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. 2008;2:e265. doi: 10.1371/journal.pntd.0000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]
- 4.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 5.WHO . Report of an informal working group on urogenital schistosomiasis and HIV transmission, 1–2 October 2009. Geneva; Switzerland: 2009. [Google Scholar]
- 6.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, Kalluvya SE, Changalucha JM, Johnson WD, Jr, Fitzgerald DW. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asin SN, Eszterhas SK, Rollenhagen C, Heimberg AM, Howell AL. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis. 2009;200:965–972. doi: 10.1086/605412. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand M, Ross WF, Blair DM, Weber MC. Distribution and extent of schistosomiasis in female pelvic organs, with special reference to the genital tract, as determined at autopsy. Am J Trop Med Hyg. 1971;20:846–849. doi: 10.4269/ajtmh.1971.20.846. [DOI] [PubMed] [Google Scholar]
- 9.Kjetland EF, Ndhlovu PD, Mduluza T, Gomo E, Gwanzura L, Mason PR, Kurewa EN, Midzi N, Friis H, Gundersen SG. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72:311–319. [PubMed] [Google Scholar]
- 10.Chen L, Jha P, Stirling B, Sgaier SK, Daid T, Kaul R, Nagelkerke N. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS ONE. 2007;2:e1001. doi: 10.1371/journal.pone.0001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shacklett BL. Immune responses to HIV and SIV in mucosal tissues: ‘location, location, location’. Curr Opin HIV AIDS. 2010;5:128–134. doi: 10.1097/COH.0b013e328335c178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 13.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 14.de Jong MA, Geijtenbeek TB. Human immunodeficiency virus-1 acquisition in genital mucosa: Langerhans cells as key-players. J Intern Med. 2009;265:18–28. doi: 10.1111/j.1365-2796.2008.02046.x. [DOI] [PubMed] [Google Scholar]
- 15.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 17.Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis. 2011;5((6)):e1170. doi: 10.1371/journal.pntd.0001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjetland EF, Poggensee G, Helling-Giese G, Richter J, Sjaastad A, Chitsulo L, Kumwenda N, Gundersen SG, Krantz I, Feldmeier H. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop. 1996;62:239–255. doi: 10.1016/s0001-706x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 19.Helling-Giese G, Sjaastad A, Poggensee G, Kjetland EF, Richter J, Chitsulo L, Kumwenda N, Racz P, Roald B, Gundersen SG, Krantz I, Feldmeier H. Female genital schistosomiasis (FGS): relationship between gynecological and histopathological findings. Acta Trop. 1996;62:257–267. doi: 10.1016/s0001-706x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- 20.Macon WR, Salhany KE. T-cell subset analysis of peripheral T-cell lymphomas by paraffin section immunohistology and correlation of CD4/CD8 results with flow cytometry. Am J Clin Pathol. 1998;109:610–617. doi: 10.1093/ajcp/109.5.610. [DOI] [PubMed] [Google Scholar]
- 21.Wright ED, Chipangwi J, Hutt MSR. Schistosomiasis of the female genital tract. A histopathological study of 176 cases from Malawi. Trans R Soc Trop Med Hyg. 1982;76:822–829. doi: 10.1016/0035-9203(82)90118-3. [DOI] [PubMed] [Google Scholar]
- 22.Berry A. A cytopathological and histopathological study of bilharziasis of the female genital tract. Path Bact. 1966;91:325–337. doi: 10.1002/path.1700910206. [DOI] [PubMed] [Google Scholar]
- 23.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 24.Furness PN. The use of digital images in pathology. J Pathol. 1997;183:253–263. doi: 10.1002/(SICI)1096-9896(199711)183:3<253::AID-PATH927>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Halama N, Zoernig I, Spille A, Westphal K, Schirmacher P, Jaeger D, Grabe N. Estimation of immune cell densities in immune cell conglomerates: an approach for high-throughput quantification. PLoS ONE. 2009;4:e7847. doi: 10.1371/journal.pone.0007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renaud G, Devidas A, Develoux M, Lamothe F, Bianchi G. Prevalence of vaginal schistosomiasis caused by Schistosoma haematobium in an endemic village in Niger. Trans R Soc Trop Med Hyg. 1989;83:797. doi: 10.1016/0035-9203(89)90333-7. [DOI] [PubMed] [Google Scholar]
- 27.Christie J, Crouse D, Pineda J, Anis-Ishak E, Smith J, Kamel I. Patterns of Schistosoma hematobium egg distribution in the human lower genital tract. I. Noncancerous lower urinary tracts. Am J Trop Med Hyg. 1986;35:743–751. doi: 10.4269/ajtmh.1986.35.743. [DOI] [PubMed] [Google Scholar]
- 28.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perona-Wright G, Jenkins SJ, MacDonald AS. Dendritic cell activation and function in response to Schistosoma mansoni. Int J Parasitol. 2006;36:711–721. doi: 10.1016/j.ijpara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Ghoneim MA. Bilharziasis of the genitourinary tract. BJU Int. 2002;89((Suppl 1)):22–30. doi: 10.1046/j.1464-4096.2001.138.138.x. [DOI] [PubMed] [Google Scholar]
- 31.Friedberg D, Berry A, Schneider J. Schistosomiasis of the female genital tract. S Afr Med J. 1991;8:S1–S16. [PubMed] [Google Scholar]
- 32.Charlewood GP, Shippel S, Renton H. Schistosomiasis in gynaecology. J Obstet Gynaecol Br Emp. 1949;56:367–385. doi: 10.1111/j.1471-0528.1949.tb07107.x. [DOI] [PubMed] [Google Scholar]
- 33.Badawy A. Schistosomiasis of the cervix. BMJ. 1962;5275:369–372. doi: 10.1136/bmj.1.5275.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams AO. Pathology of schistosomiasis of the uterine cervix due to S. haematobium. Am J Obstet Gynecol. 1967;98:784–791. doi: 10.1016/0002-9378(67)90194-9. [DOI] [PubMed] [Google Scholar]
- 35.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 36.Cheever AW, Eltoum IA, Andrade ZA, Cox TM. Biology and pathology of Schistosoma mansoni and Schistosoma japonicum infections in several strains of nude mice. Am J Trop Med Hyg. 1993;48:496–503. doi: 10.4269/ajtmh.1993.48.496. [DOI] [PubMed] [Google Scholar]
- 37.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101((Suppl 1)):327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 38.Ji MJ, Su C, Wang Y, Wu HW, Cai XP, Li GF, Zhu X, Wang XJ, Zhang ZS, Wu GL. Characterization of CD4+ T cell responses in mice infected with Schistosoma japonicum. Acta Biochim Biophys Sin (Shanghai) 2006;38:327–334. doi: 10.1111/j.1745-7270.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 39.UNAIDS Global report: 2008 report on the global AIDS epidemic. 2008.
- 40.Secor WE. Interactions between schistosomiasis and infection with HIV-1. Parasite Immunol. 2006;28:597–603. doi: 10.1111/j.1365-3024.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 41.Kjetland EF, Ndhlovu PD, Kurewa EN, Midzi N, Gomo E, Mduluza T, Friis H, Gundersen SG. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am J Trop Med Hyg. 2008;79:79–83. [PubMed] [Google Scholar]
- 42.Fenwick A. Waterborne infectious diseases–could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]


