Abstract
Both insecticide-treated bed nets (ITNs) and indoor residual spraying (IRS) reduce malaria in high malaria transmission areas.1–3 The combined effect of these interventions is unknown. We conducted a non-randomized prospective cohort study to determine protective efficacy of IRS with ITNs (ITN + IRS) compared with ITNs alone (ITN only) in preventing Plasmodium falciparum parasitemia. At baseline, participants provided blood samples for malaria smears, were presumptively treated for malaria, and received ITNs. Blood smears were made monthly and at sick visits. In total, 1,804 participants were enrolled. Incidence of P. falciparum parasitemia in the ITN + IRS and ITN only groups was 18 and 44 infections per 100 persons-years at risk, respectively (unadjusted rate ratio = 0.41; 95% confidence interval [CI] = 0.31–0.56). Adjusted protective efficacy of ITN + IRS compared with ITN only was 62% (95% CI = 0.50–0.72). The combination of IRS and ITN might be a feasible strategy to further reduce malaria transmission in areas of persistent perennial malaria transmission.
Introduction
Malaria continues to be a leading cause of morbidity and mortality in Africa. In 2008, there were over 247 million cases of malaria and nearly 1 million deaths.4 International goals have been set to dramatically reduce malaria illness and death.5 To achieve these goals, effective tools to prevent malaria, including indoor residual spraying (IRS) and insecticide-treated nets (ITNs), are being scaled up across Africa.
ITNs reduce malaria morbidity and all causes of malaria mortality across a variety of transmission settings.1 African Ministries of Health have used innovative means to increase ITN number and use, and reductions in morbidity and mortality have been observed in Kenya and elsewhere coincident with ITN scale up.6,7
IRS also reduces malaria morbidity and mortality.3,8 Until recently, IRS was not considered feasible in areas of perennial transmission because of the logistical complexity and considerable resources required to conduct a spray campaign, which in an area of perennial transmission, would need to be done several times per year. Consequently, IRS had been relegated to seasonal transmission or epidemic-prone areas. Recently, longer-lasting residual insecticide formulations have become available,9 a development that might make IRS a feasible option in areas of perennial transmission.
There has been considerable debate about the relative merits of ITNs and IRS. Review of previous intervention trials, including direct comparisons of ITNs and IRS, indicates that ITNs and IRS have similar efficacy, whereas the cost effectiveness of each intervention is dependent on the unique setting where it is implemented.8,10,11 A recent Cochrane review concluded that, in areas of perennial malaria transmission, there are insufficient data to conclude whether IRS or ITN provides better protection against malaria.2
As each intervention is scaled up within Africa, it is increasingly likely that many people will be protected by both ITNs and IRS. It is unknown whether the combination of these interventions reduces malaria transmission beyond the reduction that would be seen by one of these interventions used alone. Recent data from cross-sectional surveys seem to indicate an added benefit of IRS with ITNs.6 If ITNs and IRS have additive or synergistic effects when applied in combination, then a strategy of combined IRS plus ITNs may be an effective way to drive transmission to very low levels. However, if there is no added benefit of combining ITNs and IRS, then implementation of both is an inefficient use of scarce resources.
Kenya began an IRS program in Rachuonyo District in Western Kenya in 2008. We used this opportunity to conduct a non-randomized prospective cohort study to compare the combined effectiveness of ITNs and IRS with ITNs alone in preventing Plasmodium falciparum parasitemia in areas of perennial malaria transmission. We compared Rachuonyo District with Nyando District, an adjacent district with similar malaria transmission levels12 where IRS was not conducted.
Methods
Study site and population.
Rachuonyo and Nyando Districts are located in Nyanza Province in Western Kenya. A community-based survey conducted in April of 2008 before the initiation of IRS in Rachuonyo District showed similar malaria prevalence in the two districts (9% in Rachuonyo and 11% in Nyando) and lower ITN use in Rachuonyo, where 37% of compound members reportedly slept under an ITN the prior night compared with 50% in Nyando (Kenya Medical Research Institute [KEMRI]/Centers for Disease Control and Prevention [CDC], unpublished data). Malaria transmission in Nyanza Province is high and perennial, with seasonal peaks in April to July and November to December. The main malaria vectors in this region are Anopheles gambiae s.s., An. arabiensis, and An. funestus. Historically, An. arabiensis has been the predominant vector in Rachuonyo and Nyando.13 Both districts have benefited from heavily subsidized distributions of ITNs targeting pregnant women and children.
The majority of the population in both districts are members of the Luo ethnic group who earn their living through subsistence farming and fishing.14 The region has been described elsewhere in detail.15
Procedures.
The Kenya Ministry of Health (MOH) supported an IRS campaign from July to September of 2008 using λ-cyhalothrin capsule suspension (ICON CS; Syngenta, AG, Midrand, South Africa), a longer-lasting residual insecticide.16 Every living space in Rachuonyo District was targeted for IRS. The IRS campaign was repeated in April and May of 2009 using alphacypermethrin (Fendon).
We randomly selected households within 1 km of three health facilities in Rachuonyo and within 1 km of three health facilities in Nyando for inclusion in the cohort study. Health facilities in the two districts were similar with respect to type, approximate use rate, and elevation. We limited the radius of enrollment to 1 km around each health facility to improve likeliness that every febrile illness would present to the health facility.
In November of 2008, 2 months after the first IRS campaign was completed, all members of randomly selected compounds were informed about the study. All non-pregnant compound members aged > 6 months were eligible for enrollment, and if they provided consent (or assent if aged 13–17 years), they were enrolled for participation. Women aged 13–45 years were provided a urine pregnancy test if their last menstrual period was not within the prior 4 weeks. Pregnant women were excluded to avoid exposure of the fetus to antimalarial drugs provided to participants at baseline. A questionnaire was administered to assess the use of antimosquito measures and antimalaria drugs. A new long-lasting insecticide-treated net (LLITN) was provided for every sleeping space, and participants were encouraged to replace older ITNs to ensure uniformity of insecticide and durability. All participants provided blood samples for baseline malaria detection by microscopy and hemoglobin measurement by Hemocue.
To ensure that all P. falciparum infections were cleared, including subpatent infections, participants were provided a treatment course of artemether-lumefantrine (AL) at baseline. Participants with a positive blood smear at baseline had a repeat blood smear after 2 weeks to ensure parasite clearance. Anemic participants were provided hematinics according to MOH guidelines.
Participants were visited monthly and encouraged to present to the clinic when sick. At monthly and sick visits, a blood smear was made, and hemoglobin was measured for endpoint analysis. A rapid diagnostic test for malaria (RDT) was made for clinical care. Anyone found to be RDT-positive received AL, anyone found to be blood smear-positive received AL and completed the study, and anyone with a hemoglobin < 11 g/dL was treated with hematinics. Participants were followed for up to 9 months.
Microscopy.
All blood smears were stained using Giemsa and read independently by two microscopists. Discordant results were resolved by a third microscopist.
Entomological monitoring.
Each month, an index house was randomly selected from the study area, and pyrethrum spray catches (PSCs) were conducted on the index and neighboring houses, whether or not study participants inhabited the selected houses, to measure adult anopheline mosquito numbers. In total, 20–30 houses were included for PSC per month. All mosquitoes collected were identified to species morphologically17,18 and by polymerase chain reaction (PCR) for the identification of An. gambiae s.l.19 to sibling species level.
Throughout the study period, we measured effectiveness of residual insecticide in Rachuonyo by exposing susceptible mosquitoes in plastic cones attached to the walls of 10 houses according to recommended procedures, and measuring mosquito mortality 24 hours after exposure.20
Statistical analysis.
Primary outcome measures were incidence of P. falciparum parasitemia, incidence of moderate anemia (hemoglobin < 8), and differences in entomological indices in the ITN + IRS and the ITN only groups. Measurement of time at risk and P. falciparum parasitemia events began 10.5 days after enrollment to account for the half life of AL.21 If a participant was RDT-positive and received AL but was blood smear-negative, we decreased time at risk by 10.5 days. The primary analysis was by intention, to treat; data were also analyzed according to protocol. Kaplan–Meier survival plots were used to describe time to first P. falciparum infection censored at the subject's last study visit. We compared anemia prevalence, ITN usage (having slept under an ITN the prior night), and other categorical variables between the groups at enrollment using log-binomial regression models. We compared time to P. falciparum infection and moderate anemia using Poisson regression models. Follow-up time was truncated at last study date or first P. falciparum infection. In the adjusted analysis, we controlled for study clinic, housing type (Table 1 shows housing types), baseline P. falciparum parasitemia, and seasonality, and ITN use as time-varying covariates. Generalized estimating equations were used in the above regression models to account for correlation within compounds when calculating 95% confidence intervals. Entomological data were analyzed using Poisson regression controlling for clustering at the village levels. Comparisons of species composition and sporozoite rates were done using logistic regression controlling for clustering at the village level. All analyses were done using SAS statistical software (SAS 9.1).
Table 1.
Study population characteristics at enrollment of ITN + IRS versus ITN only
| ITN + IRS (N = 919) | ITN only (N = 885) | P value | |
|---|---|---|---|
| Median age in years (range) | 12 (0.5–105) | 12 (0.6–91) | 0.61 |
| Female (%) | 57 | 56 | 0.57 |
| Head of household completed primary school (%) | 20 | 32 | 0.03 |
| Mother completed primary school (%) | 21 | 29 | 0.05 |
| Household type | |||
| Traditional mud hut (%) | 10 | 3 | 0.002 |
| Semi-permanent (corrugated iron roof) (%) | 64 | 84 | 0.002 |
| Permanent (concrete or stone walls) (%) | 26 | 12 | 0.39 |
| Eves open (%) | 90 | 94 | 0.25 |
| Mosquito prevention methods | |||
| Mosquito coils, insecticide spray, or repellents used in prior week (%) | 6 | 6 | 0.96 |
| Received IRS (%) | 73 | 6 | < 0.001 |
| At least one bed net in house (%) | 71 | 72 | 0.95 |
| Slept under an ITN the prior night (%) | |||
| < 5 years | 36 | 30 | 0.24 |
| 5–14 years | 20 | 15 | 0.20 |
| ≥ 15 years | 30 | 22 | 0.05 |
| Overall | 28 | 21 | 0.04 |
| Fever in prior 2 weeks (%) | |||
| < 5 years | 51 | 44 | 0.18 |
| 5–14 years | 34 | 21 | < 0.002 |
| ≥ 15 years | 60 | 38 | < 0.001 |
| Overall | 49 | 33 | < 0.001 |
| Took antimalarial in prior 2 weeks (%) | 21 | 28 | 0.08 |
| Took ACT in prior 2 weeks (%) | 6 | 10 | 0.09 |
| Mean hemoglobin (g/dL) | |||
| < 5 years | 11.2 | 10.5 | < 0.001 |
| 5–14 years | 12.6 | 12.3 | 0.008 |
| ≥ 15 years | 12.6 | 12.6 | 0.95 |
| Overall | 12.3 | 12.0 | 0.009 |
| P. falciparum parasitemia prevalence (%) | |||
| < 5 years | 8 | 6 | 0.48 |
| 5–14 years | 12 | 9 | 0.37 |
| ≥ 15 years | 2 | 2 | 0.95 |
| Overall | 7 | 5 | 0.39 |
| Geometric mean P. falciparum parasite density per microliter) among participants with parasites | 1,136 | 835 | 0.45 |
| Clinical malaria (P. falciparum parasitemia with fever; %) | |||
| < 5 years | 1.9 | 0.5 | 0.24 |
| 5–14 years | 2.4 | 0.5 | 0.05 |
| ≥ 15 years | 0.3 | 0.6 | 0.55 |
| Overall | 1.4 | 0.5 | 0.05 |
Ethical review.
Informed consent was obtained from all human adult participants and the parents or legal guardians of minors. This protocol was approved by the ethical review boards of KEMRI and CDC.
Role of the funding source.
The sponsor had no role in the study design, data collection, analysis, interpretation, or writing the report.
Results
Baseline.
In total, 1,804 household members were enrolled, 919 in the ITN + IRS group and 885 in the ITN only group. Of those people enrolled, 86% completed the 9-month follow-up period, with no difference between study groups (P = 0.92) (Figure 1). Study groups differed at baseline; in the ITN + IRS group, household heads were less likely to have completed primary school education, houses were more likely to be traditional mud and less likely to be semi-permanent structures, overall ITN use was higher, and participants were more likely to have had a fever in the prior 2 weeks than those participants in the ITN only households (Table 1). Children < 15 years of age in the ITN + IRS group had a higher mean hemoglobin. Parasite prevalence did not differ significantly between the two study groups. Clinical malaria, defined as P. falciparum parasitemia accompanied by reported fever in the prior 24 hours, was low in both study groups. In total, 74% of households in Rachuonyo and 6% in Nyando reportedly received IRS.
Figure 1.
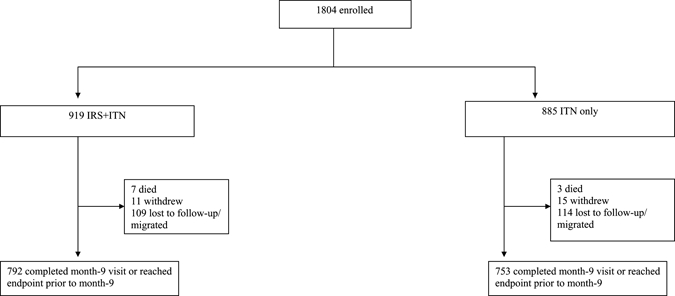
Study profile.
Follow up.
Participants were followed for 1,197 person-years, 627 and 570 person-years in the ITN + IRS and ITN only groups, respectively. There were 114 and 251 events of P. falciparum parasitemia among those participants in the ITN + IRS and ITN only groups, respectively, resulting in 18 and 44 events per 100 person-years, respectively (unadjusted rate ratio [RR] = 0.41, 95% confidence interval [CI] = 0.31–0.56). The overall adjusted protective efficacy (aPE) of ITN + IRS compared with ITN only was 62%. ITN + IRS provided significant protective efficacy in every age category, with the greatest protective efficacy (67%) observed in those 6 months to 4 years of age (aPE = 0.67, 95% CI = 0.38–0.82) (Table 2). Kaplan–Meier curves showing time to first P. falciparum parasitemia by age group are shown in Figure 2. Clinical malaria likewise was reduced in the ITN + IRS compared with ITN only group, with 57 and 157 events of clinical malaria recorded, respectively, resulting in 9 and 27 events per 100 person-years (unadjusted RR = 0.34, 95% CI = 0.24–0.49). Incidence of moderate anemia was low, with no difference between the study groups overall or by age group (Table 2).
Table 2.
Unadjusted and adjusted malaria parasitemia incidence and anemia incidence in ITN + IRS and ITN only by age group*
| ITN + IRS | ITN only | RR (95% CI) | Adjusted RR* (95% CI) | aPE* (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Person-years at risk | Rate per 100 person-years | Events | Person-years at risk | Rate per 100 person-years | ||||
| Malaria incidence | |||||||||
| Overall | 114 | 627 | 18 | 251 | 570 | 44 | 0.41 (0.31–0.56) | 0.38 (0.28–0.50) | 62 (0.50–0.72) |
| 6 months to 4 years | 21 | 146 | 14 | 57 | 133 | 43 | 0.34 (0.20–0.56) | 0.33 (0.18–0.62) | 67 (0.38–0.82) |
| 5–14 years | 70 | 220 | 32 | 137 | 186 | 74 | 0.43 (0.30–0.62) | 0.37 (0.26–0.54) | 63 (0.46–0.74) |
| ≥ 15 years | 23 | 261 | 9 | 57 | 251 | 23 | 0.39 (0.23–0.65) | 0.34 (0.18–0.64) | 66 (0.36–0.82) |
| Anemia incidence (hemoglobin < 8) | |||||||||
| Overall | 38 | 633 | 6 | 46 | 583 | 8 | 0.76 (0.49–1.19) | 0.83 (0.51–1.34) | 17 (−0.34–0.49) |
| 6 months to 4 years | 14 | 144 | 10 | 18 | 133 | 14 | 0.72 (0.35–1.47) | 0.99 (0.43–2.29) | 1 (−1.29–0.57) |
| 5–14 years | 3 | 228 | 1 | 6 | 196 | 3 | 0.43 (0.10–1.84) | 0.50 (0.15–1.70) | 50 (−0.70–0.85) |
| ≥ 15 years | 21 | 260 | 8 | 22 | 255 | 9 | 0.93 (0.50–1.73) | 0.85 (0.42–1.73) | 15 (−0.73–0.58) |
Adjusted for study clinic, baseline parasitemia, and housing type at baseline and seasonality and ITN use as time-varying variables.
Figure 2.
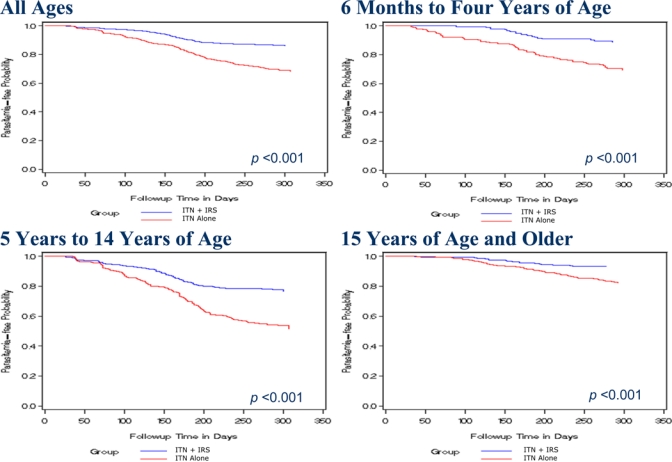
Time to first malaria parasitemia (ITN + IRS versus ITN alone) in Nyanza Province, Kenya.
We also assessed the incidence of P. falciparum parasitemia in a per-protocol analysis, removing those participants who reported at all follow-up visits that they did not sleep under an ITN the prior night (N = 58), who lived in the ITN + IRS area but did not have their house sprayed during the initial IRS campaign (N = 242), or who lived in the ITN only area who reported having their houses sprayed (N = 54) from the analysis. Results from this per-protocol analysis (data not shown) were similar to the results from the intention to treat analysis.
During follow up, participants in the ITN only group who developed P. falciparum parasitemia had significantly higher geometric mean parasite density than those participants in the ITN + IRS group (4,266 versus 1,760 parasites/μL, P < 0.0001). ITN use differed at month 9, with 72% of participants in the ITN + IRS group and 98% of participants in the ITN only group reportedly using ITNs (P < 0.001).
Incident rates for first or only P. falciparum malaria varied between study clinics; participants enrolled near the three study clinics in the ITN + IRS area experienced 10, 14, and 29 events per 100 person-years, whereas those participants enrolled near the three study clinics in the ITN only area had 17, 46, and 87 events per 100 person-years.
Entomologic data.
The average number of anopheline mosquitoes collected in pyrethrum spray catches is shown by month in Figure 3. In April of 2008, before the IRS campaign began, there was no difference in the total number of anopheline mosquitoes collected from houses in the two districts (RR = 0.91, 95% CI = 0.67–1.23, P = 0.71). After the IRS campaign, anopheline density was low in Rachuonyo relative to Nyando District through October of 2009 with the exception of two months (May and June of 2009). These months corresponded to the peak transmission period, during which the second round of IRS was being conducted, and may indicate waning efficacy of the first IRS round before the second round was completed. Overall, from September 2008 to October 2009, there were significantly fewer anopheline mosquitoes in Rachuonyo compared with Nyando District (RR = 0.40, 95% CI = 0.22–0.72, P < 0.001).
Figure 3.
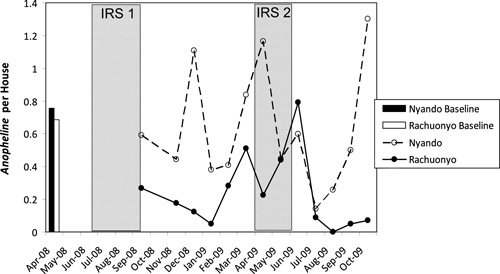
Number of anopheline mosquitoes per house before and after IRS in Rachuonyo (IRS) and Nyando Districts (no IRS), Kenya.
In both districts, An. gambiae s.l. was the primary species captured, although there was a higher proportion of An. funestus in Nyando compared with Rachuonyo both pre- and post-IRS. Before IRS was implemented, An. funestus accounted for 15.0% of the anophelines collected from Nyando and 1.6% of the anophelines collected from Rachuonyo (odds ratio [OR] = 0.09, 95% CI = 0.02–0.49, P = 0.03). From August of 2008 to October of 2009, An. funestus accounted for 9.2% of anophelines collected from Nyando and 2.7% of anophelines collected from Rachuonyo (OR = 0.36, 95% CI = 0.12–1.22, P = 0.06). Of 121 An. gambiae s.l. collected in April 2008, 120 were determined by PCR to be An. arabiensis. From August of 2008 to October of 2009, the proportion of An. gambiae s.l. that was identified as An. arabiensis was 97.5% in Rachuonyo and 96.8% in Nyando (OR = 2.27, 95% CI = 0.32–16.16, P = 0.45). Sporozoite enzyme-linked immunosorbent assays (ELISAs) were done on 483 anopheline mosquitoes collected after spraying began. Sporozoite rates were lower in Rachuonyo (1.5%) compared with Nyando (3.1%), but the difference was not statistically significant (OR = 0.48, 95% CI = 0.12–1.91, P = 0.34).
Wall bioassays were conducted each month from September of 2008 to September of 2009. Valid data were obtained for 12 of 13 months; adjusted mortality was > 50% for all 12 months and > 75% for 10 of 12 months.
Discussion
We found that the combination of IRS and ITNs provided significantly greater protection than the protection provided by ITNs alone in preventing malaria P. falciparum infection. Participants who received both interventions experienced a 61% reduction in P. falciparum parasitemia compared with those participants who had ITNs and no IRS, and the benefit extended to all household members, regardless of age. Moreover, those participants in the ITN + IRS group who developed malaria infection had a lower parasite density. The added benefit provided by IRS was observed, despite only 74% of households in the IRS + ITN group having received IRS.
Entomologic data show that IRS was effective in reducing anopheline mosquito numbers within households, and the anopheline mosquitoes remained highly susceptible to the lethal effects of the applied insecticide throughout the study period. Surprisingly, wall bioassays resulted in high mosquito mortality for the full 8 months between the first and second IRS campaigns, considerably longer than the 6-month expected effectiveness of λ-cyhalothrin described by the World Health Organization (WHO),9 suggesting that, with proper timing, a yearly application of this insecticide could be a feasible control strategy.
Despite a significant difference in malaria incidence in the two study groups, we did not find a difference in incidence of moderate anemia, and development of moderate anemia overall was infrequent. This finding likely was because of the study design; hemoglobin was measured monthly and at sick visits, iron supplementation was provided whenever hemoglobin was found to be < 11.0 g/dL, and malaria was detected and treated early.
We observed a reduction in ITN use among participants in the ITN + IRS group, which may have been a consequence of reduced mosquitoes in the house or the perception of reduced malaria risk. Any program that aims to promote the combination of the two strategies will need to include an educational campaign to promote continued ITN use.
Implementation of IRS is not suitable for every setting; it is resource-intensive and requires well-trained, well-coordinated spray teams and homes accessible to those teams. Our data show that a well-planned strategy, implemented under programmatic conditions, can result in reduced P. falciparum infection even in the setting of high ITN coverage. In fact, the combination of IRS and ITNs may be particularly effective in East Africa and similar areas where the primary malaria vectors include anthropophagic, endophilic vectors, such as An. gambiae s.s. and An. funestus, and a more zoophagic, endophilic vector such as An. arabiensis. High ITN coverage was associated with a dramatic decline of An. gambiae s.s. and An. funestus and near replacement by An. arabiensis in the KEMRI/CDC demographic surveillance area near the study site described in this report.13 Despite an overall decline in vector numbers, malaria transmission has been sustained at relatively high levels; community surveys indicate over 40% of children < 5 years of age have P. falciparum parasitemia (KEMRI/CDC, unpublished data). The current primary vector, An. arabiensis, has more varied feeding options and may survive by feeding on alternate hosts when humans are unavailable. IRS reduces the post-feeding resting place options for all anophelines, including An. arabiensis, which will often rest indoors after feeding outdoors.22 Thus, the combination of IRS and ITNs may work synergistically to eliminate both vector species simultaneously, providing a means to efficiently drive down malaria transmission.
Currently, the Kenyan Malaria Control Program relies on pyrethroid insecticides for both IRS and ITNs, and the development of insecticide resistance is a major concern. Resistance to pyrethroids in An. gambiae and An. funestus has been detected in several sites throughout Africa23–26 and has been documented to reduce the effectiveness of IRS in Bioko Island27 and South Africa.28 An advantage of IRS is that four classes of insecticides are available for application on walls; currently, only pyrethroids are considered safe, effective, and long-lasting for use on ITNs. Insecticide resistance management strategies include rotations, where insecticides are switched at regular intervals, and mosaics, where different insecticides are applied in different locations at the same time. The use of non-pyrethroids for IRS in combination with ITNs may represent a form of mosaic application of insecticides, where pyrethroids are on ITNs and non-pyrethroids are on walls, and should be explored as an approach to manage insecticide resistance.
Our study had limitations. First, because of the programmatic nature of the intervention, we were unable to randomize households or blind study staff. We controlled for known confounders, but there is always the risk that unknown confounders exist. We are reassured that the areas are adjacent, with similar population-based parasite prevalence before the introduction of IRS, and the areas have similar access to healthcare. Second, malaria incidence rates varied between study clinics within study areas. Ideally, we would have had pre-intervention incident rates from each study clinic to compare with post-intervention rates, but these rates are not available. Third, because ITNs have proven efficacy in areas of high transmission, we did not include an IRS only group, which could have provided information about whether the effects of ITNs and IRS act synergistically. Without an IRS only treatment arm, we cannot conclude whether the effects of IRS in the context of high ITN ownership would have been observed in the absence of ITNs. If so, the rationale for combining these interventions would be lacking, and vector control in Africa should be directed towards IRS. Given the progress in scaling up ITNs, this question may be difficult to answer definitively. Finally, the follow-up period was limited; over the years, insecticide resistance may develop, resulting in limited use of particular families of insecticide.28
In summary, this study provides the first prospectively collected data on the combined benefit of IRS and ITNs. These findings confirm several observational cross-sectional surveys that indicate an additive benefit from the combination of IRS and ITNs.29 The protective efficacy was substantial, suggesting that the combination of IRS and ITNs could be an effective intervention to further reduce malaria transmission in areas with persistent perennial malaria. These findings should be confirmed through a randomized controlled trial. Additionally, to help determine the best use of finite resources, a cost-effectiveness analysis would be useful and should explore the benefit of providing more protection to a limited number of homes through the combination of IRS and ITNs and less protection with ITNs alone to a larger number of homes. Until those data are available, our data support the deployment of the combination of IRS and ITNs or the introduction of IRS in an area with high ITN coverage where funds permit.
ACKNOWLEDGMENTS
This study was conducted as part of the Malaria Transmission Consortium (MTC) with support from the Bill and Melinda Gates Foundation Grant 45114. We would like to acknowledge the contributions of the MTC staff, the District Health Management Teams of Rachuonyo and Nyando, and the study participants. We would like to thank Daisy Abongo, Dr. Willis Akhwale, and Neil Lobo for their contributions. The authors thank the Director of the Center for Global Health Research, Kenya Medical Research Institute for his support. This paper was published with the approval of the KEMRI Director.
Disclaimer: Views expressed in this paper represent the authors and do not necessarily reflect the views of the US Centers for Disease Control and Prevention.
Footnotes
Financial support: This work was supported by the Bill and Melinda Gates Foundation through the Malaria Transmission Consortium (Grant 45114).
Authors' addresses: Mary J. Hamel, Laurence Slutsker, and John Gimnig, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: mhamel@cdc.gov, lslutsker@cdc.gov, and jgimnig@cdc.gov. Peter Otieno, Nabie Bayoh, Simon Kariuki, Vincent Were, Doris Marwanga, Kayla F. Laserson, and John Williamson, Kenya Medical Research Institute/Centers for Disease Control and Prevention Research and Public Health Collaboration, Kisumu, Kenya, E-mails: potieno@ke.cdc.gov, nbayoh@ke.cdc.gov, skariuki@ke.cdc.gov, vwere@ke.cdc.gov, dmarwanga@ke.cdc.gov, klaserson@ke.cdc.gov, and jwilliamson@ke.cdc.gov.
References
- 1.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne D, Grab B, Fontaine RE, Hempel JH. Impact of control measures on malaria transmission and general mortality. Bull World Health Organ. 1976;54:369–377. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Malaria Key Facts Sheet. 2010. http://www.who.int/mediacentre/factsheets/fs094/en/ Available at. Accessed May 25, 2011.
- 5.United Nations. The Millenium Development Goals Report. New York, NY: United Nations; 2009. p. 60. [Google Scholar]
- 6.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJ, Coleman M. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. [PMC free article] [PubMed] [Google Scholar]
- 7.O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis CF, Mnzava AE. Comparison of house spraying and insecticide-treated nets for malaria control. Bull World Health Organ. 2000;78:1389–1400. [PMC free article] [PubMed] [Google Scholar]
- 9.WHOPES . Report of the 10th WHOPES Working Group Meeting: Review of Spinosad 0.5% GR & 12% SC, Lambda-Cyhalothrin 10% CS, K-O TAB 1-2-3, Interceptor. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 10.Misra SP, Webber R, Lines J, Jaffar S, Bradley DJ. Malaria control: bednets or spraying? Spray versus treated nets using deltamethrin—a community randomized trial in India. Trans R Soc Trop Med Hyg. 1999;93:456–457. doi: 10.1016/s0035-9203(99)90335-8. [DOI] [PubMed] [Google Scholar]
- 11.Rowland M. Malaria control: bednets or spraying? Malaria control in the Afghan refugee camps of western Pakistan. Trans R Soc Trop Med Hyg. 1999;93:458–459. doi: 10.1016/s0035-9203(99)90336-x. [DOI] [PubMed] [Google Scholar]
- 12.Malaria Atlas Project Malaria Atlas Project. 2010. http://www.map.ox.ac.uk Available at. Accessed May 25, 2011.
- 13.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips-Howard PA, Nahlen BL, Kolczak MS, Hightower AW, ter Kuile FO, Alaii JA, Gimnig JE, Arudo J, Vulule JM, Odhacha A, Kachur SP, Schoute E, Rosen DH, Sexton JD, Oloo AJ, Hawley WA. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68:23–29. [PubMed] [Google Scholar]
- 15.Phillips-Howard PA, Nahlen B, Alaii JA, ter Kuile FO, Gimnig JE, Terlouw DJ, Kachur SP, Hightower AW, Lal AA, Schoute E, Oloo AJ, Hawley WA. The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya I. Development of infrastructure and description of study site. Am J Trop Med Hyg. 2003;68((4 Suppl)):3–9. [PubMed] [Google Scholar]
- 16.World Health Organization . Report of the 4th WHOPES Working Group Meeting. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 17.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara: South African Institute for Medical Research, Publication 55. Johannesburg, South Africa: South African Institute of Medical Research; 1987. [Google Scholar]
- 18.Gillies MT, De Meillon B. The Anophelinae of Africa, South of the Sahara. Johannesburg. South Africa: South African Institute of Medical Research; 1968. [Google Scholar]
- 19.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . Manual on Practical Entomology in Malaria. Part II. Methods and Techniques. Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- 21.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyewole IO, Awolola TS, Ibidapo CA, Oduola AO, Okwa OO, Obansa JA. Behaviour and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J Vector Borne Dis. 2007;44:56–64. [PubMed] [Google Scholar]
- 23.Cuamba N, Morgan JC, Irving H, Steven A, Wondji CS. High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe District in Mozambique. PLoS One. 2010;5:e11010. doi: 10.1371/journal.pone.0011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One. 2010;5:e11872. doi: 10.1371/journal.pone.0011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 29.Kleinschmidt I, Schwabe C, Benavente L, Torrez M, Ridl FC, Segura JL, Ehmer P, Nchama GN. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80:882–888. [PMC free article] [PubMed] [Google Scholar]


