Abstract
Determining mosquito age is important to evaluate vector control programs because the ability to transmit diseases is age dependent. Current age-grading techniques require dissection or RNA extraction. Near infrared spectroscopy has been used to rapidly and nondestructively determine the age of fresh mosquitoes and specimens stored in RNAlater, but other preservation techniques have not been examined. Thus, in this study, we investigate whether age can be predicted from insects preserved by various common methods. Results from this study show that age can be predicted from mosquitoes preserved with desiccants, ethanol, Carnoy, RNAlater, or refrigeration with confidence intervals < 1.4 days. The best results were generally obtained from mosquitoes stored using desiccants, RNAlater, or refrigeration.
Mosquito age grading, or the ability to determine age, is important in vector control programs. Because of the extrinsic incubation period of the parasites and pathogens that mosquitoes transmit, only older mosquitoes are potential vectors of mosquito-borne diseases. Indeed, novel control strategies envision a shift in population structure toward younger mosquitoes, thereby reducing the number of disease-transmitting individuals in the mosquito population.1,2 A wide variety of techniques has been used for age grading in mosquitoes. These include changes in ovarian morphology,3 cuticular hydrocarbons,4 pteridine concentrations,5 and gene transcription.6,7 Recently, near infrared spectroscopy (NIRS), which quantitatively measures organic compounds, e.g., O–H, N–H, and C–H functional groups, has been used for mosquito species identification and age grading. This method is non-invasive and was initially performed on fresh specimens from laboratory colonies or mosquitoes reared under semi-field conditions.8,9 However, the use of fresh specimens under field conditions might be impractical. The preservative RNAlater (Ambion, Austin, Texas) has been used to store mosquitoes for NIRS age grading and species identification,10 but this is an expensive storage medium. Here, we compare the effect of various lower cost storage methods commonly used for insects on our ability to predict mosquito age using NIRS.
Anopheles gambiae s.s. mosquitoes (G3 strain) were reared at Kansas State University, Manhattan, Kansas, as described previously.8 Sugar-fed females were collected at the ages of 1, 5, 9, and 13–15 days and placed in cups capped with netting, and then immobilized by placing a cotton bud imbibed with chloroform (Sigma-Aldrich Co., St. Louis, MO) on the netting cap of the cup for 7–10 min. Anesthetized mosquitoes were transferred in groups of 20 to a 9-cm Spectralon disk and scanned using the LabSpec 5000 (ASD Inc., Boulder, CO) as described previously.8 Spectra of the head and thorax were collected in absorbance mode using the ASD RS3 software. Mosquitoes were scanned fresh, and then immediately stored in 1.5-mL microcentrifuge tubes in the appropriate media for ∼1 week, 1 month, and 2 months before they were scanned again. Storage media included anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Co., Xenia, OH), ethanol (95%), Carnoy fixative (3:1 ethanol:acetic acid), silica gel, RNAlater, and refrigerated at 5°C. Approximately 40 insects were scanned for each age and storage medium. Insects stored in liquid were placed for a few minutes on paper towels that absorbed excess liquid, and to allow liquid to evaporate. Refrigerated samples were allowed to equilibrate to room temperature and any condensation was allowed to evaporate before scanning. Ages, storage media, and storage times are listed in Table 1.
Table 1.
Age and storage times of Anopheles gambiae s.s. mosquitoes used to determine the effect of preservation techniques on age-grading using near infrared spectroscopy*
| RNAlater | Ethanol | Carnoy | Drierite | Silica gel | Refrigerated | |
|---|---|---|---|---|---|---|
| Mosquito age, days | 1, 5, 9, 15 | 1, 5, 9, 14 | 1, 5, 9, 13 | 1, 5, 9, 13 | 1, 5, 9, 14 | 1, 5, 9, 14 |
| Storage time, days | 7, 42, 62 | 8, 29, 58 | 9, 28, 52 | 14, 28, 56 | 7, 28, 50 | 8, 28, 50 |
Approximately 40 insects of each age were tested, and all were scanned fresh in addition to scanning after the specified storage time.
Spectra were analyzed using the GRAMS PLSPlus/IQ (Thermo Galactic, Salem, NH) software using cross-validations as described previously.8 The wavelength region analyzed was 500–2,350 nm. Spectra outside this wavelength range were noisy because of sensor and lighting limitations. Percentages of correctly predicted mosquito ages were obtained using the ratio of the number of mosquitoes predicted to the total number of mosquitoes actually in that age for each category of a set of treatments. Age predictions were analyzed using a SAS (Cary, NC) PROC MIXED model to determine differences among storage times (fresh and about 1 week, 1 month, and 2 months). A quadratic term was added to model the nonlinearity between predicted and actual ages. Confidence intervals for mean predictions were calculated to assess prediction errors.
The effects of different storage methods of adult female An. gambiae s.s. on age grading by NIR are summarized in Table 2. There was no significant difference (P ≥ 0.01) when predicting age from fresh mosquitoes or those stored in RNAlater, Drierite, silica gel, or refrigerated. Results were similar when considering the effect of the interaction of mosquito age*storage method. A plot of the actual versus predicted age showed a nonlinear relationship (Figure 1) using a quadratic term (actual age*actual age) to account for this nonlinearity in the statistical analysis gave a statistically significant correlation (P < 0.01). However, including this quadratic term did not change any conclusions regarding whether any storage methods gave different results from those obtained using fresh insects.
Table 2.
Statistics showing the probability that age predictions from mosquitoes stored in various media differ from age predictions from fresh insects
| RNAlater | Ethanol | Carnoy | Drierite | Silica gel | Refrigerated | |
|---|---|---|---|---|---|---|
| Storage method | P = 0.0415 | P < 0.0001 | P < 0.0001 | P = 0.0745 | P = 0.6195 | P = 0.7293 |
| Actual age*Storage method | P = 0.0289 | P < 0.0001 | P < 0.0001 | P = 0.1213 | P = 0.8729 | P = 0.0478 |
Figure 1.
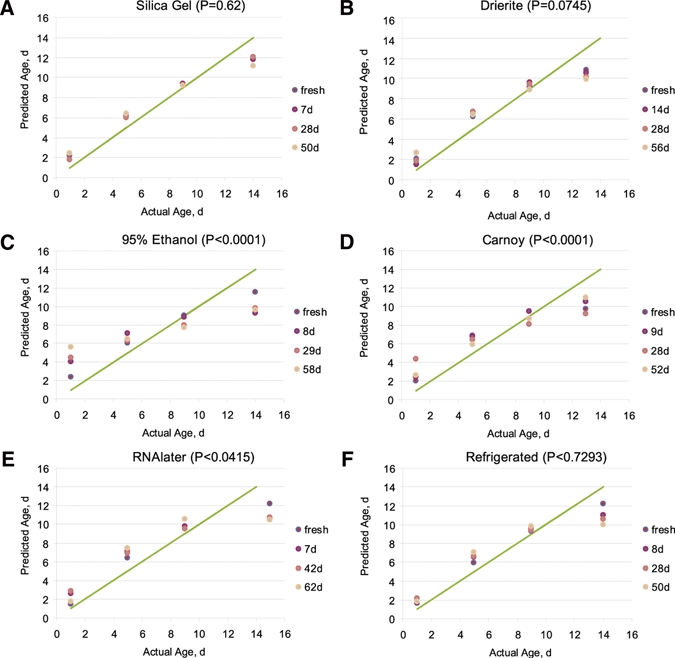
Actual versus predicted age of mosquitoes scanned fresh and after being stored with different preservatives for ∼1 week to 2 months. P values indicate whether the near-infrared spectroscopy predictions differ when comparing fresh and stored insects.
Table 3 shows results for classifying mosquitoes into “young” (< 7 days) and “old” (≥ 7 days) categories. This approach was selected so data could be condensed into one value for evaluating the impacts of storage methods and length of storage time on age-grading accuracy, and 7 days was chosen to correspond to mosquitoes approaching malaria-transmission age. The classification accuracy for fresh insects exceeded that of all stored insects, with the exception of the 7–14-day-old mosquitoes stored in Drierite. Mosquitoes stored in Drierite or silica gel, or under refrigeration, generally had the highest classification accuracy, whereas mosquitoes stored in ethanol had the lowest classification accuracy. The confidence intervals (CIs) of storage times at each actual age overlapped such that there was no significant influence of the length of storage time on classification accuracy (data not shown). Thus, storage time did not affect classification accuracy within the time range studied, suggesting a minimum of 2 months between mosquito sampling and analysis is acceptable for accurate analysis. When classifying as young or old, the 5-day insects were most likely to be misclassified (data not shown). Spectra appear to rapidly change around this age, and thus obtaining and scanning insects early or late on Day 5 may contribute to some variability in classification accuracy.
Table 3.
Accuracy (% correct) of classifying Anopheles gambiae mosquitoes as young (< 7 days) or old (≥ 7 days) when scanned fresh or after storing using different preservation techniques
| Length of preservation time | RNAlater | Ethanol | Carnoy | Drierite | Silica gel | Refrigerated |
|---|---|---|---|---|---|---|
| Fresh | 86.0 | 92.5 | 86.3 | 83.7 | 93.8 | 89.4 |
| 7–14 days | 77.0 | 69.6 | 78.3 | 85.1 | 86.5 | 85.0 |
| 28–42 days | 80.9 | 78.0 | 82.7 | 83.1 | 84.7 | 84.4 |
| 50–62 days | 75.6 | 71.4 | 81.7 | 74.6 | 81.0 | 75.0 |
Overall, the best NIRS age prediction results were achieved with mosquitoes preserved using desiccants, refrigeration, or RNAlater when considering classification accuracies, CIs, and comparison to fresh insects, with accuracies of about 80% and CIs around 1.2 days (Table 4). The poorest results were obtained from insects preserved with ethanol or Carnoy fixative, with classification accuracy of 73.0% and 80.9% and CIs of 1.36 and 1.29 days, respectively. These trends agree with those reported by Perez-Mendoza and others15 when predicting age of fly heads scanned fresh or after storing in a desiccant or ethanol, and by Sikulu and others10 for age-grading RNAlater-preserved mosquitoes. Our results indicate that calibrations for insects stored in ethanol or Carnoy would give the poorest results; however, based on our data, reasonable NIRS calibrations can likely be developed (albeit with varying classification accuracy) for any of the preservation methods that were tested. Although models using insects stored in Carnoy fixative for about 2 months were better than other preservation results at that storage time (Table 3), the CI (Table 4) and difference from fresh insects (Table 2) cause it to generally rank lower than other storage media. It is important to note that calibrations developed using one medium is valid only for insects stored in that medium. Any changes in formulation or insect treatment before storage may require new calibrations if changes affect NIR absorbance within the wavelength range being analyzed.
Table 4.
Comparison of mosquito preservation methods
| Storage method | Comparison to fresh insects* | Accuracy of classifying as young (< 7 d) or old (≥ 7 d) (%) | Avg. 95% confidence interval (days) | Preservation cost per 1.5 mL vial† | Suitability for field use | Health hazards | Suitability for DNA extraction‡ | Suitability for dissection§ |
|---|---|---|---|---|---|---|---|---|
| Fresh | – | 88.6 | 1.14 | None | Must scan immediately | None | Good | Good |
| Drierite | No significant difference | 80.9 | 1.15 | $0.01 | Must keep activated | Minimal | Fair-Good | Poor-Good |
| Silica gel | No significant difference | 84.1 | 1.16 | $0.01 | Must keep activated | Minimal | Fair-Good | Poor-Good |
| RNAlater | No significant difference | 77.8 | 1.33 | $1.00 | Must keep submerged | Minimal | Excellent | Good |
| Ethanol | Significantly different | 73.0 | 1.36 | $0.01 | OK | Low | Fair-Good | Poor-Fair |
| Carnoy | Significantly different | 80.9 | 1.29 | $0.15 | OK | Significant | Poor | Fair |
| Refrigerated | No significant difference | 81.5 | 1.15 | $0.01 | Must keep cool | None | Good | Good |
Other factors, including cost, toxicity of the storage medium, preservation of nucleic acids, and/or specific field settings may be crucial for selection of a specific mosquito preservation method (Table 4). Overall, insects stored using Drierite and silica gel has advantages over those preserved using ethanol and Carnoy in some respects. Additionally, these desiccation methods are commonly used in the field, and existing collections (dried using these methods) could be age-graded by NIRS. Rapid desiccation using silica gel results in specimens that can be difficult to determine internal musculature anatomy.12 Desiccated samples must remain dry to minimize enzymatic activity. Drierite is also a desiccating agent, but absorbs less moisture than silica gel.13 Dried samples can be used for molecular assays, but with variable results caused by shearing of the DNA during the initial drying period.11
DNA preservation in field-caught mosquitoes is crucial for monitoring of ongoing vector control programs, and polymerase chain reaction-based techniques have been established for determining a wide variety of relevant characteristics, such as vector species, infection status, and insecticide resistance. Additionally, with the ability to perform genome-wide single nucleotide polymorphism association studies for An. gambiae s.s. and the falling cost of whole-genome sequencing, these relatively new techniques can now be applied to field-collected mosquito specimens.16 However, these techniques require high-quality DNA, which is difficult to obtain from samples preserved by desiccation methods or in ethanol-based storage media.11,12,14 However, high-quality DNA can be obtained from specimens stored in RNAlater, which is non-toxic and non-flammable. Samples can be stored for 1 week at room temperature in this preservative, and for one month at 4°C, making it appropriate for use in most field settings. Although the cost of this preservative is substantially higher than desiccation methods, it is the only storage medium that allows RNA preservation at room temperature. Therefore, although RNA profiling from field-collected mosquitoes has thus far found limited application and has relied on proximal laboratory settings, novel techniques such as RNAseq are now being transferred to the field.17–19
In summary, the data shown here are further testimony to the applicability of NIRS for age grading of An. gambiae s.s. Unlike other established methods of age grading, NIRS is non-invasive and can analyze samples preserved using various storage methods. Our data indicate that it should be possible to use this technique on existing specimen collections, although further analyses are needed to determine the maximum storage time compatible with accurate results.
ACKNOWLEDGMENTS
We thank Jade Savage, Biological Sciences, Bishop's University, Canada, and Stefanie Fischnaller, Institute of Ecology, University of Innsbruck, Austria for comments on an early version of this manuscript; Mark West, Statistician, ARS, Ft. Collins, Colorado, for help with statistical analyses; and Tinea Graves and KaraJo Sprigg for mosquito rearing.
Disclaimer: The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Footnotes
Financial support: A.N. would like to thank J. A. Ajienka, Vice-Chancellor, University of Port Harcourt; O. Akaranta, Director, Center for Research Management; O. Ebong, Head, Malaria Research Laboratory; S. N. Okiwelu, Medical and Veterinary Entomology Unit, Department of Animal and Environmental Biology; and E. F. Osaisai, Director General, Nigeria Atomic Energy Commission, for providing fellowship funding to study in the United States.
Authors' addresses: Floyd E. Dowell, Engineering and Wind Erosion Research Unit, Center for Grain and Animal Health Research, U.S. Department of Agriculture, Agricultural Research Service, Manhattan, Kansas, E-mail: Floyd.dowell@ars.usda.gov. Aline E. M. Noutcha, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria, E-mail: naemekeu@yahoo.com. Kristin Michel, Division of Biology, Kansas State University, Manhattan, Kansas, E-mail: kmichel@k-state.edu.
References
- 1.Sinkins SP, O'Neill SL. In: Wolbachia as a vehicle to modify insect populations. Insect Transgenesis: Methods and Applications. Handler AM, James AA, editors. Boca Raton, FL: CRC Press; 2000. pp. 271–287. [Google Scholar]
- 2.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7:e1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugo LE, Quick-Miles S, Kay BH, Ryan PA. Evaluations of mosquito age grading techniques based on morphological changes. J Med Entomol. 2008;45:353–369. doi: 10.1603/0022-2585(2008)45[353:eomagt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Chen CS, Mulla MS, March RB, Chaney JD. Cuticular hydrocarbon patterns in Culex quinquefasciatus as influenced by age, sex, and geography. Bull Soc Vector Ecol. 1990;15:129–139. [Google Scholar]
- 5.Wu D, Lehane MJ. Pteridine fluorescence for age determination of Anopheles mosquitoes. Med Vet Entomol. 1999;13:48–52. doi: 10.1046/j.1365-2915.1999.00144.x. [DOI] [PubMed] [Google Scholar]
- 6.Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O'Neill SL. Predicting the age of mosquitoes using transcriptional profiles. Nat Protoc. 2007;2:2796–2806. doi: 10.1038/nprot.2007.396. [DOI] [PubMed] [Google Scholar]
- 7.Wang MH, Marinotti O, James AA, Walker E, Githure J, Yan G. Genome-wide patterns of gene expression during aging in the African malaria vector Anopheles gambiae. PLoS ONE. 2010;13:e13359. doi: 10.1371/journal.pone.0013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayagaya VS, Michel K, Ferguson HM, Benedict MQ, Wirtz RA, Dowell FE. Non-destructive determination of age and species of Anopheles gambiae s.l. using near-infrared spectroscopy. Am Soc Trop Med J. 2009;81:622–630. doi: 10.4269/ajtmh.2009.09-0192. [DOI] [PubMed] [Google Scholar]
- 9.Sikulu M, Killeen GF, Hugo LE, Ryan PA, Dowell KM, Wirtz RA, Moore SJ, Dowell FE. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasit Vectors. 2010;3:e49. doi: 10.1186/1756-3305-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikulu M, Dowell KM, Hugo LE, Wirtz RA, Michel K, Peiris KH, Moore S, Killeen GF, Dowell FE. Evaluating RNAlater as a preservative for using near-infrared spectroscopy to predict Anopheles gambiae s.l. age and species. Malar J. 2011;10:186. doi: 10.1186/1475-2875-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisanti M, Ganassi S, Mandrioli M. Comparative analysis of various fixative solutions on insect preservation for molecular studies. Entomol Exp Appl. 2009;130:290–296. [Google Scholar]
- 12.Quicke DL, Belshaw R, Lopez-Vaamonde C. Preservation of hymenopteran specimens for subsequent molecular and morphological study. Zool Scr. 1999;28:261–267. [Google Scholar]
- 13.Nagy ZT. A hands-on overview of tissue preservation methods for molecular genetic analysis. Org Divers Evol. 2010;10:91–105. [Google Scholar]
- 14.Mandrioli M, Borsatti F, Mola L. Factors affecting DNA preservation from museum-collected lepidopteran specimens. Entomol Exp Appl. 2006;120:239–244. [Google Scholar]
- 15.Perez-Mendoza J, Dowell FE, Broce AB, Throne JE, Wirtz RA, Xie F, Fabrick JA, Baker JA. Chronological age-grading of house flies by using near-infrared spectroscopy. J Med Entomol. 2002;39:499–508. doi: 10.1603/0022-2585-39.3.499. [DOI] [PubMed] [Google Scholar]
- 16.Neafsey DE, Lawniczak MK, Park DJ, Redmond SN, Coulibaly MB, Traoré SF, Sagnon N, Costantini C, Johnson C, Wiegand RC, Collins FH, Lander ES, Wirth DF, Kafatos FC, Besansky NJ, Christophides GK, Muskavitch MA. SNP genotyping defines complex gene-flow boundaries among African malaria vector mosquitoes. Sci. 2010;330:514–517. doi: 10.1126/science.1193036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindh JM, Terenius O, Faye I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl Environ Microbiol. 2005;71:7217–7223. doi: 10.1128/AEM.71.11.7217-7223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller P, Chouaïbou M, Pignatelli P, Etang J, Walker ED, Donnelly MJ, Simard F, Ranson H. Pyrethroid tolerance is associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in Northern Cameroon. Mol Ecol. 2008;17:1145–1155. doi: 10.1111/j.1365-294X.2007.03617.x. [DOI] [PubMed] [Google Scholar]
- 19.Mendes AM, Schlegelmilch T, Cohuet A, Awono-Ambene P, De Iorio M, Fontenille D, Morlais I, Christophides GK, Kafatos FC, Vlachou D. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog. 2008;16:e1000069. doi: 10.1371/journal.ppat.1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]


