Abstract
A field trial was conducted in a Lyme disease-endemic area of New Jersey to determine the efficacy of a doxycyline hyclate rodent bait to prophylactically protect and cure small-mammal reservoirs and reduce infection rates in questing Ixodes scapularis ticks for Borrelia burgdorferi and Anaplasma phagocytophilum. The doxycycline-laden bait was formulated at a concentration of 500 mg/kg and delivered during the immature tick feeding season in rodent-targeted bait boxes. The percentage of infected small mammals recovered from treated areas after 2 years of treatment was reduced by 86.9% for B. burgdorferi and 74% for A. phagocytophilum. Infection rates in questing nymphal ticks for both B. burgdorferi and A. phagocytophilum were reduced by 94.3% and 92%, respectively. Results from this study indicate that doxycycline-impregnated bait is an effective means of reducing infection rates for B. burgdorferi and A. phagocytophilum in both rodent reservoirs and questing I. scapularis ticks.
Introduction
Despite continued increases in the incidence of disease resulting from pathogens transmitted by Ixodes scapularis Say, including Lyme disease, human babesiosis, and human granulocytic anaplasmosis, in many parts of the United States, successful prevention efforts have not kept pace.1 As a result, the development of reliable interventions to reduce tick-borne disease risk remains a public health priority.2,3
Reducing vector tick abundance is among the most effective ways of mitigating the risk of disease transmission.3,4 Area-wide application of chemical acaricides is perhaps the most widely investigated, rapid, and reliable means of suppressing I. scapularis.2,5,6 However, the use of habitat-targeted acaricides is generally viewed by the public as having adverse health effects and undesirable environmental impacts.7,8 Consequently, it has been estimated that < 25% of households in Lyme disease-endemic communities treat their properties to control ticks.9,10 Host-targeted chemical control provides an alternative to area-wide acaricide applications. The 4-Poster (ARS, USDA, Kerrville, TX) topical treatment device, which targets adult I. scapularis parasitizing white-tailed deer (Odocoileus virginianus), and the Maxforce TMS (Tick Box Technology Corp., Norwalk, CT) bait boxes, designed to treat a variety of small-mammal hosts of immature I. scapularis, have been shown to be effective when used alone or in combination.11–13 Although these technologies have the advantage of minimizing the amount of acaricide introduced into the environment, their widespread use has been constrained by regulatory and economic considerations.1,12
We report a novel host-targeted approach using doxycycline hyclate-impregnated bait.14–16 After promising laboratory studies, we conducted a field trial in a Lyme disease-endemic area in central New Jersey to assess the ability of these baits to prophylactically protect small-mammal reservoirs, cure infected reservoirs, and reduce infection of immature I. scapularis ticks by both the Lyme disease spirochete, Borrelia burgdorferi, and the agent of human granulocytic anaplasmosis, Anaplasma phagocytophilum.
Materials and Methods
Study areas.
The study was conducted in an area of mixed hardwood forest located in Millstone Township, Monmouth County, New Jersey, where I. scapularis and its small-mammal hosts are well-established.12,17,18 The 10- to 15-m canopy was dominated by chestnut oak (Quercus prinus L.), red oak (Q. rubra L.), and white oak (Q. alba L.), with associated species including red maple (Acer rubrum L.), yellow poplar (Liriodendron tulipifera L.), black gum (Nyssa sylvatica Marsh.), and American beech (Fagus grandifolia Ehrh.). The understory and shrub layer consisted of saplings and seedlings of canopy species, highbush blueberry (Vaccinium corymbosum L.), lowbush blueberry (V. angustifolium Ait.), huckleberries (Gaylussacia spp.), sweet pepperbush (Clethra alnifolia L.), and greenbriar (Smilax glauca Walt.). The treatment site and one of the control sites were ∼2.5-ha single-family residential properties located ∼200 m apart. A second undeveloped control site (∼3 ha) was located ∼1,000 m south in nearby Assunpink Wildlife Management Area (WMA).
Deployment and maintenance of bait stations.
Beginning in May of 2007 and 2008, 50 Protecta LP bait stations (Bell Laboratories, Inc., Madison, WI) were fitted with ∼135 g extruded, wax-based bait blocks containing 500 mg/kg doxycycline (Genesis Laboratories, Inc., Wellington, CO) and deployed at the treatment site. Bait stations were placed in forested habitat at ∼20-m intervals along two concentric perimeters at distances of ∼5 and 25 m from the lawn–forest interface. Whenever possible, bait stations were deployed adjacent to natural structures, including fallen trees, stumps, brush piles, woodpiles, and outbuildings, which are considered to be likely foraging or nesting sites for small mammals. Bait stations were inspected weekly to assess overall condition and bait consumption, and they were rebaited as necessary. Consumption of bait was recorded using a five-point Activity Index (1 = no bait consumption, 2 = 25% bait consumed, 3 = 50% bait consumed, 4 = 75% bait consumed, and 5 = 100% bait consumed) (Figure 1). In addition, any bait station that was significantly damaged by eastern gray squirrels (Sciurus carolinensis) was replaced. All bait stations were withdrawn in September of both years after 19 consecutive weeks of deployment.
Figure 1.
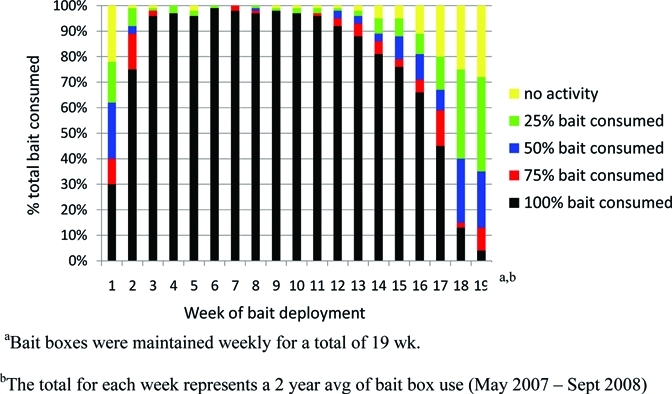
Summary of doxycycline bait station use as measured by estimates of bait consumption. Bait consumption was based on an activity index: 1 = no activity, 2 = 25% bait consumed, 3 = 50% bait consumed, 4 = 75% bait consumed, and 5 = 100% bait consumed. Bait stations were deployed from June to September for a total of 19 wk in 2007 and 2008.
Small-mammal trapping and tick burdens.
Small mammals were collected using 7.6 × 8.9 × 30.5-cm Sherman non-folding box traps (H. B. Sherman, Tallahassee, FL) baited with rolled oats for food and cotton balls for nesting material and 40.6 × 12.7 × 12.7-cm Tomahawk Model 102 rigid single-door live traps (Tomahawk Traps and Equipment, Tomahawk, WI) baited with apple slices. Pre-intervention small-mammal and tick burden data were obtained from a single trapping event conducted during May of 2007, whereas post-intervention small-mammal and tick burden data were collected during the months of June and August of 2007 and 2008, the peak activity periods of I. scapularis nymphs and larvae, respectively.12,18,19 Post-intervention trapping provided data on the (1) species composition of the small-mammal community in treated and untreated areas, (2) tick burdens, (3) prevalence of infection with B. burgdorferi and A. phagocytophilum in small mammals and attached ticks in treated and untreated sites, and (4) blood plasma levels of doxycycline in sampled small mammals. During each trapping event, 100 Sherman traps and 10 Tomahawk traps were set at each site for 3 consecutive days. All traps were deployed by mid-afternoon and checked by mid-morning on the following day. Traps remained open during the day and were checked periodically until late afternoon. Captured rodents were transported to a central processing location and anesthetized with isoflurane. Ectoparasites were removed with fine forceps and placed in 70% ethanol for species identification and evaluation for infection with B. burgdorferi and A. phagocytophilum by polymerase chain reaction (PCR). Ear biopsies were taken, surface-sterilized, and cultured in Barbour–Stoner–Kelley (BSK-H) media for isolation of B. burgdorferi.20 Cultures were read by dark-field microscopy every 7 days for 4 weeks before negative cultures were discarded.21 In addition, ∼300 μL ethylenediaminetetraacetic acid (EDTA) blood were obtained from each capture and placed in microtainers (Becton Dickinson, Franklin Lakes, NJ). Serum was subsequently used to determine pharmacokinetic levels of doxycycline hyclate in small-mammal plasma by high-pressure liquid chromatography (HPLC).15 Animals were ear-tagged (Monel Model 1005-1 or 1005-3; National Band and Tag Company, Newport, KY) and weighed, their overall body measurements were recorded (total body length, tail length, right ear length, and right hind foot length), and subsequently, they were released at the point of capture.11 Small mammals recaptured during a particular trapping event were not reprocessed.
Animals were handled according to approved protocols on file with the Centers for Disease Control and Prevention, Division of Vector-Borne Diseases Animal Care and Use Committee Protocol 08-008.
Tick collections.
Host-seeking ticks were collected using a combination of dragging and walking methods.22,23 Each site was sampled until a minimum of 50 ticks was collected. In 2007, sampling for subadult I. scapularis was performed to coincide with trapping events. Thereafter, adults were collected during the first week of November of 2007, April of 2008, and November of 2009, whereas nymphs were obtained in June of 2008 and 2009. All sampling was performed between 08:00 and 12:00 hours, when vegetation was dry and wind was judged to be below 10 km/hour.24,25 Ticks adhering to drags and coveralls were removed and placed into discrete vials containing 70% ethanol for later analyses by PCR.
Analysis of ticks and small-mammal blood for B. burgdorferi and A. phagocytophilum infection.
DNA was extracted from ticks using the 1.5-mL Kontes disposable pestle system (Kimble Chase, Vineland, NJ) and the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). Briefly, individual ticks were homogenized using a pestle and 180 μL buffer ATL plus 20 μL proteinase K in a 1.5-mL microfuge tube; the solution was incubated overnight at 56°C. Approximately 12 hours later, 200 μL buffer AL were added to each sample, and the samples were vortexed and incubated at 70°C for 10 minutes. Subsequently, 250 μL ethanol (100%) were added, and the samples were vortexed thoroughly. The samples were then loaded onto a Qiagen DNeasy column and washed with buffer AW1 and AW2 according to the manufacturer's protocol. Finally, the columns were eluted with 50 μL buffer AE. To ensure the presence of DNA, all tick extracts were tested for tick actin DNA by real-time PCR (qPCR). The primers used for the actin control PCR were actin-F 5′-CCATCCAGGCCGTGCTCTC-3′ and actin-R 5′-ATCTTCATCAGGTAGTCGGTCAGG-3′. The actin qPCR was done in FastStart CYBR Green Master mix (Roche, Indianapolis, IN) with 1 μM each primer and 2 μL sample in a 25-μL reaction. The samples, including a positive sample and a water control, were analyzed in a 96-well format with the following parameters: 1 cycle at 95°C for 10 minutes and 40 cycles at 95°C for 10 seconds, 60°C for 20 seconds, and 72° for 30 seconds, and recording at 82°C using a real-time detection system (Bio-Rad Laboratories, Hercules, CA). At the end of each run, a melting curve analysis was performed to verify the correct amplicon. Crossing threshold (CT) and melting curves were determined using Opticon Monitor 3 software. Only samples that were positive for tick actin were included in this study. To verify the presence of B. burgdorferi s.s. in a sample, we performed qPCR amplifying the fliD locus. The primers used in this TaqMan probe assay were fliD-F 5′-TGGTGACAGAGTGTATGATAATGGAA-3′, fliD-R 5′-ACTCCTCCGGAAGCCACAA-3′, and fliD-probe 5′-FAM- TGCTAAAATGCTAGGAGATTGTCTGTCGCC-BHQ-3′, which were described previously.26 The fliD qPCR was done in 25 μL using FastStart Probe Master mix (Roche, Indianapolis, IN) with 1 μM each primer, a 250-nM probe, and 5 μL sample (10% of the total sample).27 The samples, including a positive sample and water controls, were analyzed in a 96-well format with the following parameters: 1 cycle at 95°C for 10 minutes and 40 cycles at 95°C for 10 seconds and 60°C for 1 minute followed by recording using a real-time detection system (Bio-Rad Laboratories, Hercules, CA). Each 96-well plate was analyzed with a 10-fold dilution standard curve of genomic DNA of B. burgdorferi B31 (1,200 - 10 pg copies), which also served as a positive control; in addition, a water/no DNA control was performed on each plate. Spirochete numbers in each sample were calculated using Opticon Monitor 3 software, and only those samples having greater than 12 spirochetes (the smallest detectable positive control) were considered positive for B. burgdorferi s.s.
DNA extracted from tick samples were then analyzed for the presence of A. phagocytophilum as previously described.28 The primer set for A. phagocytyophilum targeted the major outer surface protein (p44) gene msp2 as previously described.28,29 Amplicons from the tick and mouse DNA were then purified with the QIAquick PCR purification kit (Qiagen, Valencia, CA) and sequenced in both directions using BigDye XTerminator Purification sequencing kits (Applied Biosystems, Carlsbad, CA) with the same primers used for PCR amplification to confirm that positive PCR samples contained A. phagocytophilum.
DNA was extracted from small-mammal blood samples (50 μL whole blood/animal) using the Qiagen DNeasy tissue kit (Valencia, CA) according to the manufacturer's protocol with a final elution volume of 200 μL. A qPCR assay was used to amplify the mouse actin gene with the Mouse ACTB (actin-β) Endogenous Control (FAM Dye/MGB Probe, Non-Primer Limited; Applied Biosystems, Carlsbad, CA) to verify the presence of murine DNA. qPCR was done in TaqMan Universal PCR Master mix (Applied Biosystems, Carlsbad, CA), with 2.5 μL primer and probe mix and 2.5 μL sample (of the 200-μL DNA purification). Samples were analyzed for mouse actin in a 96-well format with the following parameters: 1 cycle at 95°C for 10 minutes, 45 cycles at 95°C for 10 seconds and 60°C for 1 minute, and 1 cycle at 10°C for 10 seconds using a Chromo4 real-time detection system (Bio-Rad Laboratories, Hercules, CA). The presence of A. phagocytophilum was then detected by the PCR target in the p44 gene.28 Sequence analysis of positive amplicons was then determined as described for ticks above.
Analysis of small-mammal blood for doxycycline hyclate.
HPLC was performed to determine plasma pharmacokinetic levels for small mammals as previously described.14,15 Approximately 300 μL whole blood were collected in microtainers containing EDTA and centrifuged, and the plasma was run over a Beckman System Gold high-pressure liquid chromatograph (Beckman Coulter, Fullerton, CA); associated 32-Karat version 5.0 software was used combined with a C18 column (100 × 4.6-mm inside diameter; Alltech, Deerfield, IL).
Testing B. burgdorferi field isolates for doxycycline resistance.
To determine if any isolates of B. burgdorferi from doxycycline bait treatment areas had developed resistance to doxycycline during the course of bait deployment, isolates were subjected to a minimum inhibitory concentration (MIC) and minimum borreliacidal concentration (MBC) assay as previously described.30 Briefly, MBC was determined to be the lowest concentration of doxycycline in which no live spirochetes were observed by microscopy or recovered by incubation in BSK for 21 days, and MIC was determined to be the lowest concentration of cells after incubation that did not exceed the initial number of cells. Two isolates from each treatment area were subjected to testing and compared with a B31 laboratory strain (passage 6; isolated from Shelter Island, NY) as a control. B. burgdorferi strains used for control untreated areas were NJ-134 and NJ-141, pre-bait deployment areas NJ-103 and NJ-104, and post-bait deployment areas NJ-129 and NJ-130. Isolates were grown to log phase in BSK-H medium20; 100 µL B. burgdorferi culture at 3 × 105 cells/mL in log phase were placed into a 96-well cell culture plate. Doxycycline hyclate (Sigma-Aldrich, St. Louis, MO) at concentrations from 0.25 to 10 µg/mL was added to the wells in duplicate in parallel with a 0 µg/mL control. Plates were allowed to incubate at 34°C for 72 hours; 10 µL culture from each well were examined by dark-field microscopy for live spirochetes. All isolates from doxycycline concentrations ranging from 0 to 0.75 µg/mL were placed into fresh BSK-H medium for 21 days to confirm borreliacidal activity. The experiment was repeated three times.
Statistical analyses.
The modified Abbott's formula31 was used for primary comparisons between untreated control and treatment areas, including percent reduction of B. burgdorferi and A. phagocytophilum infection rates in small mammals, number of ticks removed from hosts, and questing nymphal and adult ticks. Percent control was calculated as follows: 100 - (treatment/untreated control × 100). χ2 tests were used to determine significance (at P < 0.05).
Results
Deployment and maintenance of bait stations.
After the first week of deployment in May of 2007, 34 of 50 (68%) bait stations showed some consumption of doxycycline bait. By the third week and lasting through 15 weeks, all baits were consumed from 42 (84%) to 50 (100%) bait stations. Mid-week inspections conducted at 4 and 12 weeks showed that, in high-use bait stations, baits were totally consumed after only 4 days of deployment (data not shown). With the appearance of a substantial oak mast at week 16, bait consumption steadily declined (Figure 1).
In 2007, we began to observe squirrel damage to bait stations within 2 weeks of deployment. We typically observed that the entrance ports of many bait stations were enlarged, presumably by squirrels attempting to access baits. Because the functional integrity of these bait stations was not compromised, they remained in service. However, by the end of the study, squirrel depredation resulted in complete loss and replacement of 57 bait stations (mean = 3.0 ± 3.77 bait stations lost/week, range = 0–7 bait stations/week).
Small-mammal ticks burdens.
A total of 3,960 trap nights resulted in 365 small-mammal captures (203 captures from the untreated control area and 162 captures from the treatment area). Eastern chipmunks (Tamias striatus) comprised 43.3% of captures, white-footed mice (Peromyscus leucopus) were 40.6% of captures, eastern gray squirrels (S. carolinensis) were 10.4% of captures, and short-tailed shrews (Blarina brevicauda) were 5.7% of captures. A total of 656 ticks were collected from mammal hosts (446 ticks from the two untreated areas and 210 ticks from the treatment area); 91% of the ticks were larval or nymphal I. scapularis, and the remaining 9% were immature Dermacentor variabilis. Two Amblyomma americanum larvae were collected from a squirrel trapped on the untreated control area. Over 2 years, we collected 365 ticks from 157 T. striatus (2.3 ticks/chipmunk, 56.3% infestation rate), 225 ticks from 148 P. leucopus (1.5 ticks/mouse, 48% infestation rate), 69 ticks from 38 S. carolinensis (1.8 ticks/squirrel, 47.4% infestation rate), and 7 ticks from 21 B. brevicauda (0.3 ticks/shrew, 28.6% infestation rate).
Infection rates in small mammals.
Pre-treatment (May of 2007) B. burgdorferi infection rates in small mammals were 41% and 30% for the control and treated areas, respectively (Table 1). After doxycycline bait deployment, not a single infected animal was collected from the treatment area (0% infection rate, N = 110; 100% reduction), whereas average infection rates in the control areas were 61% and 41% in June and August, respectively.
Table 1.
Impact of doxycycline hyclate-laden bait on B. burgdorferi and A. phagocytophilum infection in small mammals
| Month | Doxycycline blood level (μg/mL)* | B. burgdorferi infection rate (number positive/total number) | A. phagocytophilum infection rate (number positive/total number) | |||
|---|---|---|---|---|---|---|
| Doxy | Control | Doxy | Control | Doxy | Control | |
| 2007 | ||||||
| May | NA* | NA† | 8/26 (30%) | 9/22 (41%) | 17/26 (65%) | 14/22 (64%) |
| June | 1.17 | 0.0 | 0/40 (0.0%) | 19/31 (61%) | 3/40 (7.5%) | 6/31 (19%) |
| August | 1.42 | 0.028‡ | 0/22 (0.0%) | 11/27 (41%) | 2/22 (9.1%) | 7/27 (26%) |
| 2008 | ||||||
| May | NA* | NA† | 1/14 (7.1%) | 12/33 (36%) | 7/14 (50%) | 15/33 (46%) |
| June | 1.12 | 0.027§ | 0/34 (0.0%) | 25/42 (60%) | 5/34 (15%) | 8/42 (19%) |
| August | 1.29 | 0.025¶ | 0/16 (0.0%) | 15/34 (44%) | 0/16 (0.0%) | 8/34 (24%) |
MIC for doxycline blood levels is ∼0.5 µg/mL.15
May of 2007 and 2008 were pre-treatment trapping events, and no doxycycline bait was deployed during these periods; therefore, blood was not tested by HPLC. NA = not applicable.
Although there was no doxycycline bait deployed in the untreated control areas, three eastern chipmunks and one eastern gray squirrel had detectable doxy blood levels as determined by HPLC. These animals most likely entered from one of two control areas located ≥ 200 m from the treatment area.
A single eastern chipmunk had a detectable blood level of 1.12 µg/mL for doxycycline.
A single eastern chipmunk had a detectable blood level of 0.87 µg/mL for doxycycline.
Pre-treatment A. phagocytophium infection rates in small mammals from the untreated control and treatment areas were 64% and 65%, respectively (Table 1). Average infection rates in animals recovered from the treatment area in 2007 and 2008 declined to 13.5% (34.7% reduction; P = 0.04). The combined percentage of animals coinfected with both B. burgdorferi and A. phagocytophium from both the treated and untreated areas was 13.4%.
B. burgdorferi infection in ticks.
Average infection rates in ticks removed from small mammals in the untreated areas throughout the duration of the study was 54.5%. Average pre-treatment (May of 2007) infection rates in ticks removed from small mammals in the treatment area was 47.7%, and this percentage decreased significantly after bait deployment to 12.3% (P = 0.03, 74.2% overall reduction; data not shown).
Initial (May of 2007) average B. burgdorferi infection rate in questing nymphal ticks from the untreated areas was 33.1%, which declined slightly to an average of 23.5% in 2008 and 2009 (Table 2). In the treated area, 36.7% of questing nymphs were infected during the pre-treatment period, whereas infection rates declined significantly to 1.9% and 1.5% during 2008 and 2009, respectively, after bait deployment (92.8% control; P < 0.01) (Table 2).
Table 2.
Impact of doxycline hyclate-laden bait on B. burgdorferi and A. phagocytophilum infection in questing nymphal I. scapularis ticks
| Date of collection | B. burgdorferi infection rate (number positive/total number)* | A. phagocytophilum infection rate (number positive/total number)† |
|---|---|---|
| Doxy bait area | ||
| 2007* | 18/49 (36.7%) | 8/49 (16.3%) |
| 2008 | 1/52 (1.9%) | 4/52 (8.2%) |
| 2009 | 1/66 (1.5%) | 1/66 (1.5%) |
| Untreated area | ||
| 2007 | 17/58 (33.1%) | 13/58 (22.4%) |
| 2008 | 15/56 (26.8%) | 11/56 (19.6%) |
| 2009 | 8/42 (19.1%) | 7/42 (16.7%) |
Percent infection of nymphal I. scapularis ticks for B. burgdorferi and A. phagocytophilum was determined by PCR.
Pre-intervention.
The pre-intervention B. burgdorferi infection rate in questing adult I. scapularis from the untreated areas (65.9%) was significantly greater than the rate observed in the treatment area (30.6%, P = 0.03). This finding is most likely attributable to antibiotic baits being available to small mammals for 19 weeks before the adult tick season. Nymphal ticks that fed on treated mice may have been cleared of spirochetal infection, or they may have fed on mice that had been cleared of infection and therefore, were uninfected during the molt to the adult stage. The infection rate in questing adult ticks after 1 year of bait deployment was decreased by 26.9% (Table 3).
Table 3.
Impact of doxycline hyclate-laden bait on B. burgdorferi and A. phagocytophilum infection in questing adult I. scapularis ticks
| Date of collection | B. burgdorferi infection rate (number positive/total number)* | A. phagocytophilum infection rate (number positive/total number)* |
|---|---|---|
| Doxy bait area | ||
| 2007 | 15/49 (30.6%) | 10/49 (20.4%) |
| 2008 | 11/52 (21.2%) | 4/52 (7.7%) |
| 2009 | 6/50 (12.0%) | NA† |
| Untreated area | ||
| 2007 | 31/47 (65.9%) | 17/37 (36.1%) |
| 2008 | 49/93 (52.7%) | 13/59 (22.03%) |
| 2009 | 29/55 (52.7%) | NA |
Percent infection of adult I. scapularis ticks for B. burgdorferi and A. phagoctyophilum was determined by PCR.
Study was terminated after nymphal collection in June of 2009. NA = not applicable.
A. phagocytophilum infection in questing ticks.
Pre-intervention infection rate in questing nymphal ticks collected in the treatment area was 16.3%. After 2 years of bait deployment (38 weeks total), infection rates declined > 10-fold to 1.5% (72% reduction) (Table 2). Infection rates in adult ticks declined from 20.4% in May of 2007 to 7.7% in 2008 (1 year of bait deployment, 19 weeks) (Table 3). This finding resulted in a significant decrease of 59.7% (P = 0.04) (Table 3). Adult ticks collected in the fall of 2009 were not tested for infection. We were unable to test adult ticks for A. phagocytophilum in November of 2009, because DNA was not available from these ticks.
Doxycycline resistance to B. burgdorferi in small-mammal isolates.
A total of six field isolates were tested for B. burgdorferi resistance to doxycycline hyclate. Samples tested included two B. burgdorferi-positive cultures from the untreated control areas and four cultures from the treated area. Two of four treatment area isolates came from ear biopsy-positive mammals trapped in May of 2007 during the initial pre-treatment trapping event. During the months of bait deployment, there were no culture-positive small mammals trapped in the treatment area (N = 110). The remaining two B. burgdorferi-positive isolates tested were obtained during the month of May both in 2008 and 2009. All isolates tested, including B. burgdorferi B31 control, were completely susceptible to doxycycline hyclate at the lowest concentration tested (0.25 µg/mL), and attempts to grow spirochetes from the lowest concentrations tested were unsuccessful, indicating there was no development of resistance to doxycycline in these field trials.
Discussion
This field trial showed that targeted delivery of bait containing doxycycline hyclate reduced B. burgdorferi and A. phagocytophilum infection rates in small mammals and subsequently, questing I. scapularis populations. Consumption data showed that the 500 mg/kg doxycycline hyclate-impregnated baits were palatable and that target small mammals rapidly acclimated to the bait stations. Within several weeks of deployment, all bait was removed from > 95% of bait stations, and in most cases, this removal occurred within 4 days of deployment. Presumably, the bait was consumed within the bait station or removed and cached by small mammals, particularly squirrels and eastern chipmunks, for later consumption. During the first week of September of 2007 (week 16 post-bait box deployment), we experienced substantial oak masting, and bait consumption steadily declined from a high of 100% in week 6 to only 4% by week 19. Small mammals often switch foraging preferences as the amount and type of food available changes during a given year.32,33 In addition, the works by Jones and others34 and Ostfeld and others35 showed that interannual variation in the abundance of preferred foods (including acorns) may directly influence fluctuations in the density of tick hosts and consequently, the ecological risk of exposure to Lyme disease. Therefore, the efficacy of host-targeted tick control technologies that rely on a bait source, such as the one described here, may be less effective during times when there is an abundance of alternative food sources.
Previous laboratory trials using the same 500-mg/kg formulation of doxycycline hyclate-laden bait showed 100% prophylaxis and clearing of acute established B. burgdorferi infection in C3H/HeJ mice.14 The field trial described here suggests that consumption of this bait by wild-trapped small mammals had a significant impact on the natural enzootic cycles of both B. burgdorferi and A. phagocytophilum, impacting both I. scapularis and its reservoir hosts. Ear-biopsy cultures from small mammals showed that the enzootic cycle for both infections in this area of central New Jersey is very intense. Average B. burgdorferi and A. phagocytophilum infection rates in small mammals in the untreated area during this 2-year study were 47.2% and 33%, respectively. Placement of antibiotic bait stations early in the spring when transmission usually occurs cleared many spirochete-infected small mammals that were being fed on by infected nymphal ticks. After bait box deployment, infection rates in small mammals in the treated areas were reduced by 86.9% and 74% for B. burgdorferi and A. phagocytophilum, respectively. Our recapture data also supports the ability of doxycycline bait to prophylactically protect and cure small mammals and ultimately, impact the Lyme disease enzootic cycle. Of 365 total captures, there were a total of 46 recaptures (12.6% recapture rate). In the treatment area, 22 of 23 (95.7%) small mammals were either cured (4/23; 17%) or prophylactically protected against (18/23; 78.3%) B. burgdorferi infection compared with 15 of 23 (65.2%) that became infected during the trial or were previously infected and remained so in the untreated control areas. This rate equates to a 91.7% reduction in the number of infected small-mammal reservoirs in the doxycycline bait-treated areas. Similarly, 12 of 23 (52.2%) small mammals were cured, and 10 of 23 (43.5%) were prophylactically protected against A. phagocytophilum infection (22/23 total; 95.7%), where 11 of 23 (47.8%) small mammals from the untreated control area either acquired infection or were already infected and remained so during the trial.
Multiple microorganisms have been shown to cocirculate in I. scapularis ticks collected previously in northern New Jersey, including ticks dually infected with both B. burgdorferi and A. phagocytophilum.36 Small-mammal reservoirs are routinely found to be infected with multiple tick-borne pathogens as well.37 We trapped 33 animals that were coinfected with B. burgdorferi and A. phagocytophilum (28 animals from the untreated area and 5 animals from the treated area captured before bait box deployment). All five of the latter animals were subsequently recaptured during periods of bait deployment, and all were found to be negative for both B. burgdorferi and A. phagocytophilum infection. A single chipmunk that was found to be negative for both infections during May of 2007 was positive for both when later captured in May of 2008 during a period between bait deployments. In a laboratory study, Zeidner and others16 showed the ability of a sustained release formulation of doxycyline hyclate (Atridox) to prophylactically protect against simultaneous infection of B. burgdorferi and A. phagocytophilum in C3H/HeJ mice by tick bite. However, we believe our study to be the first field trial of a novel technology to show the simultaneous cure of both B. burgdorferi and A. phagocytophilum in wild populations of small mammalian reservoirs.
Ticks removed from small mammals in the treatment area during the months of bait deployment showed a > 3.8-fold reduction in B. burgdorferi infection rates. However, unlike in a previous laboratory study,14 we were unable to show complete clearing of ticks of B. burgdorferi infection. In the laboratory, mice were provided the doxycycline bait during the duration that ticks fed (∼96 hours to repletion). In contrast, we were unable to determine how many times individual animals visited bait boxes or how much bait they consumed. HPLC testing showed that a high percentage (77.8%) of mammal hosts from the treatment area had MIC blood levels of doxycycline hyclate in plasma. Because ticks that were removed from hosts captured in the treated area and analyzed by PCR were at various stages of feeding, some were nearly engorged, and some were newly attached and relatively flat. Ticks in early stages of feeding may not have taken in sufficient levels of antibiotic to clear them of spirochetal infection, or their hosts may not have had sufficient pharmacokinetic levels to clear infection. Future studies should include holding animals over water until all ticks are fed to repletion before PCR analysis to test for complete clearing. We tested B. burgdorferi strains collected from the test area to be certain antibiotic resistance was not selected for during our pilot study. No evidence of antibiotic resistance was found. However, the use of antibiotic-laden baits for the prevention of Lyme disease in the field would require close monitoring to be certain that antibiotic resistance did not arise.
The number of Lyme disease cases reported to the Centers for Disease Control and Prevention (CDC) continues to exceed 25,000/year. There is a continued need to develop and test novel approaches to tick control that can be effective either alone or as part of an integrated tick management (ITM) approach on residential properties and public lands that minimize the use of synthetic acaricides and emphasize the least-toxic methods.
The use of host-targeted bait boxes containing the acaricide fipronil has been shown to be effective at reducing tick burdens on mice and chipmunks, numbers of questing ticks, and infection rates in rodent reservoirs. In a 3-year study on a coastal island of Connecticut, bait boxes reduced immature I. scapularis burdens on mice by 76%, questing nymphal populations by > 50%, and the number of infected mice by > 57%.11 In a New Jersey field trial, simultaneous deployment of US Department of Agriculture 4-Poster deer feeders and MaxForce TMS bait boxes was used to passively treat deer, white-footed mice, and eastern chipmunks, and it resulted in a 94.3% reduction of host-seeking nymphal ticks after 2 years.12 In the present study, the use of doxycycline-treated bait significantly reduced the percentage of rodent reservoirs infected with B. burgdorferi by 86.9% compared with > 50% for the fipronil-treated bait boxes. However, although the use of antibiotic bait was extremely effective at reducing infection rates in small mammals and questing tick populations (> 92%), this method alone will not reduce questing tick populations and would provide the greatest benefit if used as part of an ITM approach.
A novel doxycycline hyclate-impregnated bait formulation was shown to be highly efficacious in providing prophylaxis against tick-transmitted B. burgdorferi and A. phagocytophilum, curing infected small mammals, and reducing infection rates in questing ticks. We recognize that distributing doxycycline-impregnated bait for controlling tick-borne diseases may be controversial given the fact that doxycyline is routinely prescribed for treating these infections in patients. Although the results reported here serve as proof of concept, studies are underway to evaluate alternative antibiotics with similar antispirochetal activity that do not serve as front-line drugs for the treatment of Lyme disease, Rocky Mountain spotted fever, or human granulocytic anaplasmosis.
Disclaimer: The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Mention of a method or product does not constitute an endorsement. Animals were handled according to approved protocols on file with the Centers for Disease Control and Prevention Division of Vector-Borne Diseases Animal Care and Use Committee Protocol number 08-008.
Footnotes
Authors' addresses: Marc C. Dolan, Gabrielle Dietrich, Andrias Hojgaard, Amy J. Ullmann, Cherilyn Sackal, Nordin S. Zeidner, and Joseph Piesman, Division of Vector-Borne Diseases, Bacterial Disease Branch, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: mcd4@cdc.gov, eid7@cdc.gov, fth3@cdc.gov, aff1@cdc.gov, gtf3@cdc.gov, naz2@cdc.gov, and jfp2@cdc.gov. Terry L. Schulze and Christopher J. Schulze, Terry L. Schulze, PhD, Inc., Perrineville, NJ, E-mails: tlschulze@mormouth.com and cjschulze1@gmail.com. Robert A. Jordan, Freehold Area Health Department, Freehold, NJ, E-mail: rajordanphd@optimum.net.
Reprint requests: Marc C. Dolan, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, 3150 Rampart Rd., Fort Collins, CO 80521, E-mail: mcd4@cdc.gov.
References
- 1.Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- 2.Stafford KC III, Kitron U. In: Lyme Borreliosis Biology, Epidemiology and Control. Gray J, Kahl O, Lane RS, Stanek G, editors. New York, NY: CABI Publishing; 2002. pp. 301–334. (Environmental management for Lyme borreliosis control). [Google Scholar]
- 3.Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–2430. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- 4.Hayes EB, Maupin GO, Mount GA, Piesman J. Assessing the prevention effectiveness of local Lyme disease control. J Public Health Manag Pract. 1999;5:84–92. doi: 10.1097/00124784-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Schulze TL, Jordan RA, Krivenko AJ. Effects of barrier application of granular deltamethrin on subadult Ixodes scapularis (Acari: Ixodidae) and nontarget forest floor arthropods. J Econ Entomol. 2005;98:976–981. doi: 10.1603/0022-0493-98.3.976. [DOI] [PubMed] [Google Scholar]
- 6.Schulze TL, Jordan RA, Schulze CJ, Healy SP. Suppression of Ixodes scapularis (Acari: Ixodidae) following annual habitat-targeted acaricide applications against fall populations of adults. J Am Mosq Control Assoc. 2008;24:566–570. doi: 10.2987/08-5761.1. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg HS. Lyme disease and conservation. Conserv Biol. 1994;8:343–353. [Google Scholar]
- 8.Schulze TL, Jordan RA, Hung RW, Krivenko AJ, Jr, Schulze JJ, Jordan TM. Effects of an application of granular carbaryl on non-target forest floor arthropods. J Econ Entomol. 2001;94:123–128. doi: 10.1603/0022-0493-94.1.123. [DOI] [PubMed] [Google Scholar]
- 9.Piesman J. Strategies for reducing the risk of Lyme borreliosis in North America. Int J Med Microbiol. 2006;296((Suppl 40)):17–22. doi: 10.1016/j.ijmm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Gould LH, Nelson RS, Griffith KS, Hayes EB, Piesman J, Mead PS, Cartter ML. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector Borne Zoonotic Dis. 2008;8:769–776. doi: 10.1089/vbz.2007.0221. [DOI] [PubMed] [Google Scholar]
- 11.Dolan MC, Maupin GO, Schneider BS, Denatale C, Hamon H, Cole C, Zeidner NS, Stafford KC III. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol. 2004;41:1043–1054. doi: 10.1603/0022-2585-41.6.1043. [DOI] [PubMed] [Google Scholar]
- 12.Schulze TL, Jordan RA, Schulze CJ, Healy SP, Jahn MB, Piesman J. Integrated use of 4-Poster passive topical treatment devices for deer, targeted acaricide applications, and Maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J Med Entomol. 2007;44:830–839. doi: 10.1603/0022-2585(2007)44[830:iuoppt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Fish D, Childs JE. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: background and rationale. Vector Borne Zoonotic Dis. 2009;9:357–364. doi: 10.1089/vbz.2009.0022. [DOI] [PubMed] [Google Scholar]
- 14.Dolan MC, Zeidner NS, Gabitzsch ES, Dietrich G, Borchert JN, Poche RM, Piesman J. A doxycycline hyclate rodent bait formulation for prophylaxis and cure of tick-transmitted Borrelia burgdorferi. Am J Trop Med Hyg. 2008;78:803–805. [PubMed] [Google Scholar]
- 15.Zeidner NS, Brandt KS, Dady E, Dolan MC, Happ C, Piesman J. Sustained-release formulation of doxycycline hyclate for prophylaxis of tick bite infection in a murine model of Lyme borreliosis. Antimicrob Agents Chemother. 2004;48:2697–2699. doi: 10.1128/AAC.48.7.2697-2699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidner NS, Massung R, Dolan MC, Dadey E, Gabitzsch E, Dietrich G, Levin M. A sustained release formulation of doxycycline hyclate (AtridoxTM) prevents simultaneous transmission of Anaplasma phagocytophilum and Borrelia burgdorferi transmitted by tick bite. J Med Microbiol. 2008;57:463–468. doi: 10.1099/jmm.0.47535-0. [DOI] [PubMed] [Google Scholar]
- 17.Schulze TL, Jordan RA, Hung RW, Taylor RC, Markowski D, Chomsky MS. Efficacy of granular deltamethrin against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J Med Entomol. 2001;38:344–346. doi: 10.1603/0022-2585-38.2.344. [DOI] [PubMed] [Google Scholar]
- 18.Schulze TL, Jordan RA, Schulze CJ. Host associations of Ixodes scapularis (Acari: Ixodidae) in residential and natural settings in a Lyme disease-endemic area in New Jersey. J Med Entomol. 2005;42:966–973. doi: 10.1093/jmedent/42.6.966. [DOI] [PubMed] [Google Scholar]
- 19.Schulze TL, Bowen GS, Lakat MF, Parkin WE, Shisler JK. Seasonal abundance and host utilization of Ixodes dammini (Acari: Ixodidae) and other ixodid ticks from an endemic Lyme disease focus in New Jersey, USA. J Med Entomol. 1986;23:105–109. doi: 10.1093/jmedent/23.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Sinsky RJ, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piesman J, Mather TN, Donahue JG, Levine J, Campbell JD, Karakashian SJ, Spielman A. Comparative prevalence of Babesia microti and Borrelia burgdorferi in four populations of Ixodes dammini in eastern Massachusetts. Acta Trop. 1986;43:263–270. [PubMed] [Google Scholar]
- 22.Ginsberg HS, Ewing CP. Comparison of flagging, walking, trapping, and collecting ticks from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone star ticks, Amblyomma americanum (Acari: Ixodidae) Exp Appl Acarol. 1989;7:313–322. doi: 10.1007/BF01197925. [DOI] [PubMed] [Google Scholar]
- 23.Schulze TL, Jordan RA, Hung RW. Biases associated with several sampling methods used to estimate the abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1997;34:615–623. doi: 10.1093/jmedent/34.6.615. [DOI] [PubMed] [Google Scholar]
- 24.Schulze TL, Jordan RA, Hung RW. Effects of selected meteorological factors on diurnal questing of adult Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2001;38:318–324. doi: 10.1603/0022-2585-38.2.318. [DOI] [PubMed] [Google Scholar]
- 25.Schulze TL, Jordan RA. Meteorologically mediated diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J Med Entomol. 2003;40:395–402. doi: 10.1603/0022-2585-40.4.395. [DOI] [PubMed] [Google Scholar]
- 26.Zeidner NS, Schneider BS, Dolan MC, Piesman J. An analysis of spirochete load, strain, and pathology in a model of tick-transmitted Lyme borreliosis. Vector Borne Zoonotic Dis. 2001;1:35–44. doi: 10.1089/153036601750137642. [DOI] [PubMed] [Google Scholar]
- 27.Houck JA, Hojgaard A, Piesman J, Kuchta RD. Low-density microarrays for the detection of Borrelia burgdorferi s.s. (the Lyme disease spirochete) in nymphal Ixodes scapularis. Ticks Tick Borne Dis. 2011;2:27–36. doi: 10.1016/j.ttbdis.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Zeinder NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, Biggerstaff BJ, Maupin GO. Transmission of the agent of HGE by Ixodes spinipalpis ticks: evidence of an enzootic cycle of co-infection with Borrelia burgdorferi in Northern Colorado. J Infect Dis. 2000;182:616–619. doi: 10.1086/315715. [DOI] [PubMed] [Google Scholar]
- 29.Massung RF, Slater KG. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J Clin Microbiol. 2003;41:717–722. doi: 10.1128/JCM.41.2.717-722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SE, Klein GC, Schmid GP, Feeley JC. Susceptibility of the Lyme disease spirochete to seven antimicrobial agents. Yale J Biol Med. 1984;57:549–553. [PMC free article] [PubMed] [Google Scholar]
- 31.Mount GA. Amblyomma americanum: area control of overwintered nymphs and adults in Oklahoma with acaricides. J Econ Entomol. 1981;74:24–26. [Google Scholar]
- 32.Witmer G. In: Perspectives in Ecological Theory and Integrated Pest Management. Kogan M, Jepson P, editors. Cambridge, United Kingdom: Cambridge University Press; 2007. pp. 393–410. (The ecology of vertebrate pests and integrated pest management (IPM)). [Google Scholar]
- 33.Borchert JN, Enscore RE, Eisen RJ, Atiku LA, Owor N, Acayo S, Babi N, Montenieri JA, Gage KL. Evaluation of rodent bait containing imidacloprid for the control of fleas on commensal rodents in a plague-endemic region of northwest Uganda. J Med Entomol. 2010;47:842–850. doi: 10.1603/me09221. [DOI] [PubMed] [Google Scholar]
- 34.Jones CG, Ostfeld RS, Richard MP, Schauber EM, Wolff JO. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science. 1998;13:1023–1026. doi: 10.1126/science.279.5353.1023. [DOI] [PubMed] [Google Scholar]
- 35.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006;4:e182. doi: 10.1371/journal.pbio.0040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adelson ME, Rao R-VS, Tilton RC, Cabets K, Eskow E, Fein L, Occi JL, Mordechai E. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophilum in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2002;42:2799–2801. doi: 10.1128/JCM.42.6.2799-2801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stafford KC III, Massung RF, Mangarelli LA, Ijdo JW, Anderson JF. Infection with agents of human granulocytic ehrlichiosis, Lyme disease, and babesiosis in wild white-footed mice in Connecticut. J Clin Microbiol. 1999;37:2887–2892. doi: 10.1128/jcm.37.9.2887-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


