Abstract
We report a severe case of typhus group rickettsiosis in a returning traveler from Indonesia. This typically mild illness was characterized by progressive pulmonary edema and prolonged fever, with defervescence 4 days after the commencement of doxycycline.
Case Report
A 29-year-old man presented with an 8-day history of fever, rigors, drenching night sweats, myalgia, and severe frontal headache. He subsequently developed a dry cough with shortness of breath on exertion and a maculopapular rash 2 days before presentation to hospital.
He had returned 11 days previously from a holiday that initially comprised 2 weeks in Bali and Lombok, Indonesia, and then 7 days in Hong Kong. He had visited both urban and rural environments and described exposure to fresh water, sea water, and hot springs. He had sustained lacerations to both feet from coral while scuba diving. He reported frequently seeing rats in his living environment, including in his accommodation. He denied any contact with animals. He sustained multiple insect bites, including presumptive mosquito bites, but no tick bites.
He had been vaccinated against Salmonella typhi and hepatitis A and was taking atovaquone/proguanil (Malarone® GlaxoSmithKline, Brentford, Middlesex, United Kingdom) for antimalarial prophylaxis. His only past history comprised well-controlled asthma, and he took no regular medicines.
On examination, he appeared unwell and was pyrexial at 39.6°C (103°F). He had conjunctival injection and a faint maculopapular rash confined to both arms, with sparing of the palms that resolved rapidly over the next 2 days. Respiratory examination revealed bibasal crepitations. The liver was palpable 1 cm below the costal margin, splenomegaly was noted, and a few subcentimetrer tender cervical lymph nodes were palpable.
Blood tests on admission showed a normal white cell count of 6.1 × 109/L, (differential: neutrophils = 4.6, lymphocytes = 0.6, monocytes = 0.4, and eosinophils = 0.01 × 109/L), platelets at 78 × 109/L, and hemoglobin at 13.8 g/dL. Sodium (Na) was 132 mmol/L, potassium (K) was 4.1 mmol/L, creatinine was 124 μmol/L, albumin (alb) was 31 g/L, bilirubin was 60 μmol/L, alkaline phosphatase (ALP) was 264 U/L, alanine transaminase (ALT) was 340 U/L, and C-reactive protein > 250 mg/L (Figure 1 shows trends). Three malaria films and histidine-rich protein- 2 (HRP-2) antigen were negative. Serology for human immunodeficiency virus (HIV) and hepatitis A, B, and C were negative. Admission chest X-ray was normal, but a repeat X-ray 3 days later showed bibasal reticulonodular shadowing consistent with pulmonary edema. Abdominal ultrasound showed a normal liver and biliary tree with an enlarged spleen at 15 cm.
Figure 1.
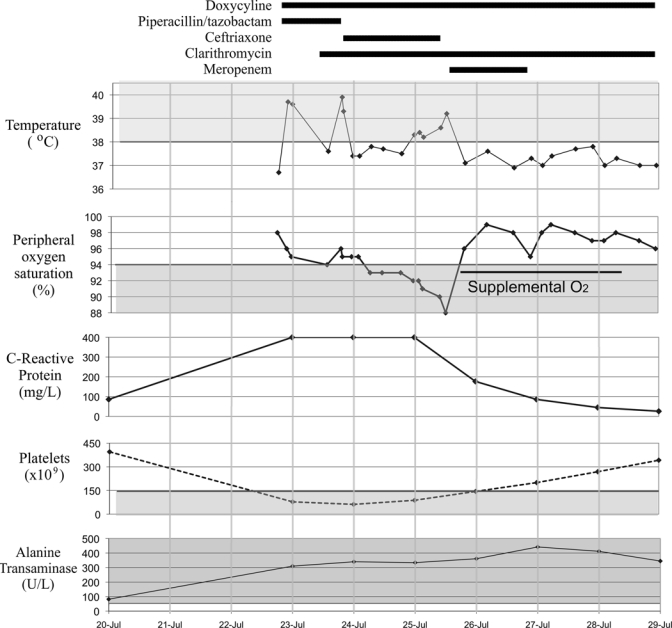
Trends in observations, blood tests and antibiotic therapy.
Our differential diagnosis included rickettsiosis, leptospirosis, typhoid, and legionella. Accordingly, he was initially treated with doxycycline, clarithromycin, and piperacillin/tazobactam (Figure 1). After 1 day, piperacillin/tazobactam was switched to ceftriaxone because of failure to improve. After 2 days, he was clinically worse, with persistent pyrexia and diminishing oxygen saturations. He needed supplemental oxygen for the next 3 days (Figure 1). Ceftriaxone was empirically switched to meropenem. Doxycycline was continued throughout. It took a total of 4 days for the fever to settle and clinical improvement to occur.
Three blood cultures and urine and stool cultures were negative. Serology for leptospirosis, spotted fever group Rickettsia, arboviruses, schistosomiasis, and Strongyloides was negative. Legionella urinary antigen was negative.
Typhus group indirect immunofluorescence (IF) was negative on admission, but convalescent serology 1 month after admission was very strongly positive for both immunoglobulin M (IgM) and IgG at titers of 1 to 1,024 and 1 to 2,048, respectively.
Discussion
Typhus is a rare cause of fever in a returning traveler. A 12-year review of the GeoSentinel database of returning international travelers seeking medical attention found that rickettsioses accounted for 211 of 13,763 travelers presenting with fever (1.5%).1 Of these travelers, the majority had spotted fever rickettsioses (82.5%); a minority had scrub typhus (16/211) or typhus group rickettsioses (10/211). Most cases of typhus group rickettsioses (6/10; 60%) occurred in travelers returning from Southeast Asia.
Making the diagnosis of murine typhus is difficult given the rarity of the presentation and the absence of specific characteristic features. Indeed, 22 different initial diagnoses were proposed for the 80 patients described in one series.2 There remains a lack of satisfactory diagnostic tests that can be used in the acute phase of the illness, and a fourfold increase in IF assay remains the gold standard for diagnosis.3 The IF antibody test used (Focus Rickettsia IF assay; Focus Diagnostics, Inc., Cypress, CA) is based on inactivated R. typhi, but it is known to cross-react with antibody to the agent of epidemic typhus, R. prowazekii.4 Given the lack of exposure to lice, incompatible geographic exposure, lack of risk factors for epidemic typhus, and very high antibody titers to R. typhi, we consider this patient to represent a case of murine typhus. It is possible that undetected coexistent diagnoses may have accounted for the prolonged time to defervesence described here, although extensive investigation effectively ruled out all of our main differential diagnoses. Reported mean time to defervescence is 2–3 days after doxycyline therapy in several case series2,5–7; delayed response to tetracycline has rarely been observed.8,9
Murine typhus is caused by R. typhi, an obligate intracellular gram-negative bacterium and a zoonosis carried by the rat flea Xenopsylla cheopis.10 R. typhi is distributed worldwide, with endemic foci in Southeast Asia, Africa, Australia, and North and South America.10 Transmission commonly occurs in urban environments with large populations of rats. The incubation period is 1–2 weeks followed typically by a mild disease with fever, headache, nausea, rash, elevated liver enzymes, and thrombocytopenia.11
A recent review collated evidence from seven case series of confirmed murine typhus comprising 553 patients from the United States, Greece, Spain, and Thailand,3 and one additional contemporaneous series is available.12 The most common symptoms were fever (98–100%), headache (41–90%), arthralgia (40–77%), and rash (20–80%). The rash is classically described as faint and maculopapular, with an initial central distribution starting on the trunk and spreading peripherally but sparing the palms and soles.3 In this case, the patient was not aware of a rash until day 6 of illness and may have failed to recognize a faint central distribution at onset. The variability in described cutaneous manifestation precludes its use in narrowing the differential diagnosis.
Murine typhus is generally a mild febrile illness.3,11 Organ-specific complications have been reported in 2–26% of cases, and the most common complications are acute renal failure or neurological abnormalities such as confusion or ataxia.2,5–7,13–15 Pulmonary involvement is rare. From the seven cases series described above, pneumonitis or pulmonary edema was seen in 0–13% of patients and comprised a total of 31 reported cases.2,5–7,13–15 Mortality in adult series ranges from 0% to 1%,3 although in an older series from the 1980s, it was 4%.2 Selection bias may account for the severity of cases seen in these series, which are hospital-based and identified retrospectively by elevated antibody titers. The exception is one series where cases were identified by care providers reporting to a surveillance system.12 Patients with mild disease may not seek medical attention, the diagnosis may be missed, or their doctors may not report it.
This case was a particularly severe case of murine typhus with a delayed response to tetracycline antibiotic treatment and the development of pulmonary edema. A definitive diagnosis was only made based on convalescent serology. The diagnosis of murine typhus should be considered in patients with an undifferentiated febrile illness returning from an endemic area, and empirical doxycycline should be considered pending serological confirmation.
Footnotes
Authors' address: Alexander J. Stockdale, Michael P. Weekes, Bridget Kiely, and Andrew M. L. Lever, Department of Infectious Diseases, Cambridge University Hospitals NHS Foundation Trust, Addenbrooke's Hospital, Cambridge, United Kingdom, E-mails: alexstockdale@doctors.org.uk, weekes@doctors.org.uk, bridgetmkiely@yahoo.co.uk, and amll1@medschl.cam.ac.uk.
References
- 1.Jensenius M, Davis X, von Sonnenburg F, Schwartz E, Keystone JS, Leder K, Lopéz-Véléz R, Caumes E, Cramer JP, Chen L, Parola P. GeoSentinel Surveillance Network. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis. 2009;15:1791–1798. doi: 10.3201/eid1511.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 3.Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913–918. doi: 10.1086/527443. [DOI] [PubMed] [Google Scholar]
- 4.La Scola B, Rydkina L, Ndihokubwayo JB, Vene S, Raoult D. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin Diagn Lab Immunol. 2000;7:612–616. doi: 10.1128/cdli.7.4.612-616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernabeu-Wittel M, Pachón J, Alarcón A, López-Cortés LF, Viciana P, Jiménez-Mejías ME, Villanueva JL, Torronteras R, Caballero-Granado FJ. Murine typhus as a common cause of fever of intermediate duration: a 17-year study in the south of Spain. Arch Intern Med. 1999;159:872–876. doi: 10.1001/archinte.159.8.872. [DOI] [PubMed] [Google Scholar]
- 6.Fergie JE, Purcell K, Wanat D. Murine typhus in South Texas children. Pediatr Infect Dis J. 2000;19:535–538. doi: 10.1097/00006454-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Psaroulaki A, Tselentis Y. Murine typhus in Greece: epidemiological, clinical, and therapeutic data from 83 cases. Trans R Soc Trop Med Hyg. 2002;96:250–253. doi: 10.1016/s0035-9203(02)90090-8. [DOI] [PubMed] [Google Scholar]
- 8.Lai CH, Huang CK, Weng HC, Chung HC, Liang SH, Lin JN, Lin CW, Hsu CY, Lin HH. Clinical characteristics of acute Q fever, scrub typhus, and murine typhus with delayed defervescence despite doxycycline treatment. Am J Trop Med Hyg. 2008;79:441–446. [PubMed] [Google Scholar]
- 9.Angelakis E, Botelho E, Socolovschi C, Sobas CR, Piketty C, Parola P, Raoult D. Murine typhus as a cause of fever in travelers from Tunisia and Mediterranean areas. J Travel Med. 2010;17:310–315. doi: 10.1111/j.1708-8305.2010.00435.x. [DOI] [PubMed] [Google Scholar]
- 10.Azad AF, Beard CB. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 1998;4:179–186. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendershot EF, Sexton DJ. Scrub typhus and rickettsial diseases in international travelers: a review. Curr Infect Dis Rep. 2009;11:66–72. doi: 10.1007/s11908-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 12.Adjemian J, Parks S, McElroy K, Campbell J, Eremeeva ME, Nicholson WL, McQuiston J, Taylor J. Murine typhus in Austin, Texas, USA, 2008. Emerg Infect Dis. 2010;16:412–417. doi: 10.3201/eid1603.091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández Cabrera M, Angel-Moreno A, Santana E, Bolaños M, Francès A, Martín-Sánchez MS, Pérez-Arellano JL. Murine typhus with renal involvement in Canary Islands, Spain. Emerg Infect Dis. 2004;10:740–743. doi: 10.3201/eid1004.030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silpapojakul K, Chayakul P, Krisanapan S, Silpapojakul K. Murine typhus in Thailand: clinical features, diagnosis and treatment. Q J Med. 1993;86:43–47. [PubMed] [Google Scholar]
- 15.Whiteford SF, Taylor JP, Dumler JS. Clinical, laboratory, and epidemiologic features of murine typhus in 97 Texas children. Arch Pediatr Adolesc Med. 2001;155:396–400. doi: 10.1001/archpedi.155.3.396. [DOI] [PubMed] [Google Scholar]


