Abstract
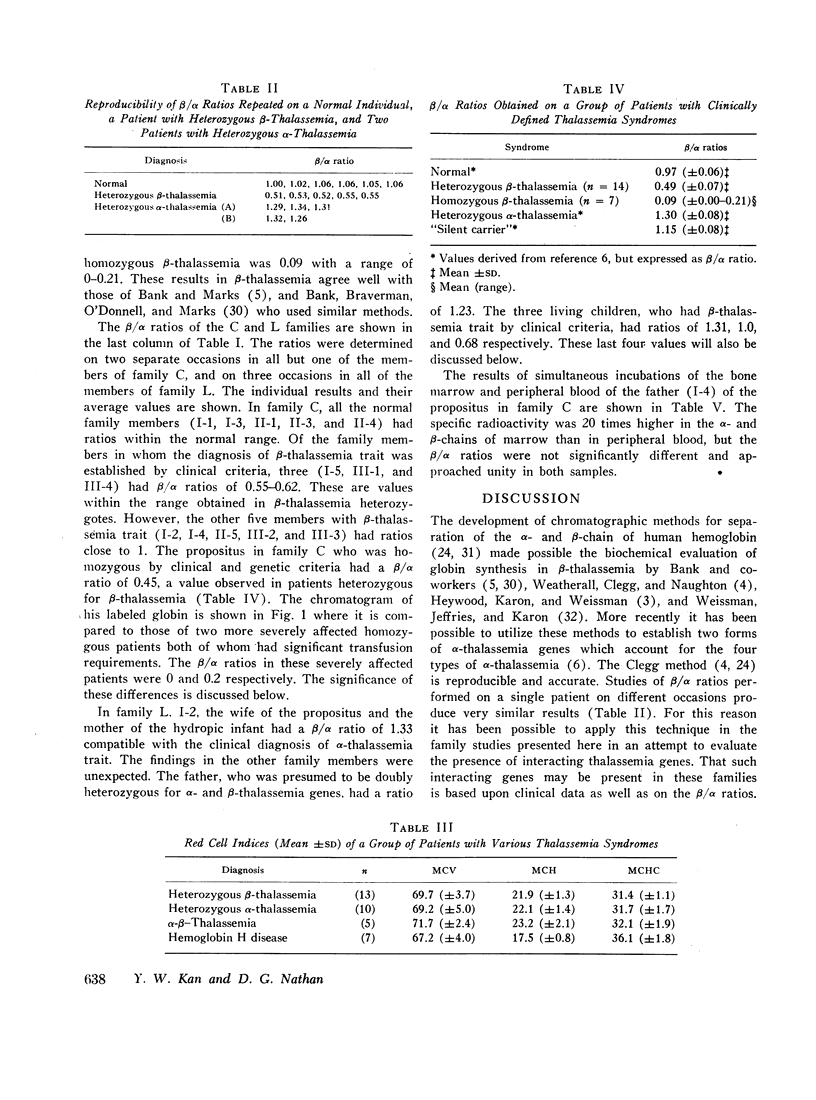
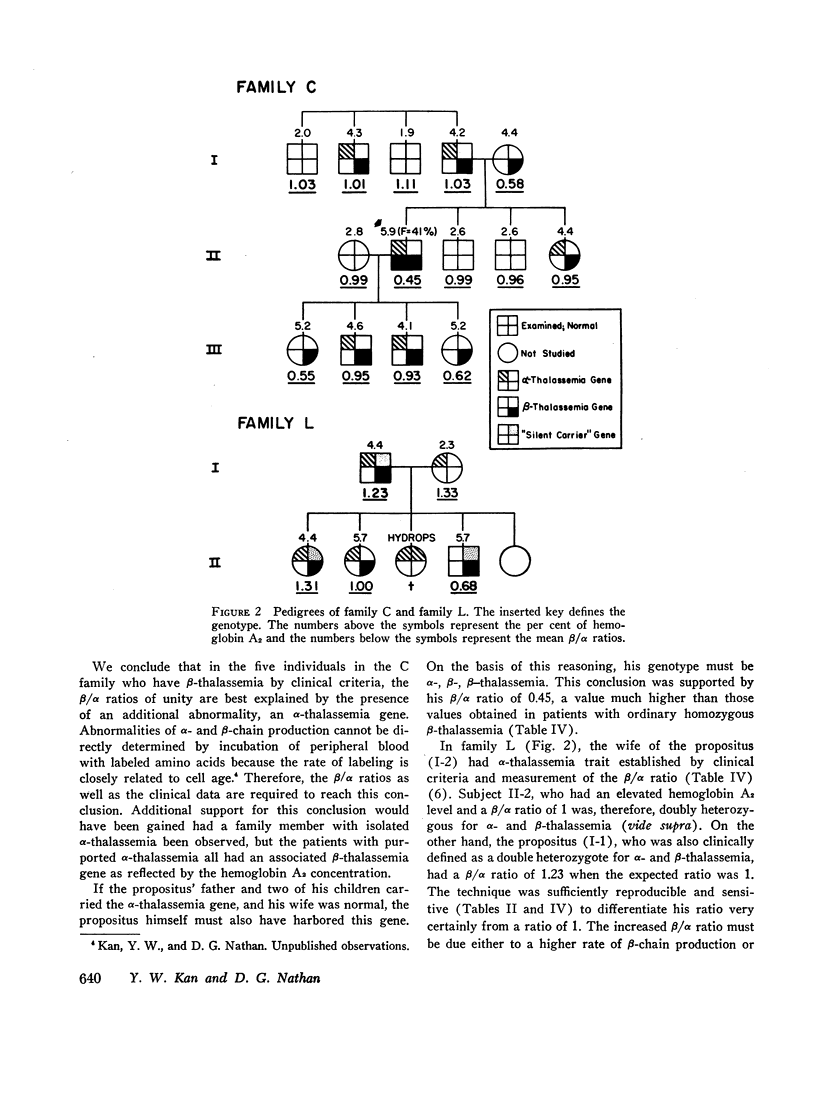
Homozygous thalassemia is due to inherited unbalanced synthesis of the α- or β-chains of hemoglobin. Clinical severity may be in part related to the extent of α:β imbalance. Two families are presented that illustrate this concept. Thalassemia in these individuals was evaluated by clinical and genetic criteria. The relative rates of α- and β-chain synthesis in their reticulocytes were estimated by the extent of incorporation of 1-leucine—U-14C into the chains. Unusual combinations of clinical and hematological data and biosynthetic ratios were obtained in certain individuals which indicated the presence of combinations of α- and β-thalassemia genes. The propositus of the first family had mild Cooley's anemia and was believed to have one α- as well as two β-thalassemia genes. Presumably the α-thalassemia gene interfered with α-chain production which lead to less accumulation of α-chains and a reduced rate of intramedullary and peripheral hemolysis. In the second family two individuals were believed to have an α-thalassemia, a “silent carrier,” and a β-thalassemia gene. Despite the fact that they appeared to have the genotype of hemoglobin H disease, their cells contained no hemoglobin H and had a normal lifespan presumably because excess β-chain production was inhibited by the β-thalessemia gene. These family studies suggest that the α:β imbalance observed in thalassemia may be favorably influenced by combinations of α- and β-thalassemia genes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank A., Braverman A. S., O'Donnell J. V., Marks P. A. Absolute rates of globin chain synthesis in thalassemia. Blood. 1968 Feb;31(2):226–233. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherall D. J. An improved method for the characterization of human haemoglobin mutants: identification of alpha-2-beta-2-95GLU, haemoglobin N (Baltimore). Nature. 1965 Aug 28;207(5000):945–947. doi: 10.1038/207945a0. [DOI] [PubMed] [Google Scholar]
- Fessas P., Loukopoulos D., Kaltsoya A. Peptide analysis of the inclusions of erythroid cells in beta-thalassemia. Biochim Biophys Acta. 1966 Aug 24;124(2):430–432. doi: 10.1016/0304-4165(66)90216-9. [DOI] [PubMed] [Google Scholar]
- Fessas P., Yatachanas X. Intraerythroblastic instability of hemoglobin beta-4 (Hgb H). Blood. 1968 Mar;31(3):323–331. [PubMed] [Google Scholar]
- GABUZDA T. G., NATHAN D. G., GARDNER F. H. THALASSEMIA TRAIT. GENETIC COMBINATIONS OF INCREASED FETAL AND A2 HEMOGLOBINS. N Engl J Med. 1964 Jun 4;270:1212–1217. doi: 10.1056/NEJM196406042702302. [DOI] [PubMed] [Google Scholar]
- GOUTTAS A., FESSAS P., TSEVRENIS H., XEFTERI E. Description d'une nouvelle variété d'anémie hémolytique congénitale; etude hématologique, électrophorétique et génétique. Sang. 1955;26(9):911–919. [PubMed] [Google Scholar]
- Gabuzda T. G., Nathan D. G., Gardner F. H. THE TURNOVER OF HEMOGLOBINS A, F, AND A(2) IN THE PERIPHERAL BLOOD OF THREE PATIENTS WITH THALASSEMIA. J Clin Invest. 1963 Nov;42(11):1678–1688. doi: 10.1172/JCI104854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYWOOD J. D., KARON M., WEISSMAN S. AMINO ACIDS: INCORPORATION INTO ALPHA- AND BETA-CHAINS OF HEMOGLOBIN BY NORMAL AND THALASSEMIC RETICULOCYTES. Science. 1964 Oct 23;146(3643):530–531. doi: 10.1126/science.146.3643.530. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M., STRETTON A. O. Genetic basis of the thalassaemia diseases. Nature. 1959 Dec 19;184:1903–1909. doi: 10.1038/1841903a0. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Allen A., Lowenstein L. Hydrops fetalis with alpha thalassemia. N Engl J Med. 1967 Jan 5;276(1):18–23. doi: 10.1056/NEJM196701052760103. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Schwartz E., Nathan D. G. Globin chain synthesis in the alpha thalassemia syndromes. J Clin Invest. 1969 Nov;47(11):2512–2522. doi: 10.1172/JCI105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Gunn R. B. Thalassemia: the consequences of unbalanced hemoglobin synthesis. Am J Med. 1966 Nov;41(5):815–830. doi: 10.1016/0002-9343(66)90039-8. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Stossel T. B., Gunn R. B., Zarkowsky H. S., Laforet M. T. Influence of hemoglobin precipitation on erythrocyte metabolism in alpha and beta thalassemia. J Clin Invest. 1969 Jan;48(1):33–41. doi: 10.1172/JCI105972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H. A. Alpha-beta thalassemia disease in a Negro family. N Engl J Med. 1966 Jul 28;275(4):176–181. doi: 10.1056/NEJM196607282750402. [DOI] [PubMed] [Google Scholar]
- RIGAS D. A., KOLER R. D., OSGOOD E. E. Hemoglobin H; clinical, laboratory, and genetic studies of a family with a previously undescribed hemoglobin. J Lab Clin Med. 1956 Jan;47(1):51–64. [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. II. Their identification by means of the method of fractional denaturation. Blood. 1951 May;6(5):429–435. [PubMed] [Google Scholar]
- Slater L. M., Muir W. A., Weed R. I. Influence of splenectomy on insoluble hemoglobin inclusion bodies in beta thalassemic erythrocytes. Blood. 1968 Jun;31(6):766–777. [PubMed] [Google Scholar]
- Todd D., Lai M. C., Braga C. A., Soo H. N. Alpha-thalassaemia in Chinese: cord blood studies. Br J Haematol. 1969 Jun;16(6):551–556. doi: 10.1111/j.1365-2141.1969.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Todd D., Lai M., Braga C. A. Thalassaemia and hydrops foetalis-family studies. Br Med J. 1967 Aug 5;3(5561):347–349. doi: 10.1136/bmj.3.5561.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigi V., Volpato S., Gaburro D., Conconi F., Bargellesi A., Pontremoli S. The correlation between red-cell survival and excess of alpha-globin synthesis in beta-thalassemia. Br J Haematol. 1969 Jan-Feb;16(1):25–30. doi: 10.1111/j.1365-2141.1969.tb00375.x. [DOI] [PubMed] [Google Scholar]
- WASI P., NA-NAKORN S., SUINGDUMRONG A. HAEMOGLOBIN H DISEASE IN THAILAND: A GENETICAL STUDY. Nature. 1964 Nov 28;204:907–908. doi: 10.1038/204907a0. [DOI] [PubMed] [Google Scholar]
- WEATHERALL D. J. BIOCHEMICAL PHENOTYPES OF THALASSEMIA IN THE AMERICAN NEGRO POPULATION. Ann N Y Acad Sci. 1964 Oct 7;119:450–462. doi: 10.1111/j.1749-6632.1965.tb54046.x. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Naughton M. A. Globin synthesis in thalassaemia: an in vitro study. Nature. 1965 Dec 11;208(5015):1061–1065. doi: 10.1038/2081061a0. [DOI] [PubMed] [Google Scholar]
- Weissman S. M., Jeffries I., Karon M. The synthesis of alpha, beta, and delta peptide chains by reticulocytes from subjects with thalassemia or hemoglobin Lepore. J Lab Clin Med. 1967 Feb;69(2):183–193. [PubMed] [Google Scholar]
- Wennberg E., Weiss L. Splenic erythroclasia: an electron microscopic study of hemoglobin H disease. Blood. 1968 Jun;31(6):778–790. [PubMed] [Google Scholar]


