Abstract
We have previously demonstrated that exposure of adult rat to a type of enriched environment, known as ‘naturalistic habitat’ (NH), induces extensive functional plasticity in the whiskers’ representations within the primary somatosensory cortex. Here we have investigated the molecular basis for such functional plasticity involved in this model. Based on the role of BDNF on synaptic plasticity and neuronal growth, the focus of this study is on BDNF and its downstream effectors CREB, synapsin I, and GAP-43. In particular, we determined the effects of natural whiskers use during 2, 7 or 28 days exposure to a NH on barrel cortex and hippocampus, as compared to standard cage controls. Naturalistic whiskers use resulted in increased levels of mRNAs and proteins for BDNF and its downstream effectors. Level changes for these markers were already detected after 2 days in the naturalistic habitat and grew larger over longer exposures (7 and 28 days). The cerebral cortex was found to be sensitive to the naturalistic habitat exposure at all time points, and more sensitive than the hippocampus to the trimming of the whiskers. Trimming of the whiskers decreased the level of most of the markers under study suggesting that whiskers exert a tonic influence on plasticity markers that can be further enhanced by naturalistic use. These results implicate BDNF and its downstream effectors in the plasticity induced by the naturalistic habitat. The critical action of experience on molecular substrates of plasticity seems to provide molecular basis for the design of experienced-based rehabilitative strategies to enhance brain function.
Keywords: Rat, somatosensory cortex, hippocampus, environmental enrichment, cortical plasticity
1. Introduction
Over the last few decades, adult cortical plasticity has been increasingly recognized as a fundamental feature of the CNS involving functional and structural changes at the level of synapses, neuronal circuits and their representational maps (Xerri, 2008). Transferring an animal to an ‘enriched environment’ (EE; mainly composed of various objects, a running wheel and the ability to interact with other animals) (Sale et al., 2009) is a powerful stimulator of cortical plasticity. We have previously demonstrated that exposing an adult rat to a type of enriched environment, known as ‘naturalistic habitat’ (NH), induces extensive functional plasticity in the whiskers’ functional representations within the primary somatosensory cortex (SSC) (Polley et al., 2004). NH seems advantageous over the traditional EE to study the somatosensory system because it promotes sensorimotor behaviors more commonly displayed in the rat natural environment like tunnel digging, 3-D underground navigation, and foraging (Polley et al., 2004).
The molecular mechanisms involved in the NH-induced cortical plasticity have yet to be revealed. In previous studies, exposure to the NH resulted in contraction of whiskers unctional representation such that this size reduction resulted in sharper responses to whisker stimulation (Polley et al., 2004). These findings were interpreted as a refinement of the functional organization of the cortex that is achieved by an increase in stimulus-induced inhibition within the barrel cortex (Frostig, 2006). Notably, similar findings (i.e., contraction and weakening of whisker functional representations) were obtained following topical application of brain-derived neurotrophic factor (BDNF) to the adult barrel cortex (Prakash et al., 1996). These data have suggested to us that BDNF could be an underlying player in NH-induced plasticity. Therefore, the current study was designed to investigate the hypothesis that augmented whiskers use in the NH can involve BDNF mRNA and protein, and downstream effectors of BDNF such as cAMP response element-binding (CREB), synapsin I, and growth-associate protein 43 (GAP-43). The actions of these molecules have been associated with circuit modification and synaptic plasticity involved in behavioral tasks including learning and memory (Barth et al., 2000; Molteni et al., 2002) and therefore could underlie functional plasticity following NH exposure. Because behaviors such as foraging and three-dimensional tunnel navigation are known to involve the hippocampus (Churchwell et al., 2010; Kennedy and Shapiro, 2009), our studies were extended to the hippocampus.
We found that a 28 days of exposure to a NH induced a major increase in expression of BDNF mRNA and associated molecular systems, but, even shorter exposures (2 and 7 days) were already enough to induce an increase. In addition, the exposure to NH had a differential influence on the cerebral cortex vs. the hippocampus, i.e., the cortex exhibited faster and more extensive changes in BDNF and associated molecules than the hippocampus for all the time periods studied. Finally, we demonstrate that NH-induced cortical and hippocampal modifications are specifically dependent on whisker use.
2. Results
Our previous results describe extensive functional plasticity 28 days after transfer to the NH (Polley et al., 2004). The results (described below) encouraged us to question whether shorter exposure to the NH could also be significant, and thus, we shortened the time to 2 and 7 days.
2.1 Somatosensory Cortex (SSC)
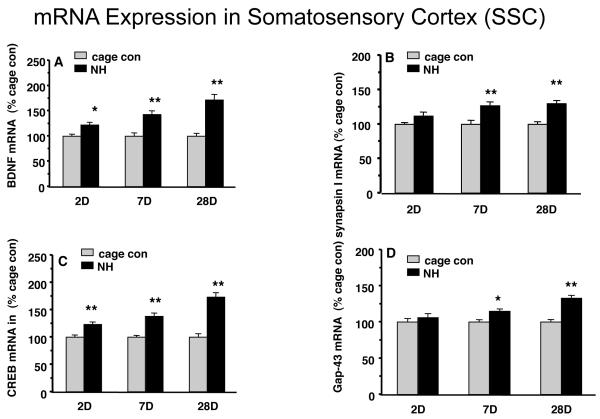
We determined the time course for the changes in mRNA levels within SSC after exposing the animals to the NH for 2, 7, or 28 days. BDNF mRNA levels (Fig.1A) were increased progressively with the time animals spent in the NH, compared to their matched cage controls. BDNF mRNA levels were increased to 122% (p<0.05) after 2 days of NH, 143 % (p<0.01) after 7 days of NH, 172% (p<0.01) after 28 days of NH exposure. As compared to the cage control animals, the mRNA levels of synapsin I (Fig. 1B), CREB (Fig. 1C) and GAP-43 (Fig. 1D) showed a similar pattern to that of BDNF mRNA. 2 and 7 days of NH significantly increased the mRNA levels, 28 days of NH exhibited the highest effects on synapsin I mRNA (130%, p<0.01, Fig.1B), CREB mRNA (173%, p<0.01, Fig.1C) and GAP-43 mRNA (133%, p<0.01, Fig.1D) within SSC compared to cage control group. Finally, we measured the NT-3 mRNA expression in SSC and the results shown that NT-3 mRNA remained unchanged for all the time points in SSC (data not shown).
Figure 1.
The naturalistic habitat (NH) affected mRNA levels of (A): BDNF; (B): synapsin I; (C): CREB; and (D): GAP-43 in the adult somatosensory cortex (SSC). Adult male Sprague-Dawley rats were exposed to NH for 2, 7 and 28 days, and the effects were compared to a cage control group. NH increased the mRNA levels of all the markers in proportion to the time spent on the NH housing. Values are expressed as mean ± SEM. Independent sample T-test (SPSS 16.0) was carried out between NH and cage con groups (*p<0.05, **p<0.01, n=9/group).
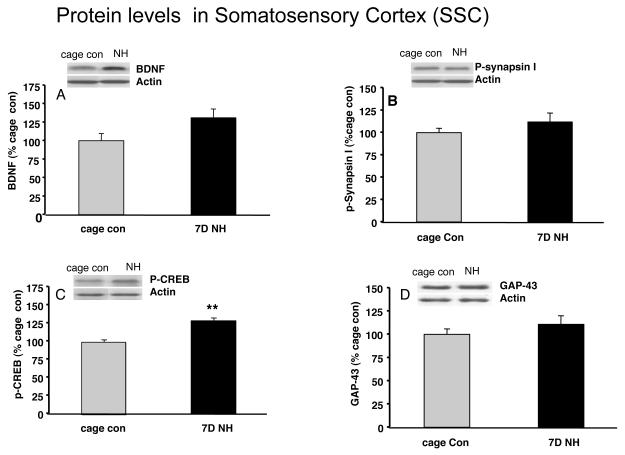
We chose to assess protein levels within SSC for plasticity markers after 7 days of NH exposure (Fig. 2) as to protein synthesis generally takes longer than mRNA synthesis. BDNF levels increased to 131% (p=0.07, Fig. 2A) of the cage control group. Levels of phospho-synapsin I and GAP-43 remained unchanged at day 7, but phospho-CREB levels were significantly increased (27%, p<0.01, Fig. 2C).
Figure 2.
Effects of 7 days exposure to the naturalistic habitat (NH) on protein levels of (A): BDNF; (B): phospho-synapsin I (p-synapsin I); (C); phospho-CREB (p-CREB); and (D): GAP-43 in the adult somatosensory cortex (SSC). The NH promoted a trend for an increase in all protein studied but p-CREB was the only one to reach significance compared to cage control group. Values are expressed as mean ± SEM Independent sample T-test (SPSS 16.0) was carried out between NH and cage con groups (**p<0.01, n=9/group).
2.2 Hippocampus
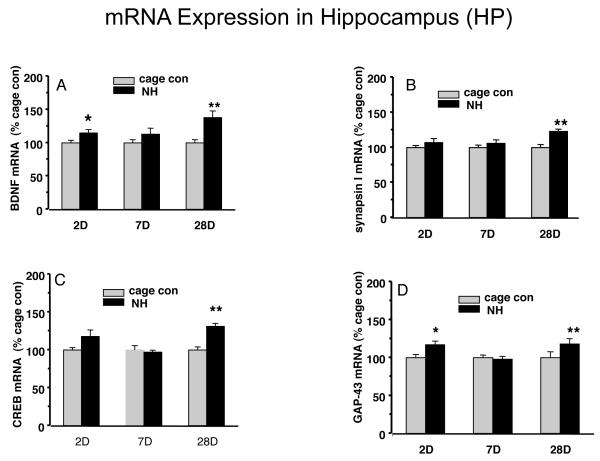
We measured hippocampal mRNA levels for BDNF, synapsin I, CREB, and GAP-43 in the same animals used for SSC assessments. As shown in Fig. 3, 28 days of NH showed an increase in the mRNA levels for BDNF (138%, p<0.01, Fig. 3A); synapsin I (123%, p<0.01, Fig. 3B); CREB (131%, p<0.01, Fig. 3C); and GAP-43 (118%, p<0.01, Fig. 3D). We didn’t find changes in mRNA levels of BDNF (Fig. 3A), synapsin I (Fig. 3B), CREB (Fig. 3C) and GAP-43 (Fig. 3D) after 7 days of NH (Fig.3A-D). Interestingly, at 2 days of NH, BDNF mRNA and GAP-43 mRNA were significantly increased to 115% and 117% respectively, but synapsin I and CREB mRNA levels remained same as the cage control group. There were no changes in NT-3 mRNA in hippocampus at any of the time points (data not shown).
Figure 3.
The naturalistic habitat (NH) affected mRNA levels in the hippocampus (A): BDNF; (B): synapsin I; (C): CREB; and (D): GAP-43. Adult male Sprague-Dawley rats were housed in NH for 2, 7 and 28 days, or standard cages. The effects of NH were mostly evident at the 28 days timepoint. Values are expressed as mean ± SEM.; Independent sample T-test (SPSS 16.0) was carried out between NH and cage con groups (*p<0.05, **p<0.01, n=9/group).
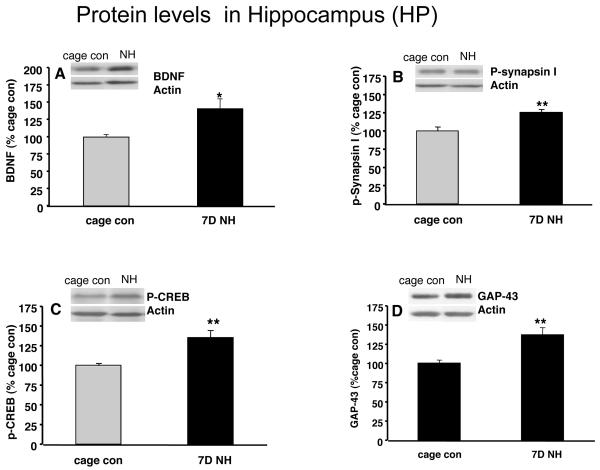
Protein levels were measured after 7 days exposure to the NH. As shown in Fig. 4, BDNF levels in the NH group increased to 141% (p<0.05, Fig. 4A) compared to the cage control group. We found that levels of phospho-synapsin I (125%, p<0.01Fig. 4B), phospho-CREB (135%, p<0.01, Fig. 4C), and GAP-43 (137%, p<0.01, Fig. 4D) were significantly increased (p<0.01) compared to the cage control group.
Figure 4.
Effects of 7 days of naturalistic habitat (NH) on protein levels of (A): BDNF; (B): phospho-synapsin I (p-synapsin I); (C): phospho-CREB (p-CREB); and (D): GAP-43, in the hippocampus (HP). The increase in protein levels reached significant for all markers. Values are expressed as mean ± SEM. Independent sample T-test (SPSS 16.0) was carried out between NH and cage con groups (*p<0.05, **p<0.01, n=9/group).
2.3 Whisker trimming experiments
To examine the role of whisker use in NH-induced mRNA and protein, we trimmed the whiskers of rats (n=18), and half of the rats (n=9) remained in the original cage (cage-whk group), while the other half were exposed to the NH (NH-whk group), during two days. Results of whisker-trimmed rats were compared to those of intact cage control rats (n=9, cage con group).
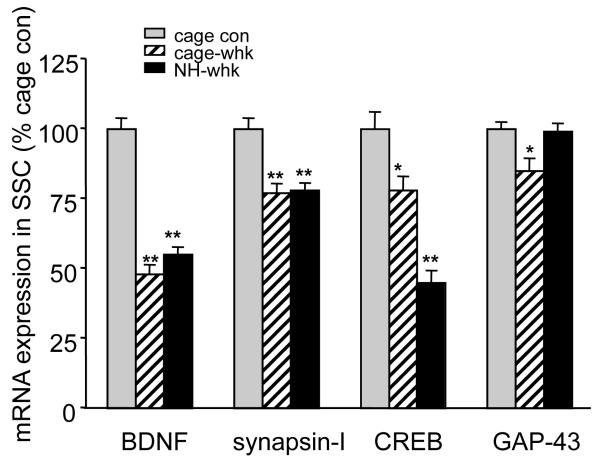
In SSC, BDNF mRNA levels (F(2,24) = 108.422, p<0.01) were decreased to 48% (p<0.01) in cage-whk group and decreased to 55% (p<0.01) in NH-whk group as compared to the cage control group (Fig. 5). Synapsin I mRNA levels (F(2,24) = 15.641, p<0.01) were decreased to 77% in cage-whk (p<0.01) and decreased to 78% (p<0.05) in the NH-whk rats as compared to the cage control group (Fig. 5). CREB mRNA levels (F(2,24) = 20.722, p<0.01) were decreased in cage-whk animals (78%, p<0.05) and in NH-whk animals (45%, p<0.01) as compared to the cage control group (Fig.5). GAP-43 mRNA levels (F(2,24) = 6.180, p<0.01) were also decreased in cage-whk (85%, p<0.05), but no difference was found in NH-whk animals as compared to the cage control group (Fig. 5).
Figure 5.
The effects of whiskers trimming on the mRNA levels for BDNF; synapsin I; CREB and GAP-43 in the rat somatosensory cortex (SSC) region. Separate groups of whiskers trimmed rats were exposed to the naturalistic habitat (NH-whk) or standard cage (cage-whk) for two days. The trimming of the whiskers affected mRNA levels in rats exposed to NH and normal cages (cage-whk). Results were compared to rats with intact whiskers exposed to normal cages. Values are expressed as mean ± SEM. One-way ANOVA was conducted followed by Bonferroni post hoc comparisons between groups. (*p<0.05, **p<0.01, n=9/group).
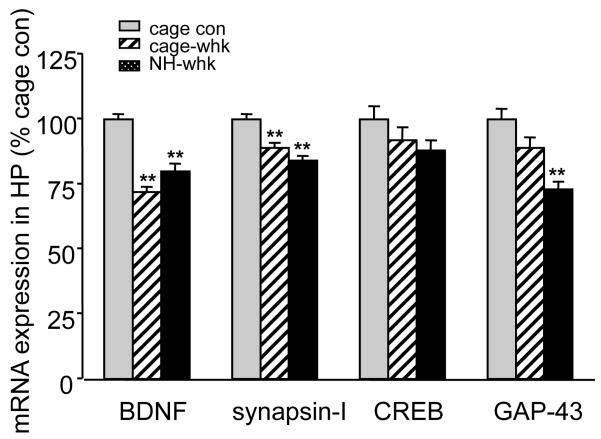
In hippocampus, BDNF mRNA levels (F(2,24) = 22.772, p<0.01) were decreased in cage-whk (72%, p<0.01) and in NH-whk (80%, p<0.01) groups as compared to the intact cage control group (Fig. 6). Synapsin I mRNA levels (F(2,24) = 22.593, p<0.01) were decreased to 89% (p<0.01) in cage-whk and to 84% (p<0.01) in NH-whk compared to the cage control animals (Fig. 6). GAP-43 mRNA levels (F(2,24) = 13.394, p<0.01) were 89% (p>0.05) in cage-whk group and decreased to 73% (p<0.01) in NH-whk group (Fig. 6). There were no changes in CREB mRNA levels. In both SSC and HP, the cage-whk group and NH-whk group remained the same levels of NT-3 mRNA as cage con group (data not shown).
Figure 6.
The effects of whisker trimming on the mRNA levels for BDNF; synapsin I; CREB and GAP-43 in the rat hippocampus (HP). Whiskers were trimmed and separate groups of rats were exposed to the naturalistic habitat (NH-whk) or normal control cage (cage-whk) for two days. The trimming of the whiskers affected mRNA levels in rats exposed to NH and normal cages (cage-whk). Results were compared to rats with intact whiskers exposed to normal cages. Values are expressed as mean ± SEM. One-way ANOVA was conducted followed by Bonferroni post hoc comparisons between groups. (**p<0.01, n=9/group).
3. Discussion
We have previously shown that exposure of rats to an environment similar to what they encounter in their natural habitat (NH) fosters plasticity in the whiskers receptive fields within the somatosensory cortex (Polley et al., 2004). Here we show that the same behavioral paradigm promotes an elevation of BDNF mRNA and protein levels, as well as levels of molecular systems associated to the function of BDNF in synaptic plasticity such as CREB, synapsin I, and GAP-43. Based on previous evidence that the NH promotes functional and structural plasticity in the somatosensory cortex, our results strengthen the case for BDNF as a mediator for the NH-induced cortical plasticity. This possibility is further supported by our findings that trimming of the whiskers reduced the effects of the NH on BDNF expression. In addition, our results provide evidence for the involvement of the hippocampus in the molecular plasticity elicited by the NH exposure.
3.1 NH exposure plasticity in cerebral cortex and hippocampus
The current results suggest the possibility that BDNF may underlie the effects of the NH on somatosensory cortex plasticity. This is supported by our previous studies showing that topical application of BDNF to the barrel cortex promoted cortical plasticity as evidenced by a sharpening of the whiskers functional representations (Prakash et al., 1996). There have been previous attempts directed to evaluate the effects of whisker stimulation on BDNF in the adult barrel cortex. These studies were based on short, passive stimulation of the whiskers (Rocamora et al., 1996), such that their relevance for understanding mechanisms involved in spontaneous use of the whiskers remains unclear. Others studies have shown that exposure of adult amblyoptic rats for several weeks to EE increased the level of BDNF in their visual cortices and restored normal visual acuity and ocular dominance (Sale et al., 2007). Based on the results of our whisker trimming experiments, there seem to be a clear association between whiskers use and the levels of BDNF (mRNA and protein) and associated plasticity markers in the cortex and in the hippocampus. It is interesting that whisker trimming also resulted in molecular plasticity in the hippocampus, which may reflect some of the newly discovered functions of the hippocampus. For example, recent research indicates that the hippocampus is highly involved in behaviors that are continuously expressed in the NH such as 3-D navigation and foraging (Churchwell et al., 2010; Kennedy and Shapiro, 2009). In addition, whisker stimulation in freely behaving rats has been shown to elicit strong evoked neuronal hippocampal activity (Pereira et al., 2007).
We have found that the NH influences BDNF-related plasticity with a particular temporal profile. Increasing NH exposure periods resulted in progressive increases in the cortical and hippocampal mRNA levels compared to matched SC controls. These studies, also established the remarkable finding that an exposure as short as two days to the NH was sufficient to elevate the levels of some of the plasticity markers. Previous studies using the “classical EE” report that the duration of EE exposure leading to elevated BDNF is in the range of weeks or months (Bindu et al., 2007; Ickes et al., 2000; Sale et al., 2007). Finally, our studies suggest that the cortex seems to be particularly sensitive to the NH exposure, as changes in BDNF and associated molecules were consistently growing stronger over time. Increases in hippocampal BDNF protein levels at day 7, a time where only small mRNA elevations were detected, may suggest that these BDNF protein increases could be the result of translational regulation, accumulation, or transport from other regions such as the septum or entorhinal cortex (Altar et al., 1997; Canals et al., 2001; Smith et al., 1997).
3.2 Activity-dependent modulations of BDNF and associated molecules
We found changes at the mRNA and protein levels in molecules implicated with various aspects of synaptic plasticity such as BDNF, CREB, synapsin 1, and GAP-43. The finding that the other member of the neurotrophin family NT-3 mRNA did not exhibit any changes over exposure periods to the NH suggests some level of specificity of the BDNF system. Interestingly, some patterns could be found in the expressions of CREB and BDNF in the cortex. Both showed the strongest increases in their mRNA levels over all exposure periods to the NH, and both showed the strongest decreases when the whiskers were trimmed. The potential interaction between cortical BDNF and CREB may be significant for regulation of cortical activation. Recent studies show that a subtle knocking mutation in the mouse Bdnf gene that specifically blocks the ability of activity-regulated CREB to bind Bdnf promoter IV (the most common promoter in cortex), disrupts the effects of sensory stimulation on Bdnf in the cortex (Hong et al., 2008).
The results of trimming whiskers experiments suggest that whiskers exert a constant (tonic) influence on mRNA expression, even in the standard cage, and this basal level can be further modulated by whisker use (upregulated) or disuse (downregulated). It is noteworthy that the trimming did not equally influence all molecules under study. For example, CREB mRNA showed just a decreasing trend in the hippocampus. Although GAP-43 mRNA had a significant reduction in the hippocampus, it showed only a reducing trend in the cortex, following NH exposure. These findings may be associated with the different roles of CREB and GAP-43 in hippocampus and cortex. The results of trimming experiments may have a potential behavioral correlate: we have noticed that the rats with trimmed whiskers do not dig tunnels as compared to normal, non-deprived rats that were exposed to the NH for the same time period. This observation may also explain why similar drops in molecular markers where found in both caged and NH animals following whisker trimming. Indeed, trimmed and intact caged animals experienced low sensorimotor stimulation (tunnel digging and navigation activity involving intense whisker use). The major difference between the two groups is mainly the free roaming and potential social interactions, which involve minimal use of whiskers, suggest that a reduction in whisker use may be the main cause of the molecular changes. In addition, the fact that the trimming of the whiskers reduced the effects of the NH on the various molecular systems seems to rule out the potential effects of social interaction, which remained the same in the NH/trimmed animals.
3.3 Implications for NH-dependent increase in BDNF
Our findings indicate that NH exposure exerts a powerful influence on the homeostatic levels of BDNF mRNA and protein in the barrel cortex. How could this influence be further interpreted in terms of cortical function? Currently, there seem to be opposing views on the role of BDNF on maintaining the balance between excitation and inhibition in the cortex, a balance that is believed to be critical for normal function of the cortex and its plasticity. One view promotes the concept that an increase in BDNF level is paralleled by a reduction in cortical inhibition, which would allow for EE-induced functional plasticity in the visual cortex (Sale et al., 2009; Spolidoro et al., 2009). The opposing view holds that an increase in the level of BDNF leads to an increase in cortical inhibition (Huang et al., 1999; Ohba et al., 2005). Notably, adult mice with reduced BDNF failed to increase the numbers of inhibitory synapses following passive whisker stimulation, an increase seen in the wild-type (Genoud et al., 2004). A knockin mutation in the mouse Bdnf gene that blocks the ability of CREB to bind Bdnf results in disruption in the sensory experience-dependent induction of Bdnf in the cortex (Hong et al., 2008). The association between elevated BDNF levels and elevated cortical inhibition seems also to fit with our previous findings demonstrating smaller and weaker whisker functional representations following exposure to the NH (Polley et al., 2004) or topical application of BDNF to the cortex (Prakash et al., 1996).
3.4 Conclusions and implications
The naturalistic whisker use upregulates BDNF and molecular systems implicated in control of synaptic plasticity in the somatosensory cortex and hippocampus. The exposure to the NH promoted molecular plasticity in the cerebral cortex at all time points and was heavily affected by the trimming of the whiskers. Although the hippocampus showed more modest responses to the NH, most of the molecular systems were sensitive to the whisker trimming. These results emphasize the involvement of the hippocampus in the effects of somatosensory stimulation. It appears that minimal use of the whiskers involves basal expression of BDNF and related molecules in both cortex and hippocampus, and this level is increased by enhanced use of the whiskers in the NH or is decreased by trimming the whiskers. The observed molecular changes could potentially serve as the underlying mechanism for NH-induced functional plasticity in barrel cortex and other brain regions (Fig. 7). These results are in line with sustained observations in the rehabilitation field indicating the capacity of sensory stimulation to counteract various neurological disorders such as stroke, visual abnormalities, tinnitus, brain trauma, etc. In addition, the results of this study add to the growing body of literature that questions the value of caged animals as a model of normal or pathologic human states.
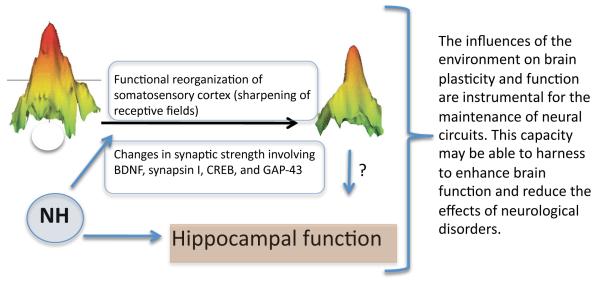
Figure 7.
Potential effects of the naturalistic habitat (NH) on cortical and hippocampal plasticity. Based on our previous findings, we propose that exposure to the NH promotes changes in the reorganization of cortical fields in the somatosensory cortex involving the action of BDNF and downstream effectors on synaptic plasticity such as synapsin I,CREB, and GAP-43. It is proposed that the BDNF system would act on sharpening receptive field in the somatosensory cortex. Based on the current results, we propose that the navigation in the NH engages circuits in the hippocampal formation. However, it is not clear at this point whether information reaching the hippocampus is relayed in the cerebral cortex.
4. Materials and Methods
4.1 Naturalistic Habitat paradigm
The naturalistic habitat (NH) is a novel living environment for rodents that was designed to imitate the rats’ natural environment The NH is built from a 2 m in diameter and 1 m in height steel tank that was filled with packed sterilized topsoil. The NH promotes the expression of natural, innate behaviors such as tunneling and foraging in addition to social interactions among the rats – activities that also promote natural whisker use. Rats were transferred to the NH from their standard cage – a small, standard plastic cage, where it is impossible, to express innate behaviors like the ones expressed in the NH.
In the current study, adult male Sprague-Dawley rats (Charles River) (300-350 g) were randomly exposed to the NH or to cage control groups for 2, 7, 28 days (n= 9 animals per each time point). The cage control rats (n=9) were individually housed in standard polyethylene cages. All rats were maintained in a 12-h light/dark cycle at 22-24 °C, with food and water ad libitum until they were killed by decapitation in the morning of the last day of experimental period. The somatosensory cortex (SSC) and hippocampi (HP) were rapidly dissected out, immediately placed on dry ice, and stored at −70 °C. The somatosensory cortex used for the analysis was contained in a wedge of 3.5 mm diameter centered on the whisker representation area. All procedures were approved by University of California animal research committees and followed the guidelines of the US National Institute of Health Guide for the Care and Use of Laboratory Animals.
4.2 Whisker trimming
Under gas anesthesia (isofluorane (3-4%)) whiskers on both sides of the face were completely trimmed with scissors as close as possible to the skin. The intact whiskers standard cage control group was also anesthetized for the same time as the two other trimmed groups (standard cage and NH); therefore, all three groups that took part in this experiment experienced the same short (~ 3 min) anesthesia period.
4.3 Isolation of Total RNA and Real-Time Quantitative RT-PCR
Total RNA was isolated using an RNA STAT-60 kit (TEL-TEST, Inc., Friendswood, TX) as per the manufacturer’s protocol, and quantification was carried out by absorption at 260 nm. The method used for mRNA quantification was real-time quantitative RT-PCR using an ABI PRISM 7700 sequence detection instrument (Applied Biosystems, Foster City, CA), which directly detects the RT-PCR product without downstream processing by monitoring the increase in fluorescence of a dye-labeled DNA probe specific for the factor of interest. As the control, we employed a probe specific for glyceraldyehyde-3-phosphate dehydrogenase (GAPDH) gene, which has been used previously as a successful endogenous control for the assay (Molteni et al, 2002). Processes were fully automated and carried out using the ABI sequence detector software (Applied Biosystems). Total RNA (100 ng) was converted into cDNA using TaqMan EZ RT-PCR Core reagents (Applied Biosystems, Foster City, CA). The sequences of probes, forward and reverse primers were: BDNF probe: 5′-AGTCATTTGCGCACAACTTTAAAAGTCTGCATT-3′ forward primer: 5′-GGACATATCCATGACCAGAAAGAAA-3′; reverse primer: (5′-GCAACAAACCACAACATTATCGAG-3′; synapsin I probe: 5′ -CATGGCACGTAATGGAGACTACCGCA-3; forward primer: 5′ -CCGCCAGCTGCCTTC-3′, reverse primer: 5′ -TGCAGCCCAATGACCAAA-3′; CREB probe: (5′-CATGGCACGTAATGGAGACTACC GCA-3′), forward primer: 5′-CCGCCAGCATGCCTTC-3′, reverse primer: (5′-TGCAGCCCAATGACCAAA-3′); GAP-43 probe: 5′-CTCATAAGGCTGCAACCAAAATTCAGGCT-3′, forward primer: 5′ -GATGGTGTCAAACCGGAGGAT-3′, reverse primer: 5′ -CTTGTTATGTGTCCACGGAAGC-3′; NT-3 probe: 5′-TGACCGACAAGTCCTCAGCCATTGAC-3′; forward primer: 5′-TGTGACAGTGAGAGCCTGTGG-3′, reverse primer: 5′-TGTAACCTGGTGTCCCCGAA-3′. GAPDH probe and primers for rodent were purchased from Applied Biosystems (Foster City, CA). RT reaction steps consisted of an initial 2-min incubation step at 50°C to activate uracil glycosylase (UNG) and were followed by 30 min of reverse transcription at 60°C. A completion step for UNG deactivation was carried out for 5 min at 95°C. The 40 cycles of two-step PCR reaction consisted of a 20-sec period at 94°C and a 1-min period at 62°C. A threshold cycle (CT) was designated as the amplification cycle at which the first significant increase in fluorescence occurred. Quantification of the Taqman RT-PCR results was carried out by the comparative CT method (protocol #042681, Applied Biosystem). All samples were individually prepared and were duplicated in each assay.
4.4 Protein measurements
Hippocampal and somatosensory cortex extracts were prepared in lysis buffer (137 mM NaCl, 20 mM tris-HCl pH 8.0, 1% NP-40, 10% glycerol 1 mM Phenylmethyl sulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 0.5 mM Sodium Vanadate). Homogenates were centrifuged to remove insoluble material (12,500 g for 20 min at 4 °C) and total protein concentration was determined according to the Micro BCA procedure (Pierce, Rockford, IL 61105, USA). BDNF, phospho-synapsin I, phospho-CREB and GAP-43 proteins were analyzed by Western blot as previously described (Wu et al., 2008). All samples were prepared individually. Membranes were incubated with the following primary antibodies: anti-BDNF (1:1,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-phospho synapsin I (1:2,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-actin (1:4,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-phospho CREB (1:1,000, Santa Cruz Biotechnology Inc., CA, USA); anti-GAP-43 (1:5,000, Santa Cruz Biotechnology Inc., CA, USA) followed by anti-primary IgG horseradish peroxidase conjugate. Immunocomplexes were visualized by chemiluminescence using the ECL plus kit (Amersham Phamacia Biotech Inc., Piscatawy, NJ, USA) according to the manufacturers instructions. The film signals were digitally scanned and then quantified using NIH image software and normalized for actin levels.
4.5 Statistical analysis
Software package SPSS (version 16.0) was used for statistical analysis. Independent sample T-test was used to compare means of standard cage and NH groups for the time points of 2 days, 7 days and 28 days. One-way ANOVA was conducted followed by Bonferroni post hoc comparisons with standard cage group as control for whisker trimming groups. The results were expressed as mean percent of cage control values and represent the mean±standard error of the mean (S.E.M.) of 9 independent determinations. The statistical differences were considered significant when P< 0.05.
Research Highlights.
Naturalistic habitat stimulates cortical plasticity
Whisker use promotes elevation of BDNF
Whisker use involves plasticity in cerebral cortex and hypothalamus
Acknowledgements
We thank Yumei Zhuang for assistance in the protein analysis.
The present study was supported by National Institute of Health awards R01 NS50465 and RC1 NS068473 to FGP, and NS48350 and NS055832 to RDF.
Abbreviations
- EE
Enriched environment
- NH
naturalistic habitat
- SSC
somatosensory cortex
- CREB
cAMP response element-binding
- GAP-43
growth associated protein 43
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain [see comments] Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Barth AL, McKenna M, Glazewski S, Hill P, Impey S, Storm D, Fox K. Upregulation of cAMP response element-mediated gene expression during experience-dependent plasticity in adult neocortex. J Neurosci. 2000;20:4206–16. doi: 10.1523/JNEUROSCI.20-11-04206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindu B, Alladi PA, Mansooralikhan BM, Srikumar BN, Raju TR, Kutty BM. Short-term exposure to an enriched environment enhances dendritic branching but not brain-derived neurotrophic factor expression in the hippocampus of rats with ventral subicular lesions. Neuroscience. 2007;144:412–23. doi: 10.1016/j.neuroscience.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Canals JM, Checa N, Marco S, Akerud P, Michels A, Perez-Navarro E, Tolosa E, Arenas E, Alberch J. Expression of brain-derived neurotrophic factor in cortical neurons is regulated by striatal target area. J Neurosci. 2001;21:117–24. doi: 10.1523/JNEUROSCI.21-01-00117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010 doi: 10.1016/j.nlm.2009.12.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Frostig RD. Functional organization and plasticity in the adult rat barrel cortex: moving out-of-the-box. Curr Opin Neurobiol. 2006;16:445–50. doi: 10.1016/j.conb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Genoud C, Knott GW, Sakata K, Lu B, Welker E. Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J Neurosci. 2004;24:2394–400. doi: 10.1523/JNEUROSCI.4040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–24. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ickes B, Pham T, Sanders L, Albeck D, Mohammed A, Granholm A. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental Neurology. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106:10805–10. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex. 2005;15:291–8. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, Nicolelis MA. Processing of tactile information by the hippocampus. Proc Natl Acad Sci U S A. 2007;104:18286–91. doi: 10.1073/pnas.0708611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Prakash N, Cohen-Cory S, Frostig RD. RAPID and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature. 1996;381:702–6. doi: 10.1038/381702a0. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Pascual M, Acsady L, de Lecea L, Freund TF, Soriano E. Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J Neurosci. 1996;16:3991–4004. doi: 10.1523/JNEUROSCI.16-12-03991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Vetencourt J.F. Maya, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–81. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32:233–9. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Smith MA, Zhang LX, Lyons WE, Mamounas LA. Anterograde transport of endogenous brain-derived neurotrophic factor in hippocampal mossy fibers. Neuroreport. 1997;8:1829–34. doi: 10.1097/00001756-199705260-00008. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res. 2009;192:335–41. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–9. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C. Imprinting of idiosyncratic experience in cortical sensory maps: neural substrates of representational remodeling and correlative perceptual changes. Behav Brain Res. 2008;192:26–41. doi: 10.1016/j.bbr.2008.02.038. [DOI] [PubMed] [Google Scholar]