Abstract
Given the robust influence of diet and exercise on brain plasticity and disease, we conducted studies to determine their effects on molecular systems important for control of brain homeostasis. Studies were centered on a battery of proteins implicated in metabolic homeostasis that have the potential to modulate brain plasticity and cognitive function, in rat hypothalamus and hippocampus. Adult male rats were exposed to a docosahexaenoic acid (DHA) enriched diet (1.25% DHA) with or without voluntary exercise for 14 days. Here we report that the DHA diet and exercise influence protein levels of molecular systems important for the control of energy metabolism (primarily phospho - AMPK, silent information regulator type 1), food intake (primarily leptin and ghrelin receptors), stress (primarily glucocorticoid receptors, and 11beta-hydroxysteroid dehydrogenase 1 (11βHSD1). Exercise or DHA dietary supplementation had differential effects on several of these class proteins, and the concurrent application of both altered the pattern of response elicited by the single applications of diet or exercise. For example, exercise elevated levels of glucocorticoids receptors in the hypothalamus and the DHA diet had opposite effects, while the concurrent application of diet and exercise counteracted the single effects of diet or exercise. In most of the cases, the hypothalamus and the hippocampus had a distinctive pattern of response to the diet or exercise. The results harmonize with the concept that exercise and dietary DHA exert specific actions on the hypothalamus and hippocampus, with implications for the regulations of brain plasticity and cognitive function.
Keywords: Stress, metabolism, synaptic plasticity, homeostasis, mood, depression, anxiety
Introduction
It is becoming well established that exercise (Vaynman and Gomez-Pinilla, 2006; Hillman et al., 2008)and dietary factors, i.e., the omega-3 fatty acid docosahexanoic acid (DHA) (Kitajka et al., 2004; Fedorova and Salem, 2006; Wu et al., 2008), can influence brain plasticity and cognitive function. In addition, recent studies in humans indicate that diet quality (Jacka et al., 2010) and amount of exercise (Laske et al., 2010) are important factors for fighting the risk of mood disorders such as depression illnesses and anxiety. Given that diet and exercise are concurrent components of the daily living, it is likely that their combined actions are crucial for brain homeostasis. This complementary influence of diet and exercise can be exerted on mechanisms that regulate energy balance as to exercise (Gomez-Pinilla et al., 2008) and diet (Peters et al., 2004; Gomez-Pinilla, 2008; Shin et al., 2009) are inherently associated with energy consumption and expenditure. Allostasis, originally defined as the process of achieving homeostasis through physiological and behavioral adaptations (Sterling and Eyer, 1988), likely applies to the action of diet and exercise on the maintenance of homeostasis. Molecular systems crucial to mobilize energy and promote adjustments in brain plasticity and behavior have the promise to be main players in the process of allostasis.
Exercise and DHA dietary supplementation have been shown to affect hippocampal plasticity and function (Wu et al., 2008; Chytrova et al., 2009). The hippocampus is classically recognized for its involvement in learning and memory, and its influence on energy balance is beginning to be understood (Davidson et al., 2007; Davidson et al., 2009). In addition, the hippocampus is part of the loop by which neurocognitive mechanisms are involved in the manifestation of mood disorders such as depression (Clark et al., 2009). The hypothalamus plays a crucial role on control of metabolism in conjunction with the function of higher order centers such as the hippocampus to modulate a range of functions including stress, food intake, and cognition. We have conducted studies in the hypothalamus and hippocampus to determine how exercise interacts with DHA dietary supplementation to influence molecular systems important for the control of cellular homeostasis and brain plasticity.
We have focused our attention on molecular systems recognized for their dual roles on metabolism and synaptic plasticity (reviewed in (Berthoud, 2007; Gomez-Pinilla, 2008; Shin et al., 2009), which have the potential to mediate the action of diet and exercise on brain homeostasis. Receptors for peripheral hormones such as glucocorticoids, leptin and ghrelin are abundant in the hypothalamus and hippocampus. Leptin and ghrelin, which are produced in the adipose tissue and the gut can signal hypothalamic and hippocampal neurons to regulate feeding (reviewed in (Nogueiras et al., 2008) and cognition (Gomez-Pinilla et al., 2008). In addition, glucocorticoids influence the hypothalamus and hippocampus, and regulate cellular stress, appetite and energy balance (Savontaus et al., 2002; Nishida et al., 2006; Shimizu et al., 2008). We have also assessed molecules involved in the control of cellular energy and implicated in cognitive function such as AMP-activated protein kinase (AMPK) (Minokoshi et al., 2004; Minokoshi et al., 2008). The silent information regulator type 1 (SIRT1), a NAD-dependant deacetylase involved in several physiological functions (Wojcik et al., 2009) has also been related to the regulation of energy homeostasis and brain plasticity (Ramadori et al., 2008). The molecular mechanisms that interface stress and cognition are poorly understood and may account for unsolved mental illnesses such as post-traumatic stress disorders. Leptin, ghrelin, glucocorticoids, SIRT1, and AMPK are a kind of metabolic molecules that may influence cognitive events, thereby important to understand the action of diet and exercise on mental health.
Experimental Procedures
Male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA, USA) weighing 350 g on average were housed in cages and maintained in groups of three in environmentally controlled rooms (22–24 °C) with a 12-h light/dark cycle. After acclimatization for 2 weeks on standard rat chow the rats were placed in individual cages. One set of rats was fed on DHA-enriched diet (DHA), while another set was fed on regular diet (RD). Half of the rats maintained on each diet were allowed free accesses for 14 days to running wheels (diameter 31.8 cm, width 10 cm) that freely rotated against a resistance of 100 g. Revolutions of the running wheels were recorded automatically throughout the experiment period by using VitalViewer Data Acquisition System software (Mini Mitter, Bend, OR). The rats were divided into four groups (n=6 in all groups): (1) Regular Diet/Sedentary (RD/Sed), (2) DHA supplemental diet/Sedentary (DHA/Sed), (3) Regular Diet/Exercise (RD/Exc) and (4) DHA supplemental diet/Exercise (DHA/Exc). RD/Sed group was regarded as control. After 14 days the rats were sacrificed by decapitation; the fresh tissues including hippocampus (HP) and hypothalamus (HYP) were dissected, frozen in dry ice and stored at −70 °C until use for protein analyses. Experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of California at Los Angeles Chancellor’s Animal Research Committee. The suffering and number of animals used were minimized.
The diets, fed ad libitum, were provided in the form of powder. The control diet was the standard chow with a ratio of n-6/n-3 at 6:1 (#5001, PMI Nutrition, USA); total fat: 4.5%; arachidonic acid: <0.01%; calorie: 4.07 kcal/gm. DHA enriched diet included 1.25% DHA and 0.25% EPA was prepared freshly everyday by supplementation of capsules provided by Nordic Naturals, Inc. (Watsonville, CA, USA) to the standard chow using a mixer. Food intake was measured daily at the same time at the end of the light phase. In order to standardize food intake values, the average of the food intake for the last week was divided by the body weight for individual animals. The results of the ratio for each group are: RD/Sed: 62.21 (g/kg); DHA/Sed: 57.38; RD/Exc: 64.70; DHA/Exc: 60.6.
Protein Analysis
Hippocampal and hypothalamic tissues were homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. The homogenates were then centrifuged; the supernatants collected and total protein concentration was determined according to MicroBCA procedure (Pierce, Rockford, IL, USA), using BSA as standard. Levels of glucocorticoids receptor (GR), the in-situ glucocorticoids activating enzyme 11-beta hydroxysteroid dehydrogenase type 1 (11βHSD1), ghrelin receptor (GHR; growth hormone secretagogue receptor type 1 GHS-R1a), leptin receptor (Ob-R), phospho-AMPK and SIRT1 were analyzed by Western blot. Briefly, protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to a PVDF membrane (Millipore, Bedford, MA). Non-specific binding sites were blocked in TBS 5% low-fat milk and 0.1% Tween-20. Membranes were rinsed in buffer (0.1% Tween-20 in TBS) and then incubated with anti-actin or anti-GR, 11βHSD1, GHR, Ob-R, phospho-AMPK, SIRT1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by anti-rabbit or anti goat IgG horseradish peroxidase-conjugate (1:200,000; Santa Cruz Biotechnology). After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) for GR, GHR, Ob-R, phospho-AMPK, SIRT1, and SuperSignal West femto kit (Thermo Scientific, Rockford, IL) for 11βHSD1 according to the manufacturer’s instructions. The film signals were digitally scanned and then quantified using ImajeJ software. Actin was used as an internal control for Western blot such that data were standardized according to actin values.
Statistical Analysis
Data are presented as means and their standard errors. Data were analyzed using statistics software SPSS16. Two-way ANOVA (diet vs. exercise) followed by Fisher LSDs post hoc comparisons with the RD/Sed group as control. Criterion for significance was set to p = 0.05 in all comparisons.
Results
Leptin (Ob-R) in the hypothalamus and hippocampus (Figure 1A, 1B)
Figure 1.
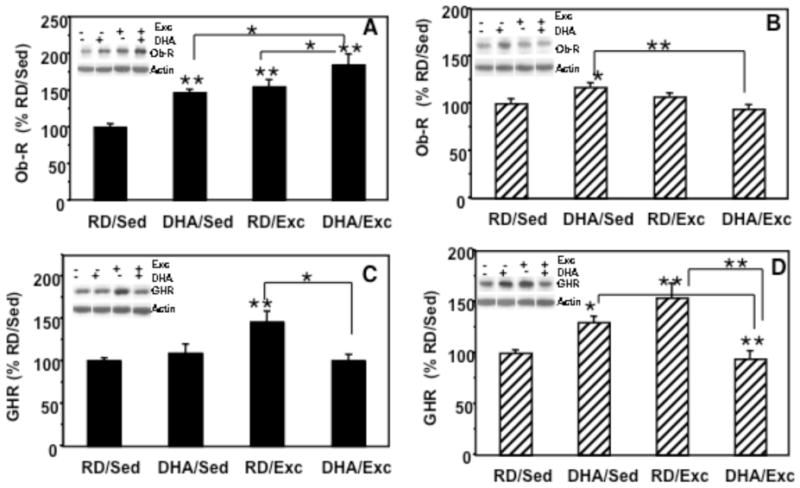
Effects of two weeks of exercise and DHA dietary supplementation on protein levels of leptin receptor (Ob-R) and ghrelin receptor (GHR). (A) In the hypothalamus, Ob-R levels were increased in the exercise group (RD/Exc, 154%) and in the animals fed DHA diet (DHA/Sed, 146%), while the combination of the DHA diet and exercise (DHA/Exc) showed an additive effect (184%). (B) In the hippocampal region, the levels of Ob-R were increased to 117% in the DHA/Sed rats. (C) In the hypothalamus, GHR levels were increased in response to exercise (146%). (D) In the hippocampus GHR levels were elevated in the DHA/sed and RD/EXc groups (154%), and decreased in the DHA/Exc group. Values are expressed as mean ± SEM. LSD’s post hoc comparisons were carried out between groups (*p<0.05, **p<0.01). RD: regular diet; DHA: DHA dietary supplementation; Exc: voluntary wheel running exercise; Sed: sedentary control.
In the hypothalamus, a significant group effect (F4,23 = 10.70, p<0.01) was found for levels of Ob-R protein. Two-way ANOVA (diet vs. exercise) indicated that diet or exercise (diet: F=12.067, p<0.01; exercise: F=17.968, p<0.01) had an influence on Ob-R levels; however, there was not significant interaction between these two factors (F1,23=0.54, p>0 05; Fig. 1A). Multiple comparison analysis using Fisher LSDs post hoc test indicated a significant increase in Ob-R levels in DHA/Sed rats (146%, p<0.05) and RD/Exc rats (154%, p<0.05) compared to the RD/Sed control group (100%). The combination of the DHA diet and exercise (DHA/Exc) showed an additive effect (184%, p<0.01) on Ob-R levels compared to control rats (RD/Sed). In the hippocampus (Fig. 1B), a significant group effect (F=4.293, p<0.05) was found for the levels of Ob-R. Two-way ANOVA (diet vs. exercise) indicated that diet or exercise (diet: F=0.191, p=0.667; exercise: F=2.871, p=0.107) had no significant influence on Ob-R levels; however, there was a significant interaction between diet and exercise (F1,23=0.9.345, p<0.01). Multiple comparisons analysis (Fisher LSDs post hoc test) showed that there was a significant increase in Ob-R in DHA/Sed rats (117%, p<0.05) compared to the RD/Sed control group (100%). Exercise alone (RD/Exc: 107%, p=0.358) or the combination of the DHA diet and exercise (DHA/Exc: 94%, p=0.396) had no significant effect on the level of Ob-R protein compared to control rats (RD/Sed).
Ghrelin receptors (GHR) in the hypothalamus and hippocampus (Figure 1C, 1D)
Hypothalamic GHR protein levels were analyzed using two-way ANOVA (diet vs. exercise) and found a significant group effect (F4,23=5.092, p<0.01). Although there was a significant interaction between diet and exercise (F1,23=8.146, p<0.01), these two factors (diet: F=3.598, p=0.072; exercise: F=3.533, p=0.075) had no significant influence on the levels of GHR. Multiple comparisons analysis (Fisher LSDs post hoc test) showed a significant increase in GHR levels in the RD/Exc group compared to RD/Sed control rats (146%, p<0.01; Fig. 1B). DHA by itself (DHA/Sed, 109%, p>0.05) or combined with exercise (DHA/Exc, 100%) showed no effects on GHR levels relative to the RD/Sed group (Fig. 1C). In the hippocampus, two-way ANOVA revealed a significant interaction of diet and behavior on the levels of GHR (F=25.14, p<0.01). Fisher LSDs post hoc comparisons revealed a significant increase in GHR in the RD/Exc group (154%, p<0.001), and in DHA/Sed group (130%, p<0.05) compared to the RD/Sed group. GHR levels in the Exc/DHA (106%, p=0.647) were no significant different to the RD/Sed control group (Fig. 1D).
Phospho-AMPK in the hypothalamus and hippocampus (Figure 2A, 2B)
Figure 2.
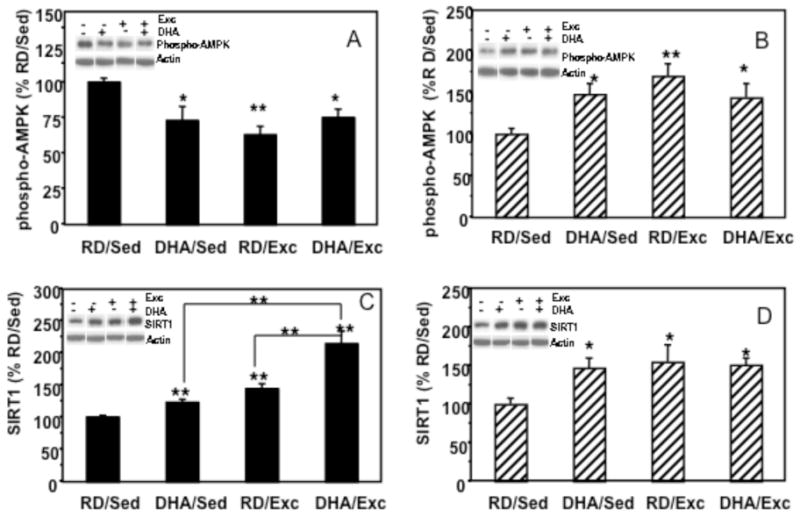
Effects of exercise and DHA dietary supplementation on phospho-AMPK and SIRT1 protein levels in hypothalamus and hippocampus. (A) In the hypothalamus, phospho-AMPK expression was significantly decreased in the three experimental groups as compared to controls. (B) In hippocampus, phospho-AMPK levels were increased in all experimental groups. (C) SIRT1 levels were significantly increased in response to exercise (RD/Exc, 123%) or DHA supplementation (DHA/Sed, 144%) in the hypothalamus, and the combination of DHA and exercise treatment showed a synergistic effect on SIRTI protein levels (DHA/Exc, 214%). (D) In the hippocampus, SIRT1 protein levels were increased in all experimental groups (DHA/Sed, 147%); DHA/Sed, 155%; DHA/Exc, 151%). Values are expressed as mean ± SEM. LSD’s post hoc comparisons were carried out between groups (*p<0.05, **p<0.01). RD: regular diet; DHA: DHA dietary supplementation; Exc: voluntary wheel running exercise; Sed: sedentary control.
In the hypothalamus, a significant group effect (F4,23 = 5.422, p<0.01) was found for levels of phospho-AMPK. Two-way ANOVA (diet vs. exercise) revealed a significant effect of exercise on AMPK levels (F=6.818, P<0.05), and a significant interaction between diet and exercise (F=8.595, p<0.01). Fisher LSDs post hoc comparisons revealed a significant decrease in phospho-AMPK levels in all the experimental groups compare to the RD/Sed control group (DHA/Sed: 73%, p<0.05; RD/Exc: 63%, p<0.001; and DHA/Exc: 75%, p<0.05, respectively; Fig. 2A). In the hippocampus, a significant group effect was found (F=4.689, p<0.01). Two-way ANOVA (diet vs. exercise) showed a significant group effect in exercise animals (F=5.746, P<0.05) but not in diet animals (F=0.649, p=0.432). There was a significant interaction between diet and behavior (F=7.400, p<0.01). Fisher LSDs post hoc analysis indicated a significant increase in phospho-AMPK in all experimental groups (DHA/Sed: 148%, p<0.05; RD/Exc: 170%, p<0.01; DHA/Exc: 144%, p<0.05, respectively, Fig. 2B) compared to the RD/Sed control group.
SIRT1 in the hypothalamus and hippocampus (Figure 2C, 2D)
In the hypothalamus, two-way ANOVA (diet vs. behavior) showed a significant effect of diet or exercise (diet; F=20.154, p<0.01; exercise: F=41.847, p<0.01) on SIRT1 protein levels, and showed an interaction for the diet and exercise (F=5.082, P<0.05). Fisher LSD analysis showed an increase in SIRT1 in the exercise alone group (RD/Exc; 144%, p<0.01), and in the group that combined exercise and DHA treatments (DHA/Exc; 214%, p<0.01). There were no significant changes in the DHA alone group (DHA/Sed; 123%, p=0.131). In the hippocampus, 2-way ANOVA revealed no significant interaction between diet and exercise (F=3.047, p=0.098), but post hoc comparisons using Fisher LSD showed a significant increase in SIRT1 in the DHA/Sed (147%, p<0.05), RD/Exc (156%, p<0.01), and DHA/Exc (151%, p<0.05) groups.
Glucocorticoid receptors (GR) and 11βHSD1 in the hypothalamus and hippocampus (Figure 3A–3D)
Figure 3.
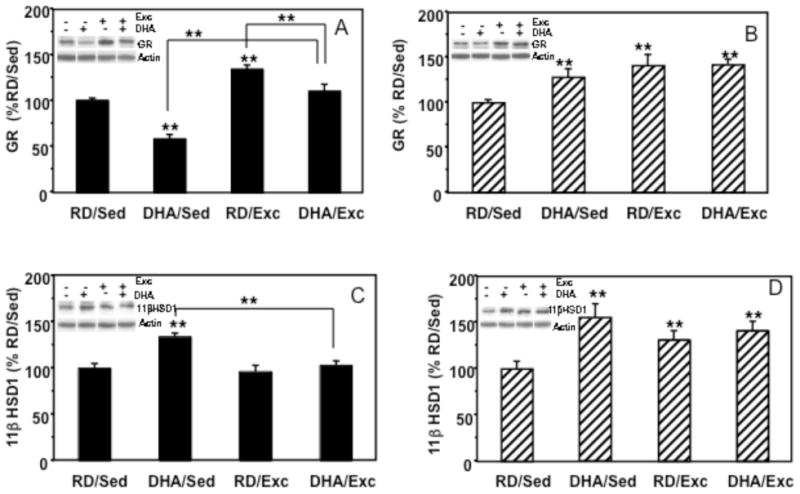
Effects of exercise and DHA dietary supplementation on levels of glucocorticoid receptors (GR) and 11βHSD1 in the hypothalamus and the hippocampus. (A) In the hypothalamus, GR expression was decreased to 58% in the DHA group (DHA/Sed) but increased in the exercise group (RD/Exc, 134%) as compared to the control group. The combination of exercise and DHA diet resulted in an increasing trend of GR (DHA/Exc, 110%). (B) In the hippocampus, GR levels were significantly increased in all experimental groups, DHA/Sed (128%), RD/Exc (141%) and DHA/Exc (142%). (C) In hypothalamus, 11βHSD1 expression was increased to 134% in response to DHA supplementation. (D) In hippocampus, 11βHSD1 levels were increased in all three experimental groups compared to controls. Values are expressed as mean ± SEM. LSD’s post hoc comparisons were carried out between groups (*p<0.05, **p<0.01). RD: regular diet; DHA: DHA dietary supplementation; Exc: voluntary wheel running exercise; Sed: sedentary control.
In the hypothalamus, a significant group effect was found for the level of GR (F=5.807, p<0.01). Two-way ANOVA detected a significant effect for the exercise factor (F=52.918, P<0.0001) and for the diet factor (DHA< RD; F=30.448, p<0.0001), but no significant interaction was found between the factors (F=2.36, p=0.141). Multiple comparisons analysis (Fisher LSD) showed an increase in GR levels in the RD/Exc (134%, p<0.001), a decrease in the DHA/Sed (58%, p<0.0001), and a no significant elevation in the Exc/DHA (110%, p=0.24) group (Figure 3A). In the hippocampus, 2-way ANOVA of the GR data revealed a significant effect for exercise (F=11.235, p<0.01), no significant effect for diet (F=3.343, p=0.820, and no interaction between diet and behavior (F=2.843, p=0.107). Multiple comparisons revealed a significant increase in GR protein level in all the experimental groups as compared to control (DHA/Sed: 128%, p<0.05; RD/Exc: 141%, p<0.01; DHA/Exc: 142%, p<0.01, respectively; Figure 3B).
In the hypothalamus, two-way ANOVA (diet vs. behavior) analysis of the glucocorticoid activating enzyme 11 beta hydroxysteroid dehydrogenase (11βHSD1) showed a significant effect of exercise (F=11.437, P<0.01), diet (F=15.482, P<0.001), and a significant interaction between diet and exercise (F=7.285, P<0.05). Fisher LSD test revealed a significant increase in 11βHSD1 only in the DHA/Sed group (134%, p<0.01), no significant change in RD/Exc (96%, p=0.971) as well as in Exc/DHA (103%, p=0.984; Figure 3C). In the hippocampus, 11βHSD1 protein level was analyzed by two-way ANOVA, finding a significant effect for the diet factor (F=22.463, P<0.001) and a significant interaction between diet and behavior (F=10.766, p<0.01). Closer examination using post-hoc LSD’s test revealed that 11βHSD1 expression was significantly higher than control in the three experimental groups (Sed/DHA: 155%, p<0.001; Exc/RD: 131%, p<0.01; Exc/DHA: 141%, p<0.001; Figure 3D).
Discussion
Food ingestion and physical activity are integral aspects of the daily living and emerging research indicates that their action can be complementary. The purpose of the present study was to understand how the interaction between DHA dietary supplementation and exercise influence important aspects of brain homeostasis. The concept of allostasis, was coined to describe the process of achieving homeostasis through physiological and behavioral adaptations (Sterling and Eyer, 1988), making it very appealing to understand the homeostatic function of diet and exercise. We found that the DHA diet and exercise exerted differential effects on molecular systems controlling important aspects of brain homeostasis, associated with food intake (Ob-R, GHR), energy metabolism (AMPK, SIRT1), and stress (GR, 11βHSD1); and all of them with the capacity to influence cognition. In agreement with the involvement of the hypothalamus and the hippocampus on energy homeostasis and behavior, these two regions showed distinct susceptibilities to the actions of diet and exercise. Results showed that the DHA diet or exercise had differential effects on the protein levels of most of the systems examined, and the concurrent applications of diet and exercise resulted in distinct protein values. Results suggest that the interactive actions of diet and exercise on the hypothalamus and hippocampus play a role on the regulation of homeostasis.
Exercise and DHA effects in the hypothalamus
Our results showed that exercise elevated levels of receptor proteins for leptin (Ob-R) in the hypothalamus. The DHA diet showed a small effect while the combination of diet and exercise had an additive effect on Ob-R. It has been previously described that exercise elevates the sensitivity of the hypothalamus to leptin action (Flores et al., 2006). It is plausible that that the combination of diet and exercise may render the hypothalamus more sensitive to the effect of leptin. Based on the well-described anorexic action of leptin, the complementary action of the DHA diet and exercise may have implications for the control of food intake. In turn, the hypothalamic receptor levels for ghrelin (GHR), a major orexic molecule (Gil-Campos et al., 2006), were elevated only in the exercise rats. The results showing that the DHA diet elevated Ob-R levels while did not affect GHR levels; seem to harmonize with the described opposed roles for leptin and ghrelin in energy metabolism and food intake.
Exercise and DHA effects in the hippocampus
Increasing evidence indicates that the hypothalamus works in concert with the hippocampus to regulate energy homeostasis, food intake (Fehm et al., 2004; Peters et al., 2004) and cognitive function (Davidson et al., 2007). Our results showed that the DHA diet but not exercise promoted increases in Ob-R in the hippocampus. These results in the hippocampus contrast those observed in the hypothalamus, in which exercise and DHA exhibited significant effects on Ob-R levels, and suggest some level of regional specificity for the effects of diet Vs exercise. It is known that leptin has also the ability to influence hippocampal synaptic plasticity and learning and memory via the activation of Ob-R (Harvey, 2007; Morrison, 2009). Therefore, the effects of the DHA diet on hippocampal leptin are significant for interpreting the reported action of the DHA diet on synaptic plasticity and cognitive function (Wu et al., 2008).
Interaction between DHA and exercise
Our results showed that the DHA diet or exercise elevated GHR levels in the hippocampus, and that the concurrent DHA supplementation reduced the effects of exercise on GHR. The finding that the diet attenuated the effects of exercise on GHR seems an excellent illustration for how the interaction of diet and exercise may affect homeostatic regulation. Ghrelin originally described as a gut hormone that signals food intake in the brain, has more recently been found to influence synaptic plasticity and cognitive function (Diano et al., 2006; Gomez-Pinilla et al., 2008). Brain-derived neurotrophic factor (BDNF) may be involved in these events as it has recently been found that voluntary exercise promoted cognitive enhancement in association with elevated levels of ghrelin mRNA and BDNF in the hippocampus (Gomez-Pinilla et al., 2008).
Given the preponderant role of DHA as an integral component of plasma membranes, it is possible that exercise may influence incorporation of DHA into the brain or affect the fate of DHA in the plasma membrane. It has been recently reported that DHA (Cansev et al., 2008; Chytrova et al., 2009) influences the expression of synaptic proteins such as syntaxin-3 that are important for DHA metabolism and function in the membrane, and that exercise enhances these effects (Chytrova et al., 2009). A potential role of exercise on the metabolism of DHA or other membrane phospholipid can have fundamental implications for neuronal signaling events.
Metabolism and plasticity
Although foods and exercise are major modulators of energy metabolism, the metabolic signaling influencing brain plasticity are poorly understood. In our search for molecular systems that may integrate metabolism and plasticity, we previously found that voluntary exercise elevated hippocampal AMPK mRNA in proportion to BDNF levels and learning performance (Gomez-Pinilla et al., 2008). The current results show that exercise or the diet increased hippocampal phospho-AMPK levels and had opposite effects in the hypothalamus. These results point out a contrasting aspect of the hypothalamic Vs hippocampal function on the processing of metabolic signals derived from diet and exercise. In addition, the increase in phospho-AMPK in the hippocampus seems in general agreement with the described action of AMPK on supporting hippocampal learning and memory performance (Winder and Thomson, 2007). In turn, the reduction in hypothalamic levels of phospho-AMPK seems in agreement with the described modulator role of AMPK on food intake (Minokoshi et al., 2004), which is exerted by interacting with leptin in the hypothalamus (Minokoshi et al., 2008).
Our results in the hypothalamus show that exercise or the diet elevated levels of SIRT1 with even greater effects for the combined applications of the DHA diet and exercise. In turn, in the hippocampus, all the interventions elevated levels of SIRT1. The results on the hippocampus are in harmony with previous findings that an omega-3 enriched diet restores levels of SIRT1 and phospho-AMPK disrupted after traumatic brain injury (Wu et al., 2007). Emerging evidence seems to indicate that SIRT1 supports cellular energy metabolism and plasticity (Fulco and Sartorelli, 2008), in particular regulation of plasticity under conditions of limited energy (reviewed in (Coppari et al., 2009). For example, reduced energy status during fasting, associated with enhanced plasticity, elevates levels of SIRT1 in the hypothalamus (Ramadori et al., 2008). It is possible that the excess of hypothalamic SIRT1 after the co-application of diet and exercise may create a metabolic stage that fosters plasticity. It would be important for future studies to dedicate special efforts to determine SIRT1 activity that can complement protein assessments.
Based on the involvement of the glucocorticoid system on metabolism and stress, we evaluated the possible involvement of this system in our paradigm. It is intriguing the fact that exercise elevated, while the diet moderated, glucocorticoid receptor levels in the hypothalamus. Glucocorticoids have multiple functions in the brain and some of them can turn detrimental when homeostasis is loss.
The action of glucocorticoids has been implicated in hippocampal learning and memory, in which moderate levels of glucocorticoids may enhance long-term memory consolidation, but higher levels may do the opposite (reviewed in (de Quervain et al., 2009).
In addition, glucocorticoids have been shown to have a stimulatory action on food intake (Freedman et al., 1985; Tataranni et al., 1996), which is influenced by the enzyme 11βHSD1 (Densmore et al., 2006). The fact that the actions of glucocorticoids are susceptible to the effects of a large array of systems may require special control to remain healthy; therefore, the potential homeostatic action of the DHA diet and exercise can be fundamental. It is interesting that the diet and exercise interaction was exerted at both glucocorticoid receptor and 11βHSD1 in the hypothalamus and hippocampus, which may finely modulate glucocorticoid function; however, more information is required to determine mechanisms involved.
The term allostasis coined to describe the process of achieving homeostasis through physiological and behavioral adaptations (Sterling and Eyer, 1988), clearly entails the function of diet and exercise as these are vital components of our daily routine. Our results harmonize with the concept that exercise and certain diets have the potential to influence long-term plasticity during allostatic load (McEwen, 2007). The current results indicate that diet and exercise influence hormones associated with the brain and body and other systems important for the mobilization of energy and execute adaptive changes in brain function. Previous studies have shown that DHA dietary supplementation enhances the supportive action of exercise on learning and memory capacity and synaptic plasticity (Wu et al., 2008; Chytrova et al., 2009). The current results showing that the diet and exercise interaction extends to the modulation of elements of allostasis in the hypothalamus and hippocampus (Fig. 4) emphasize the function of exercise and diet on maintaining brain plasticity and health.
Figure 4.
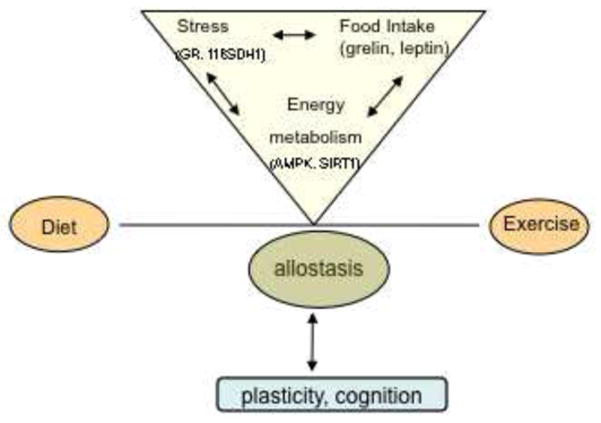
A schematic representation of a potential mechanism by which the balance between exercise and diet could affect brain allostasis. The term allostasis refers to processes involved in achieving homeostasis through physiological and behavioral adaptations (Sterling and Eyer, 1988). Diet and exercise influence several molecular systems in the brain implicated in the control of energy metabolism, which functions also overlay cellular stress and food intake. These events taking place in the hypothalamus and hippocampus can be fundamental for control of brain plasticity and cognitive function.
Acknowledgments
This work was funded by NIH awards R01 NS50465 and R01 NS056413. The authors would like to thank Dr. Amnon Katz for insightful discussions and Yumei Zhuang for invaluable help with the protein assays.
Abbreviations
- 11βHSD1
11beta-Hydroxysteroid dehydrogenase1
- GHR
ghrelin receptor
- DHA
docosahexaenoic acid
- Ob-R
leptin receptor
- SIRT1
silent information regulator type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 2008;4:S153–168. doi: 10.1016/j.jalz.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2009 doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ramadori G, Elmquist JK. The role of transcriptional regulators in central control of appetite and body weight. Nat Clin Pract Endocrinol Metab. 2009;5:160–166. doi: 10.1038/ncpendmet1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Densmore VS, Morton NM, Mullins JJ, Seckl JR. 11 beta-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: A novel constraint to hyperphagia? Endocrinology. 2006;147:4486–4495. doi: 10.1210/en.2006-0106. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Salem N., Jr Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Born J, Peters A. Glucocorticoids and melanocortins in the regulation of body weight in humans. Horm Metab Res. 2004;36:360–364. doi: 10.1055/s-2004-814568. [DOI] [PubMed] [Google Scholar]
- Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–2561. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- Freedman MR, Castonguay TW, Stern JS. Effect of adrenalectomy and corticosterone replacement on meal patterns of Zucker rats. Am J Physiol. 1985;249:R584–594. doi: 10.1152/ajpregu.1985.249.5.R584. [DOI] [PubMed] [Google Scholar]
- Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–226. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and Traditional Diets With Depression and Anxiety in Women. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Kitajka K, Sinclair AJ, Weisinger RS, Weisinger HS, Mathai M, Jayasooriya AP, Halver JE, Puskas LG. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc Natl Acad Sci U S A. 2004;101:10931–10936. doi: 10.1073/pnas.0402342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, Fritsche A, Hipp A, Niess A, Eschweiler GW. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010:1–8. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Shiuchi T, Lee S, Suzuki A, Okamoto S. Role of hypothalamic AMP-kinase in food intake regulation. Nutrition. 2008;24:786–790. doi: 10.1016/j.nut.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Yoshioka M, St-Amand J. Regulation of hypothalamic gene expression by glucocorticoid: implications for energy homeostasis. Physiol Genomics. 2006;25:96–104. doi: 10.1152/physiolgenomics.00232.2005. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann N Y Acad Sci. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28:143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. doi: 10.1016/s0006-8993(02)03674-0. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. 2008;149:4544–4553. doi: 10.1210/en.2008-0229. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav. 2009;97:572–580. doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: Wiley; 1988. pp. 629–649. [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84:699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- Wojcik M, Mac-Marcjanek K, Wozniak LA. Physiological and pathophysiological functions of SIRT1. Mini Rev Med Chem. 2009;9:386–394. doi: 10.2174/1389557510909030386. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


