Abstract
It is likely that the capacity of the brain to remain healthy during ageing depends upon its ability to adapt and nurture in response to environmental challenges. In these terms, main principles involved in hormesis can be also applied to understand relationships at a higher level of complexity such as those existing between the CNS and the environment. This review emphasizes the ability of diet, exercise, and other lifestyle adaptations to modulate brain function. Exercise and diet are discussed in relationship to their aptitude to impact systems that sustain synaptic plasticity and mental health, and are therefore important for combating the effects of aging. Mechanisms that interface energy metabolism and synaptic plasticity are discussed, as these are the frameworks for the actions of cellular stress on cognitive function. In particular, neurotrophins are emerging as main factors in the equation that may connect lifestyle factors and mental health.
Introduction
We interact with a transforming environment that continuously shapes our biological functions including mental health. The brain is a plastic system that derives its functional organization from interaction with environmental factors. In these terms, hormesis defined as the capacity of low doses of a potentially harmful stimulus to promote beneficial changes in adaptive plasticity, takes action. The same principles that apply at the molecular and cellular levels seem to apply at the levels of whole organism physiology. Here, I discuss the mechanisms by which lifestyle factors mold the efficacy of neuronal connections and synaptic plasticity. The ability of specific aspects of lifestyle such as diet, exercise and other challenges to modulate mental function is becoming increasingly recognized. New evidence suggests that the supporting role of brain-derived neurotrophic factor (BDNF) on synaptic plasticity and learning and memory may be achieved by interfacing with mechanisms that modulate cell energy metabolism (Fig. 1). New provocative evidence suggests that the involvement of BDNF with synaptic plasticity and energy metabolisms, may underlie even more profound biological processes such as those related to the epigenetic inheritance of cognitive traits.
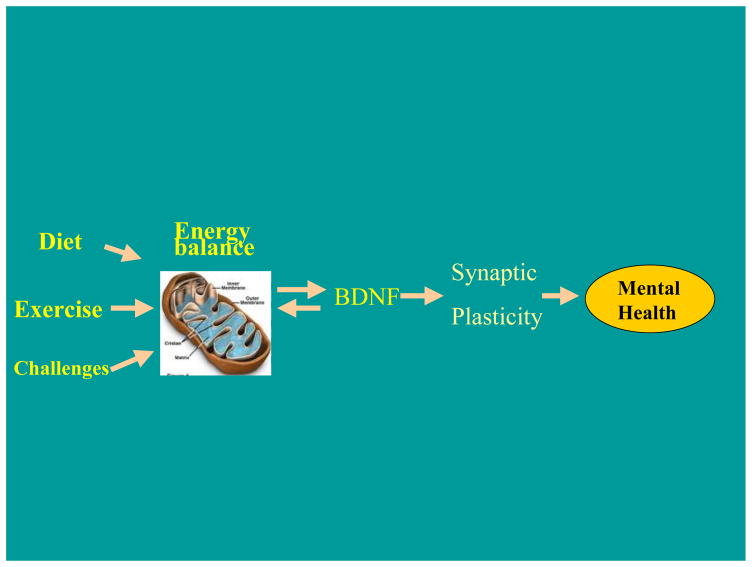
Fig. 1.
Diagram illustrating a general mechanism by which diet, exercise, and other environmental challenges can affect mental health. It is postulated that control of cellular energy balance is a confluent point for the effects of environmental factors. Energy balance via interactions with BDNF, in conjunction with other factors, can modulate synaptic plasticity underlying cognitive processes.
The Influence of Oxidative Metabolism on Synaptic Plasticity and Mental Capacity
Reactive oxygen species (ROS) are generated during cellular respiration, and their levels are greatly increased as a result of abnormal cell metabolism. Cells normally have buffering mechanisms to defend against damage induced by ROS. However, when ROS production exceeds the buffering capacity, cell function and viability are at risk (Gilgun-Sherki et al., 2002). Increase in ROS production has been identified as an important mechanism by which neuronal plasticity is compromised during aging (Gilgun-Sherki et al., 2002). A crucial principle involved with hormesis is that the re-establishment of disrupted homeostasis can result in an adaptive condition that is more beneficial than the prior stage. As discussed below, in this fashion, diet and exercise can affect synaptic plasticity and cognition by involving mechanisms proper of energy balance. New research indicates that oxidative stress (OS) and synaptic plasticity are interrelated events such that an imbalance in free radical formation influences synaptic plasticity and cognitive function. Neuronal and cognitive processes rely on an energy supply to maintain neuronal excitability and synaptic function (Mattson et al., 2004). Emerging evidence indicates that a disruption in energy balance can impact synaptic and cognitive function (Wu et al., 2004b, Vaynman et al., 2006). Recent studies show that molecules typically implicated in serving synaptic plasticity such as BDNF, are affected by cellular energy metabolism. New findings indicate that the interaction between OS and BDNF can be a fundamental mechanism by which aging threaten neuronal plasticity (Cheng and Mattson, 1994, Connor et al., 1997). These studies indicate that elevated ROS decreases BDNF-mediated synaptic plasticity (Wu et al., 2004b). The impact that energy metabolism has on BDNF can be seen by examining disorders of energy balance. Obesity and hyperglycemia in mice are associated with reduced BDNF levels (Lyons et al., 1999, Kernie et al.). Moreover, mitochondrial activity and BDNF are strongly interconnected (El Idrissi and Trenkner, 1999), such that by-products of mitochondrial metabolism as ROS, can limit BDNF protein expression (Wu et al., 2004b). It has also been shown that the BDNF receptor TrKB, mediates signaling mechanisms coupled with the melanocortin-4 receptor (MC4R), a critical hypothalamic element involved in energy balance. MC4R has been shown to regulate the expression of BDNF in the ventral medial hypothalamus (Xu et al., 2003)
Dietary Effects on Brain Plasticity
The effects of oxidative metabolism on cellular physiology can be clearly perceived by observing the mechanisms by which food consumption modulates levels of oxidative stress. From animal studies, it is known that the amount of calories per meal or the frequency of meals affect mental health. This is not surprising considering the feeding habits of our ancestors that have likely imprinted our genome. The early man life was cycled by times of feast and famine, such that those individuals who would convert more of their caloric intake into fat during times of food abundance would be more proficient to survive the times of famine (Booth et al., 2002).
Rodent studies indicate that a caloric restricted (CR) diet or alternating feeding with periods of fasting reduces deficits in motor and cognitive function associated with aging (Ingram et al., 1987, Means et al., 1993). Rodents maintained on an intermittent diet regimen for 2–4 months have shown hippocampal neurons that were much more resistant to degeneration and the degree of neuronal resistance, and this correlated with learning and memory on a water maze task (Bruce-Keller et al., 1999). CR and intermittent feeding increase the production of molecules involved in promoting cell survival, such as the stress proteins heat-shock protein 70 and glucose-regulated protein 78 (Heydari et al., 1996, Duan and Mattson, 1999, Yu and Mattson, 1999). It is also known that CR elevates levels of BDNF (Lee et al., 2002, Duan et al., 2003), and that BDNF may mediate the effect of CR on increasing hippocampal neurogenesis (Lee et al., 2002). In general, it seems that CR can act by reducing the amount of oxidative stress that cells are exposed to, as evidenced by a reduction in oxidative damage to cellular proteins, lipids, and nucleic acids (McCay et al., 1989).
Besides caloric intake per se, the composition of the diet has a strong influence on the molecular substrate for plasticity, and this may involve aspects of oxidative metabolism. Rats exposure to a diet high in saturated fat and sucrose (HFS), similar in composition to “junk food” consumed in fast food restaurants, show decreased BDNF levels in the hippocampus and deficiency in learning and memory tasks (Molteni et al., 2002a). These studies have also shown high contents of OS in the hippocampus of animals exposed to the HFS diet. The fact that antioxidant therapy reduces the effects of this diet on synaptic plasticity and cognition has prompted to the idea that OS is an intermediate factor for the effects of this diet on the brain (Wu et al., 2004a). BDNF has a critical role in maintaining neural function by affecting neuronal excitability, synaptic transmission, and protecting neurons against insult. Our new studies showing that OS may affect BDNF-mediated synaptic plasticity, suggest that proper balance between OS and BDNF is important to maintain brain function.
An increasing number of studies have shown that anti-oxidant rich foods such as blueberries increase multiple parameters of hippocampal synaptic plasticity, and these parameters correlated with improvements in spatial memory (Casadesus et al., 2004). In contrast, decreasing serum levels of vitamin E were found to be associated with poor memory performance in older people (Perkins et al., 1999). Moreover, a recent study found that vitamin E improves lifespan, mitochondrial function, and tests of neurological performance in aging mice (Navarro et al., 2005). Another line of research has shown that curcumin, the yellow curry spice associated with Indian food, may be neuroprotective. Curcumin has been found to inhibit the formation of amyloid beta oligomers and fibrils, bind plaques, and reduce amyloid in an animal model of Alzheimer’s disease (Yang et al., 2005). We have recently found that the curry spice curcumin, when supplemented to the diet, counteracts cognitive dysfunction resulting from elevated ROS after brain trauma (Wu et al., 2004b).
Conversely to the effects of a HFS diet, omega-3 fatty acids -- primary constituents of fish oils -- have been found to increase hippocampal BDNF and enhance cognitive function while reducing oxidative stress under challenging conditions (Wu et al., 2004a). This is consistent with a prospective study in which high fish consumption was inversely associated with cognitive impairment (Kalmijn et al., 1997). Omega-3 fatty acids belong to the family of polyunsaturated fatty acids (PUFAs) that are essential for normal brain function. A diet low in omega-3 content results in decreased learning and memory (Bourre et al., 1989, Moriguchi et al., 2000) and is associated with mental disorders such as attention deficit, dyslexia, dementia, depression, bipolar disorder, and schizophrenia (Hibbeln and Salem, 1995, Adams et al., 1996, Peet et al., 1996, Birch et al., 1998, Hibbeln, 1998, Horrobin, 1998, Fernstrom, 1999, Hoffman et al., 1999). PUFAs are prominent components of neuronal membranes at sites of high signal conduction activity (Crawford and Sinclair, 1972). In this capacity, omega-3 fatty acids, ecosapentaenoic (EPA) and docosahexaenoic acid (DHA) control the function of cells by influencing receptor and intracellular pathways. As omega-3 fatty acids are also components of mitochondrial membranes, they may modulate mitochondrial function. Omega-3 can alter neuronal function by increasing glucose metabolism (Pifferi et al., 2005) such that a deficiency in omega-3 fatty acids reduces brain glucose utilization and transport (Pifferi et al., 2005). As glucose utilization is tightly coupled with neuronal activity (Ip et al., 2003), the ability of omega-3 fatty acids to affect cognitive function may be associated with their capacity to modulate energy metabolism. In addition to increasing glucose metabolism, omega-3 fatty acids may stimulate cellular metabolism by modulating mitochondrial genes and biogenesis (Flachs et al., 2005) and reduce the by-products of dysfunctional metabolism by limiting oxidative stress (Wu et al., 2004a).
Exercise Benefits Mental Health
Studies in humans (Suominen-Troyer et al., 1986, Kramer et al., 1999) and in rodents (Fordyce and Farrar, 1991, van Praag et al., 1999a) have demonstrated the beneficial effects of exercise on cognitive function. These studies have shown that exercise has the capacity to enhance learning and memory (Suominen-Troyer et al., 1986, Rogers et al., 1990, van Praag et al., 1999b) under a variety of conditions, from counteracting the mental decline associated with aging (Kramer et al., 1999) to facilitating functional recovery in patients suffering from brain injury or disease (Lindvall et al., 1992, Bohannon, 1993, Grealy et al., 1999). An analysis of 18 longitudinal fitness-training studies revealed that cardiovascular fitness training improves overall cognitive function regardless of task type (Colcombe and Kramer, 2003). The finding that exercise increases BDNF levels in the hippocampus --an area vital for learning and memory formation-- has provided insight about the molecular mechanisms responsible for the effects of exercise on cognition (Neeper et al., 1996, Gomez-Pinilla et al., 2002, Vaynman et al., 2003). Blocking BDNF action using specific immuno adhesive chimeres abolished the ability of exercise to augment learning and memory in the rat (Vaynman et al., 2004), in conjunction with abolishing the capacity of exercise to elevate BDNF-mediated synaptic plasticity.
Recent findings illustrate the interdependency of metabolic processes with synaptic plasticity during exercise (Fig. 2). Proteomic studies were conducted to evaluate the effect of voluntary exercise on the expression pattern and post-translational modification of multiple protein classes in the rat hippocampus (Ding et al., 2006b). A mass spectrometry analysis of 80 protein spots of relative high abundance on two-dimensional gels revealed that approximately 90% of the proteins identified were associated with energy metabolism and synaptic plasticity. The fact that most of the proteins that were found up-regulated have been implicated in cognitive function, supports a mechanism by which exercise uses processes of energy metabolism and synaptic plasticity to promote brain health.
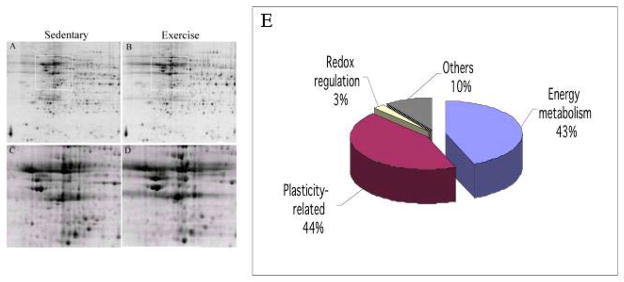
Fig. 2.
Proteomic analysis of hippocampal proteins influenced by exercise. Two-dimensional gel electrophoresis maps of the hippocampus from sedentary (A, C) and exercised (B, D) rats showing the location of protein spots. C and D are high magnifications of A and B, respectively. (E) Mass spectrometry analysis was used to identify specific protein spots, and revealed that a total of approximately 90% of the proteins identified are associated with energy metabolism or synaptic plasticity (Ding et al., Eur. J. Neurosci., 24 (2006) 1265.
Separate studies (Vaynman et al., 2006) have found that in the hippocampus, voluntary exercise decreases oxidative stress and increases the levels of cytochrome c oxidase-II, a specific component of the mitochondrial machinery. Infusion of 1,25-dihydroxyvitamin D3 -- a modulator of energy metabolism -- directly into the hippocampus during voluntary wheel running decreased exercise-induced BDNF and abolished the effects of exercise on end-products of BDNF action (i.e. cyclic AMP response element-binding protein and synapsin I) and modulated phosphorylated calmodulin protein kinase II, a signal transduction cascade downstream to BDNF action that is important for learning and memory. Exercise also significantly increased the expression of the mitochondrial uncoupling protein 2 (UCP2) -- an energy-balancing factor concerned with ATP production and free radical management (Kramer et al., 1999, Laurin et al., 2001) -- to suggest that mitochondrial cellular energy metabolism interacts with the BDNF-mediated system (Ding et al., 2006b). It has been suggested that the aptitude of UCP2 to decrease OS, generate ATP, and buffer calcium may contribute to the ability of the mitochondria to modulate synaptic release and gene expression.
The physiological events involved with the actions of exercise and diet integrate molecular mechanisms of proper energy metabolism to influence brain function. Inherently, exercise and diet are associated with the metabolism of energy throughout the body. The nervous system possesses the capacity to integrate signals with the periphery that modulate energy metabolism (i.e., feeding behaviors, food breakdown, energy acquisition, expenditure, utilization, storage, and transformation). The autonomic nervous system informs the brain about various aspects of digestion such as motility, secretion, and blood flow. Vagal afferents are both sensitive to chemicals released in to blood as well as visceral distension and pain. Interestingly, it has been found that vagus stimulation enhances memory in animal and human subjects (Clark et al., 1995, Clark et al., 1999). Thus, behaviors such as exercise and feeding can affect the CNS through via neural connections from the visceral, in addition to their ability to alter energy metabolism. Recent findings show that neurotrophic factors may comprise key molecular components of a system that engages brain cellular and whole body energy metabolism to impact gene expression and interface with learning and memory mechanisms.
Mind and Body Interaction, and Hormesis
While the brain influences the body, the opposite scenario also occurs (Fig. 3). Ancient and medieval anatomists and philosophers recognized the importance of the autonomic or visceral nervous system to maintain the harmony between internal organs and the brain. Interestingly, the early observation that the gut plays an important role on emotions has not been completely overridden in the modern age. In fact, the influence of the viscera on emotions, feelings, and sorrows is emphasized in current psychiatry. The possibility to dissect the molecular mechanisms involved with the effects of diet and exercise on the CNS provides a new window to interpret the contribution of the gut to emotions and cognition in the context of modern neuroscience. Several proteins that modulate brain function such as serotonin, dopamine, glutamate, norepinephrine, and nitric oxide have been found in the viscera.
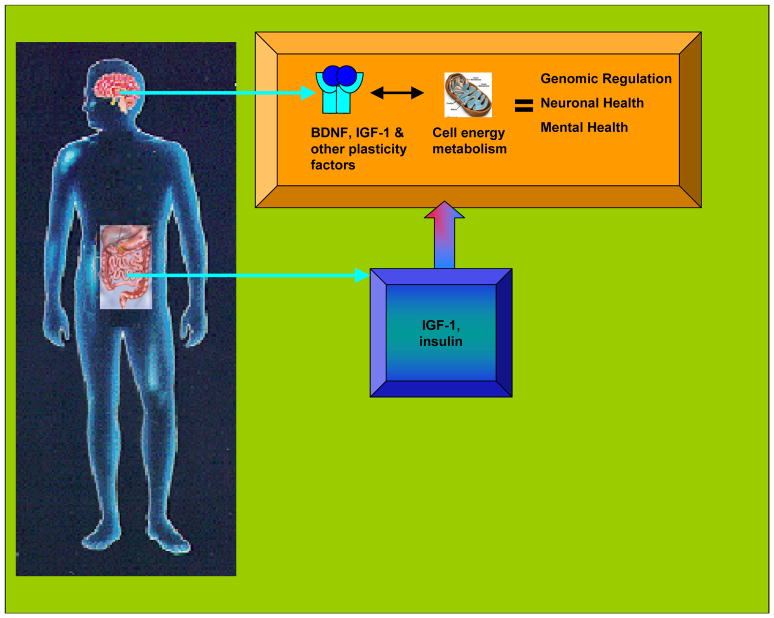
Fig. 3.
Diagram exemplifying the interaction between the gut and the brain on mental health. It is postulated that this interaction is accomplished by involving molecular and cellular mechanisms associated with energy metabolism. As discussed in the text, many molecular mechanisms attributed exclusively to the gut have been found to occur in the brain. For example, IGF-1 and insulin that are produced in the periphery can enter the CNS and modify brain function.
Insulin-like growth factor I (IGF-I) plays a major role in general body metabolism such as regulating plasma lipid concentration (Zenobi et al., 1993)and insulin action (Cusi et al., 1995). Transgenic mice with reduced IGF-I signaling are hyperglycemic and insulin resistant (Murphy and Nolan, 2000). Infusion of insulin IGF-I into the brain results in decreased plasma insulin levels and increased insulin sensitivity (Foster et al., 1991). The IGF-I receptor is expressed in the hippocampus (Islam et al., 1998) and seems to be involved in modulation of synaptic plasticity and cognitive function (Ding et al., 2006a). A decrease of IGF-I may substantially contribute to neurodegenerative diseases as reduced IGF-I levels have been found to exacerbate age-related increases in Aβ accumulations (Carro et al., 2002). IGF-I can also protect the brain from ischemic, oxidative, and amyloid β–peptide insults (Cheng and Mattson, 1992, Carro et al., 2002, Guan et al., 2003). Interestingly, IGF-I has been shown to entrain similar downstream pathways to BDNF action (Roudabush et al., 2000). Moreover, other conditions that intrinsically deal with energy expenditure, such as exercise, stimulate the uptake of blood born IGF-I into the brain, especially into the hippocampus (Trejo et al., 2001). Recent evidence from our own lab revealed that IGF-I is important hippocampal dependent learning and memory and that it may interact with BDNF by modulating the precursor to BDNF during exercise (Ding et al., 2006a).
Ghrelin is another peripheral metabolic protein that has a profound influence on hippocampal architecture and cognitive function (Diano et al., 2006). Ghrelin is defined as an adipogenic hormone that is secreted from the stomach when the stomach is empty (van der Lely et al., 2004), but can also be produced centrally (Cowley et al., 2003). It has been described that peripheral and central ghrelin administration increases food consumption (Wren et al., 2001, Faulconbridge et al., 2003). Ghrelin affects cognitive functions, in addition to its involvement in endocrine and metabolic regulation. This is notably demonstrated by the finding that injections of ghrelin into the hippocampus increase memory retention in rats (Carlini et al., 2004) and is consistent with the earlier finding that the receptors for ghrelin are present in the hippocampus (Guan et al., 1997). Indeed, a recent study shows that ghrelin may have a profound action on hippocampal synaptic plasticity, altering morphology and electrophysiological parameters such as long-term potentiation, and hippocampal-dependent behavioral functions, enhancing learning and memory (Diano et al., 2006). Aging is associated with decreased ghrelin levels (Rigamonti et al., 2002). Ghrelin may be one of a set of factors that serve as molecular interfaces between energy metabolism and neuronal and cognitive function.
Dietary constraints have been shown to enhance learning and memory. For example, either reducing the amount of calories per meal (CR) or every-other-day-fasting (EODF) demonstrated an effect on mental health. Maintaining rodents on a caloric restricted diet or EODF arrested or delayed the deficits in motor and cognitive function associated with ageing (Ingram et al., 1987, Means et al., 1993). Both of these forms of dietary restriction models seem to protect hippocampal and basal cholinergic neurons against excitotoxicity-induced death (Bruce-Keller et al., 1999, Contestabile and Ciani, 2004). In addition, EODF rats exhibited a greater preserved memory than rats fed ad lib and their degree of hippocampal neuronal resistance correlated with learning and memory on a water maze task (Bruce-Keller et al., 1999). Excessive energy intake is associated with an enhanced risk for Alzheimer’s and Parkinson’s disease. A cohort study showed that people who ate a low-calorie or low-fat diet had a significantly lower risk for acquiring these neurodegenerative diseases than those who maintained a high-caloric intake. It is interesting to note that this increased risk factor was more strongly correlated with caloric intake than with weight or body mass index (Logroscino et al., 1996, Luchsinger et al., 2002). These findings support the contention that proper energy balance is related to healthiness in the brain machinery that maintains cognitive abilities.
Energy Expenditure, Metabolism, and BDNF
BDNF provides an excellent example of a signaling mechanism, which is both intimately connected with cognitive function and energy metabolism. BDNF is a recognized arbitrator of metabolic efficiency, eating behavior, synaptic plasticity, and learning and memory. In the mature CNS, the BDNF protein is most abundant in brain areas associated with cognitive and neuroendocrine regulation -- the hippocampus and hypothalamus, respectively (Nawa et al., 1995). BDNF function has been shown to regulate obesity (Nawa et al., 1995, Lyons et al., 1999, Kernie et al., 2000), insulin sensitivity (Pelleymounter et al., 1995, Nakagawa et al., 2002), glucose (Tonra, 1999) and lipid metabolism (Tsuchida et al., 2002), and oxidative stress (OS) levels, the harmful by-products of metabolism (Lindvall et al., 1992, Lee et al., 2002, Wu et al., 2004b). A prime example is the effect of BDNF on multiple parameters of energy metabolism in a rodent model of diabetes. Central administration of BDNF to diabetic mice lowered blood glucose levels and simultaneously increased insulin levels, enhanced thermogenesis, and upregulated the mRNA expression of the uncoupling protein 1(UCP-1) in brown adipose tissue (Nonomura et al., 2001).
Studies of transgenic mice heterozygous for BDNF, show that BDNF insufficiency results in hyperphagia, obesity, and hyperinsulinemia (Lyons et al., 1999, Kernie et al., 2000). The peripheral or central administration of BDNF reduces body weight, normalizes glucose levels (Tonra, 1999), ameliorates lipid metabolism in diabetic rodents (Tsuchida et al., 2002), and increases insulin sensitivity (Pelleymounter et al., 1995, Nakagawa et al., 2002). Hyperphagia and high oxidative stress (OS) levels, the harmful by-products of energy metabolism, decrease BDNF levels, while hypoglycemia and intermittent fasting both increase BDNF levels (Lindvall et al., 1992, Lee et al., 2002, Wu et al., 2004b). In humans, a de novo mutation affecting TrkB, the consort receptor to BDNF, is linked with both hyperphagia and obesity and developmental delays and other defects in higher order neurological functions (Yeo et al., 2004).
The Energy Metabolism - Mind Connection
It has been postulated that as a result of environmental pressures of ‘feast and famine’ in man’s early evolution, mechanisms that modulate cellular energy metabolism have evolved to maximize survival rates during challenging situations (Holliday, 1999). Basically, individuals who were successful in managing food resources became the fittest and this involved adaptations in hypothalamic neuro-metabolic mechanisms. Indeed, new research shows that metabolic signals interface with the hippocampus, to affect the mechanisms of synaptic plasticity underlying cognitive function (Vaynman et al., 2006). Learning and memory are central to the ability of animals to acquire energy sources and ultimately to survive. The discordance between our genes and the environment manifests on the level of diseases related to higher order cognitive function. Numerous studies have found that there may be a link between abnormal glucose metabolism, particularly an increased risk for diabetes type II and psychiatric disorders. Psychiatric disorders such as depression, bipolar, and schizophrenia are associated with cognitive deficits and in many instances in severe cognitive impairment (O’Brien, 2005). Similar findings have been reported for other psychiatric illnesses, such as manic depression. A study of 203 inpatient manic-depressive subjects, (Lilliker, 1980) reported a three fold increased rate of diabetes as compared to other psychiatric inpatients and the general US population. Schizophrenia shows the same increasing rates of diabetes as compared to controls (Tabata et al., 1987, Mukherjee et al., 1996, Dixon et al., 2000). Controlling for the confounding factor of psychotropic medication, many of which are associated with disturbances in glucose metabolism and the onset of diabetes, it has been reported (Regenold et al., 2002) that there is an intrinsic relationship between abnormal glucose metabolism and bipolar disorder type I as well as schizoaffective disorders. Interestingly, the association between metabolic dysfunction and psychiatric disorders, especially type 2 diabetes, may be related to a decrease in BDNF expression (Krabbe et al., 2007). Illustrating the relationship between metabolism and genetics, it has been found that a BDNF polymorphism contributes to a genetic vulnerability to the development of eating disorders such as bulimia nervosa and binge eating disorder (Monteleone et al., 2006).
A specific BDNF genotype polymorphism has been identified in the etiology of psychiatric disorders. The Val66Met BDNF polymorphism is a common single nucleotide missense change (G196A) that produces a non-conservative amino acid substitution of valine to methionine at codon coding exon of the BDNF-gene at position 66 (Val66Met). The Val66Met BDNF gene polymorphism has recently been linked with cognitive impairment and brain morphometric correlates in schizophrenia (Ho et al., 2006). This study found that schizophrenic subjects exhibited impairment in medial temporal lobe-related memory performances, which were associated with the specific BDNF genotype effects on gray matter volumes. Specifically, Met allele carriers exhibited smaller temporal and occipital lobar gray matter volumes (Ho et al., 2006). The Val66Met BDNF gene polymorphism has also been linked with geriatric depression and cognitive performance (Hwang et al., 2006). From the standpoint of affective disorders, BDNF has been identified as the most important neurotrophin contributing to the pathogenesis of the depressive disorders. Preclinical and clinical studies demonstrate altered BDNF expression during chronic stress and increased BDNF activity during antidepressant treatment (Filus and Rybakowski, 2005). Stress models of depression have proposed that stress-induced BDNF downregulation is a result of a repression in the transcription of the BDNF gene promoter by the activated corticosteroid receptor in the hippocampus (Schaaf et al., 2000).
Neurotrophins and Cognitive Function
Neuronal activity enhances the expression, secretion, and actions of BDNF at the synapse to result in the modification of synaptic transmission and connectivity. Sensory stimulation regulates BDNF with visual input in the visual cortex (Castren et al., 1993), and whisker stimulation in the barrel cortex (Rocamora et al., 1996). Additionally, physiological levels of activity such as exercise (Neeper et al., 1996, Vaynman et al., 2003), learning (Kesslak et al., 1998) and sleep and circadian rhythm (Bova et al., 1998, Liang et al., 1998) modulate BDNF levels. Transfection experiments employing BDNF-GFP (green fluorescent protein) fusion constructs have enabled the actual visualization of BDNF in hippocampal and cortical neurons. Accordingly, these studies have revealed that BDNF is packaged in secretory vesicles (Haubensak et al., 1998, Kojima et al., 2001). Colocalization of BDNF with specific markers -- the presynaptic secretory protein synapsin I and the postsynaptic scaffolding protein PSD95 -- revealed that the BDNF-GFP fluorescence was found to be concentrated at synaptic junctions (Haubensak et al., 1998, Kojima et al., 2001). The BDNF-GFP fluorescence spots were found to quickly disappear when depolarization or high frequency stimulation was applied, therefore suggesting that BDNF was secreted from these synaptically localized secretory vesicles (Haubensak et al., 1998, Kojima et al., 2001). Chen et al. have reported an interesting finding revealing aspects of the mechanism involved in the regulation of BDNF gene by activity (Chen et al., 2003). Using a chromatin immunoprecipitation technique, they found that the transcriptional repressor Mecp2 is bound to the rat BDNF promoter III in resting cortical neurons. However, upon the application of activity (i.e., membrane depolarization and subsequent calcium influx) BDNF transcription occurs concurrent with the dissociation of Mecp2 repression from the BDNF promoter.
Numerous studies have documented the role of BDNF in supporting learning and memory, from findings that the hippocampal BDNF expression is increased during learning tasks (Kesslak et al., 1998, Hall et al., 2000) to studies showing that genetic deletion of the BDNF gene impairs memory formation (Linnarsson et al., 1997, Ma et al., 1998, Mizuno et al., 2000). It has also been shown that hippocampal BDNF mediates the ability of exercise to enhance learning and memory (Vaynman et al., 2004). BDNF expression is higher in the hippocampi of rats that underwent hippocampal-dependent learning paradigms such as the Morris water maze task or contextual fear conditioning (Kesslak et al., 1998, Hall et al., 2000). BDNF mRNA levels have been found to be significantly increased in the CA1 region of the hippocampus during contextual fear conditioning, another hippocampal dependent learning paradigm (Hall et al., 2000). An association between hippocampal BDNF levels and learning and memory was found to exist when measuring the performance of rats on a learning and memory task (Molteni et al., 2002b). The results of this study suggest that hippocampal levels of BDNF may be directly related to learning efficiency and memory stability. Clinical studies reveal that the val66met BDNF genotype polymorphism seems implicated in abnormal hippocampal functioning and memory processing (Egan et al., 2003, Hariri et al., 2003). Egan et al. (Egan et al., 2003) found that this BDNF polymorphism seems to be linked with specific deficits in episodic memory and that this may subside with abnormal intracellular trafficking and secretion of BDNF in neuronal cells (Chen et al., 2004).
Downstream BDNF Systems Supporting Learning and Memory
Blocking experiments have identified some of the pathways that contribute to the elevation of BDNF during exercise involving molecules related to gene transcription and synaptic transmission, such as cAMP response element binding protein (CREB) and synapsin I, respectively (Vaynman et al., 2003). Evaluation of the pathways activated downstream to BDNF induction provide further insight into how exercise is capable of orchestrating its beneficial effects on brain health and learning and memory. The two main intracellular signaling cascades found to be activated by BDNF -- CAMKII and MAPKII -- have important roles in neuronal and behavioral plasticity. TrkB receptor activation has been shown to lead to the launching of the MAPK cascade (Stephens et al., 1994), which serves as an intracellular signaling mechanism that integrates multiple signals (Sweatt, 2001) and leads to the activation of CREB mediated transcription, protein synthesis, and voltage/ion gated channels. Exercise-induced BDNF seems also to activate CAMKII (Blanquet and Lamour, 1997), which has been shown to converge on the MAPK cascade (Blanquet et al., 2003).
The Inheritable Potential for the Effects of Lifestyle on the Brain
Given the importance of lifestyle factors to modulate the health of body and mind, it is likely that the effects of lifestyle can be transmitted across generations as epigenetic phenomena. Indeed, novel findings indicate that exercise in a pregnant mother can also have a positive effect on the brain and spatial learning ability of the offspring. (Parnpiansil et al., 2003). It has been reported that the pups of pregnant rats who run on a treadmill regimen had elevated hippocampal BDNF mRNA and showed better performance on spatial learning tests than pups from sedentary mothers. Although the mechanism by which exercise in the pregnant mother benefits the newborn remains elusive, it is known that maternally derived neurotrophic factors may cross the placenta to influence the health and development of the fetus (Uchida et al., 2000, Parnpiansil et al., 2003). A more recent study on the effect of exercise during pregnancy on the offspring has found that maternal swimming exercise increases neurogenesis in the offspring (Lee et al., 2006). As hippocampal neurogenesis is very well correlated with learning and memory abilities (Kempermann et al., 1998, Gould et al., 1999, Shors et al., 2001), it may well play a significant role in the exercise-induced enhancement of cognitive function in the offspring. Exercise is already known for its ability to induce neurogenesis in the active animal (van Praag et al., 1999a). It is also possible that exercise may influence the new generation by exerting changes at the epigenetic level.
The epigenetic platform integrates with BDNF–mediated plasticity, being methyl-CpG-binding protein (MeCP2) the strongest connection to date. MeCP2 belongs to the family of methylcytosine-binding proteins that are abundantly expressed in the central nervous system and contribute to the gene silencing effect of DNA methylation (Lewis et al., 1992, Ng and Bird, 1999). The dynamic regulation of DNA methylation and MeCP2 modification contribute to the activity driven regulation of the BDNF gene. Findings indicate that MeCP2 occupies a site on the BDNF promoter in the absence of stimulation (Martinowich et al., 2003). When membrane depolarization is applied, MeCP2 dissociates from the BDNF exon IV promoter and methylation of several cysteine residues within the core promoter that results in the transcriptional repression of BDNF (Chen et al., 2003). MeCP2 extends the importance of BDNF to neural and cognitive plasticity. Mutations in the MeCP2 gene have been linked to a neuro-developmental disorder, Rett syndrome (Amir et al., 1999). Multiple studies demonstrating that MeCP2 deficiency in mice results in Rett syndrome–like abnormalities substantiate the role of MeCP2 in neuronal function (Amir et al., 1999, Chen et al., 2001, Guy et al., 2001). New findings point out that the activity dependent BDNF transcription is also regulated by the phosphorylation of MePC2 at serine 421 (S421) (Zhou et al., 2006). Neuronal activity and subsequent calcium influx was found to selectively induce a CAMKII dependent mechanism of MeCP2 phosphorylation at S421 in the brain that was required for dendritic patterning, spine morphogenesis, and activity-dependent gene expression. As MeCP2 functions as a BDNF transcriptional repressor, MeCP2 S421 phosphorylation relieves its transcriptional repressor function on the BDNF promoter IV (Zhou et al., 2006).
Conclusions
The influences of environmental factors on the brain are manifested by their abilities to promote adaptive changes using principles in common with hormesis. The novelty associated with many environmental stimuli represents a physiological challenge for affected individuals. The sustained pressure of these environmental factors results in the activation of adaptive mechanisms that can become beneficial for neuronal health and plasticity. A crucial factor for determining the ultimate biological significance of the challenge is related to the genetic disposition of affected individuals for the type of stimulus. If there is strong discordance between the challenge and the genome, the homeostasis is lost and the brain becomes vulnerable to diseases. Indeed, it is likely that many modern diseases that affect our society subside in incongruence between our genome and the environment. For example, the Western population has experienced an increase in the incidence of metabolic disorders ((Flegal et al., 1998, Sothern et al., 1999, Mokdad et al., 2001, Ogden et al., 2002). In the United States alone, 65% of adults over the age of 20 are overweight or obese (Hedley et al., 2004). The necessity for physical activity imprinted in our genome, in addition to contributing to the prevalence of obesity in modern industrialized societies (Wendorf and Goldfine, 1991, Booth et al., 2002), also imposes a risk factor for metabolic dysfunctions such as type II diabetes, hypertension, and cardiovascular disease (Jung, 1997, Must et al., 1999, Booth et al., 2002). Recent findings illustrate the interdependency of energy metabolic processes and synaptic plasticity, and this may provide a mechanism to explain how metabolic disturbances can affect mental health. Indeed, there are various psychiatric disorders that have a strong association with abnormal metabolism.
Acknowledgments
Supported by NIH awards NS45804 and NS 50465
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res. 1998;44:201–209. doi: 10.1203/00006450-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Blanquet PR, Lamour Y. Brain-derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J Biol Chem. 1997;272:24133–24136. doi: 10.1074/jbc.272.39.24133. [DOI] [PubMed] [Google Scholar]
- Blanquet PR, Mariani J, Derer P. A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience. 2003;118:477–490. doi: 10.1016/s0306-4522(02)00963-6. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Physical rehabilitation in neurologic diseases. Curr Opin Neurol. 1993;6:765–772. doi: 10.1097/00019052-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Francois M, Youyou A, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- Bova R, Micheli MR, Qualadrucci P, Zucconi GG. BDNF and trkB mRNAs oscillate in rat brain during the light-dark cycle. Brain Res Mol Brain Res. 1998;57:321–324. doi: 10.1016/s0169-328x(98)00092-8. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Ciani E. Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity. Brain Res. 2004;1002:162–166. doi: 10.1016/j.brainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Sinclair AJ. The limitations of whole tissue analysis to define linolenic acid deficiency. J Nutr. 1972;102:1315–1321. doi: 10.1093/jn/102.10.1315. [DOI] [PubMed] [Google Scholar]
- Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care. 1995;18:843–851. doi: 10.2337/diacare.18.6.843. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006a;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006b;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Dixon L, Weiden P, Delahanty J, Goldberg R, Postrado L, Lucksted A, Lehman A. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26:903–912. doi: 10.1093/oxfordjournals.schbul.a033504. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD. Effects of dietary polyunsaturated fatty acids on neuronal function. Lipids. 1999;34:161–169. doi: 10.1007/s11745-999-0350-3. [DOI] [PubMed] [Google Scholar]
- Filus JF, Rybakowski J. [Neurotrophic factors and their role in the pathogenesis of affective disorders] Psychiatr Pol. 2005;39:883–897. [PubMed] [Google Scholar]
- Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: Current state [Review] Pharmacological Reviews. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of Neurophysiology. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Archives of Physical Medicine and Rehabilitation. 1999;80:661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Heydari AR, You S, Takahashi R, Gutsmann A, Sarge KD, Richardson A. Effect of caloric restriction on the expression of heat shock protein 70 and the activation of heat shock transcription factor 1. Dev Genet. 1996;18:114–124. doi: 10.1002/(SICI)1520-6408(1996)18:2<114::AID-DVG4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, Birch EE, Birch DG, Uauy R. Fatty acid profile of buccal cheek cell phospholipids as an index for dietary intake of docosahexaenoic acid in preterm infants. Lipids. 1999;34:337–342. doi: 10.1007/s11745-999-0371-y. [DOI] [PubMed] [Google Scholar]
- Holliday R. Ageing in the 21st century. Lancet. 1999;354(Suppl):SIV4. doi: 10.1016/s0140-6736(99)90347-1. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Schizophrenia: the illness that made us human. Med Hypotheses. 1998;50:269–288. doi: 10.1016/s0306-9877(98)90000-7. [DOI] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Ip EY, Zanier ER, Moore AH, Lee SM, Hovda DA. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Islam A, Ayer-LeLievre C, Heigenskold C, Bogdanovic N, Winblad B, Adem A. Changes in IGF-1 receptors in the hippocampus of adult rats after long-term adrenalectomy: receptor autoradiography and in situ hybridization histochemistry. Brain Res. 1998;797:342–346. doi: 10.1016/s0006-8993(98)00389-8. [DOI] [PubMed] [Google Scholar]
- Jung RT. Obesity as a disease. Br Med Bull. 1997;53:307–321. doi: 10.1093/oxfordjournals.bmb.a011615. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HK, Lee TH, Shin MC, Lee MH, Shin MS, Park S, Baek S, Kim CJ. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006;28:147–154. doi: 10.1016/j.braindev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Walline R, Earnest DJ. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;242:89–92. doi: 10.1016/s0304-3940(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Lilliker SL. Prevalence of diabetes in a manic-depressive population. Compr Psychiatry. 1980;21:270–275. doi: 10.1016/0010-440x(80)90030-9. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Ernfors P, Bengzon J, Kokaia Z, Smith ML, Siesjo BK, Persson H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci U S A. 1992;89:648–652. doi: 10.1073/pnas.89.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson’s disease: a population-based, case-control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McCay PB, Brueggemann G, Lai EK, Powell SR. Evidence that alpha-tocopherol functions cyclically to quench free radicals in hepatic microsomes. Requirement for glutathione and a heat-labile factor. Ann N Y Acad Sci. 1989;570:32–45. doi: 10.1111/j.1749-6632.1989.tb14906.x. [DOI] [PubMed] [Google Scholar]
- Means LW, Higgins JL, Fernandez TJ. Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav. 1993;54:503–508. doi: 10.1016/0031-9384(93)90243-9. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. Jama. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard JR, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002a doi: 10.1016/s0306-4522(02)00123-9. in press. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002b;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Zanardini R, Tortorella A, Gennarelli M, Castaldo E, Canestrelli B, Maj M. The 196G/A (val66met) polymorphism of the BDNF gene is significantly associated with binge eating behavior in women with bulimia nervosa or binge eating disorder. Neurosci Lett. 2006;406:133–137. doi: 10.1016/j.neulet.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Decina P, Bocola V, Saraceni F, Scapicchio PL. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37:68–73. doi: 10.1016/s0010-440x(96)90054-1. [DOI] [PubMed] [Google Scholar]
- Murphy E, Nolan JJ. Insulin sensitiser drugs. Expert Opin Investig Drugs. 2000;9:1347–1361. doi: 10.1517/13543784.9.6.1347. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ono-Kishino M, Sugaru E, Yamanaka M, Taiji M, Noguchi H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab Res Rev. 2002;18:185–191. doi: 10.1002/dmrr.290. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1392–1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Ng HH, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9:158–163. doi: 10.1016/s0959-437x(99)80024-0. [DOI] [PubMed] [Google Scholar]
- Nonomura T, Tsuchida A, Ono-Kishino M, Nakagawa T, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Int J Exp Diabetes Res. 2001;2:201–209. doi: 10.1155/EDR.2001.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. Dementia associated with psychiatric disorders. Int Psychogeriatr. 2005;17(Suppl 1):S207–221. doi: 10.1017/s1041610205002036. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Jama. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Parnpiansil P, Jutapakdeegul N, Chentanez T, Kotchabhakdi N. Exercise during pregnancy increases hippocampal brain-derived neurotrophic factor mRNA expression and spatial learning in neonatal rat pup. Neurosci Lett. 2003;352:45–48. doi: 10.1016/j.neulet.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Peet M, Laugharne JD, Mellor J, Ramchand CN. Essential fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation. Prostaglandins Leukot Essent Fatty Acids. 1996;55:71–75. doi: 10.1016/s0952-3278(96)90148-9. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Perkins AJ, Hendrie HC, Callahan CM, Gao S, Unverzagt FW, Xu Y, Hall KS, Hui SL. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- Pifferi F, Roux F, Langelier B, Alessandri JM, Vancassel S, Jouin M, Lavialle M, Guesnet P. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr. 2005;135:2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Thapar RK, Marano C, Gavirneni S, Kondapavuluru PV. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Muller EE. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Pascual M, Acsady L, de Lecea L, Freund TF, Soriano E. Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J Neurosci. 1996;16:3991–4004. doi: 10.1523/JNEUROSCI.16-12-03991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sothern MS, Hunter S, Suskind RM, Brown R, Udall JN, Jr, Blecker U. Motivating the obese child to move: the role of structured exercise in pediatric weight management. South Med J. 1999;92:577–584. doi: 10.1097/00007611-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Suominen-Troyer S, Davis KJ, Ismail AH, Salvendy G. Impact of physical fitness on strategy development in decision-making tasks. Percept Mot Skills. 1986;62:71–77. doi: 10.2466/pms.1986.62.1.71. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tabata H, Kikuoka M, Kikuoka H, Bessho H, Hirayama J, Hanabusa T, Kubo K, Momotani Y, Sanke T, Nanjo K, et al. Characteristics of diabetes mellitus in schizophrenic patients. J Med Assoc Thai. 1987;70(Suppl 2):90–93. [PubMed] [Google Scholar]
- Tonra JR. Classical and novel directions in neurotrophin transport and research: anterograde transport of brain-derived neurotrophic factor by sensory neurons. Microsc Res Tech. 1999;45:225–232. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<225::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Nonomura T, Nakagawa T, Itakura Y, Ono-Kishino M, Yamanaka M, Sugaru E, Taiji M, Noguchi H. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes Metab. 2002;4:262–269. doi: 10.1046/j.1463-1326.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62:585–590. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between BDNF and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Wendorf M, Goldfine ID. Archaeology of NIDDM. Excavation of the “thrifty” genotype. Diabetes. 1991;40:161–165. doi: 10.2337/diab.40.2.161. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gómez-Pinilla F. Dietary Omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004a;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gómez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004b;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Zenobi PD, Holzmann P, Glatz Y, Riesen WF, Froesch ER. Improvement of lipid profile in type 2 (non-insulin-dependent) diabetes mellitus by insulin-like growth factor I. Diabetologia. 1993;36:465–469. doi: 10.1007/BF00402285. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]