Abstract
Hollow fiber membrane (HFM)-based artificial lungs can require a large blood-contacting membrane surface area to provide adequate gas exchange. However, such a large surface area presents significant challenges to hemocompatibility. One method to improve carbon dioxide (CO2) transfer efficiency might be to immobilize carbonic anhydrase (CA) onto the surface of conventional HFMs. By catalyzing the dehydration of bicarbonate in blood, CA has been shown to facilitate diffusion of CO2 toward the fiber membranes. This study evaluated the impact of surface modifying a commercially available microporous HFM-based artificial lung on fiber blood biocompatibility. A commercial poly(propylene) Celgard HFM surface was coated with a siloxane, grafted with amine groups, and then attached with CA which has been shown to facilitate diffusion of CO2 toward the fiber membranes. Results following acute ovine blood contact indicated no significant reduction in platelet deposition or activation with the siloxane coating or the siloxane coating with grafted amines relative to base HFMs. However,HFMs with attached CA showed a significant reduction in both platelet deposition and activation compared with all other fiber types. These findings, along with the improved CO2 transfer observed in CA modified fibers, suggest that its incorporation into HFM design may potentiate the design of a smaller, more biocompatible HFM-based artificial lung.
Keywords: Artificial lung, Carbonic anhydrase, Hemocompatibility, Hollow fiber membrane, Plasma polymerization
Artificial lungs composed of bundles of micro-porous hollow fiber membranes (HFMs), which are made from polymeric materials such as poly (methylpentene) and poly(propylene), are routinely employed to both oxygenate blood and remove carbon dioxide (CO2) in an extraluminal blood flow format. The efficiency of CO2 and oxygen (O2) gas exchange in the current artificial lung model, which is based on passive diffusion, is limited by the fiber surface area to blood volume ratio with devices requiring approximately 1–2 m2 of HFM surface to provide adequate gas exchange (1,2). Such large blood-contacting surfaces present significant challenges to hemocompatibility, necessitating aggressive anticoagulation and stimulating research into devices with improved efficiency (i.e., smaller) and hemocompatibility.
A wide variety of surface modification techniques have been evaluated to reduce the thrombogenicity of blood-contacting biomaterials, although there have been relatively few reports specifically focused on the HFMs utilized in artificial lung applications. Siloxane-grafted HFMs prepared by a plasma polymerization process with 1,3,3,7-tetramethyhydrocyclosiloxane (TMCTS)-coated fibers have shown reduced thrombogenesis relative to unmodified fibers (3). Plasma polymerization techniques have a number of advantages with regard to surface modification including: facile preparation of a thin, conformal, and pinhole-free coating; amenability to a wide variety of substrates; achievement of good adhesion between coating and substrate; and the ability to generate coatings that present excellent thermal and chemical resistance (4,5).
Another approach to improving artificial lung biocompatibility is to effectively reduce the required HFM surface area by increasing the gas exchange rate of HFM-based devices. Increasing the efficiency of CO2 removal is especially important because the natural concentration gradient for CO2 diffusion is much smaller than that for O2 addition. Furthermore, in many patients with respiratory failure, the need for CO2 removal is more important clinically, as oxygenation can be provided by nasal cannula or lung-protective ventilation (1,6–8). In a previous study, we reported the development of a bioactive HFM that could improve CO2 removal rates in lung failure patients (9). Carbonic anhydrase (CA) was covalently immobilized to the surface of a conventional HFM, and by catalyzing the dehydration of bicarbonate in blood was shown to facilitate diffusion of CO2 toward the fiber membranes, essentially mimicking the function of the enzyme on lung capillary surfaces. Results indicated that CO2 exchange rates from buffer were increased by as much as 75% in the model device.
In this brief report, we addressed whether the attachment of CA onto a HFM surface increased fiber thrombogenicity, which would negate the biocompatibility benefits associated with a smaller device. A commercial poly(propylene) HFM (Celgard, Charlotte, NC,USA) was used as the base material and for control purposes. Fibers representing the intermediate modification steps of siloxane coating and amine grafting on siloxane (Alung Technologies, Pittsburgh, PA, USA) as well as the final CA-modified fiber were evaluated. A second control of a commercial poly (methyl pentene) HFM (Oxyplus, Membrana, Wuppertal, Germany) was also included.
Materials and Methods
Materials
Oxyplus fibers (Type PMP 90/200, OD: 380 μm, ID: 200 μm) were obtained from Membrana GmbH. Celgard fibers (Type x30-240, OD: 300 μm, ID: 240 μm) were purchased from Celgard. Two types of customized composite HFMs, in which a nonporous siloxane was applied as a skin to the Celgard fibers, were supplied by Alung Technologies as follows: 1,3,3,7-TMCTS monomer plasma polymerized fibers (A-TMCTS), and N-tetramethylsilylallylamine-grafted fibers (A-TMSAA). To prepare the CA-immobilized fiber (A-TMSAA-CA), A-TMSAA fibers were modified with 0.5% glutaraldehyde (v/v) in deionized water for 1 h with mild shaking. After the treatment, the fibers were washed three times with deionized water for 10 min each. CA from bovine erythrocytes (CA, Sigma-Aldrich, St. Louis, MO, USA) was then conjugated to the fibers by incubating the enzyme (2 mg/mL) in a buffer solution (0.05 M phosphate buffer, pH 7.5) containing the HFMs for 3 h at room temperature.
Blood collection and assessment of acute thrombotic deposition and activation
Whole ovine blood was collected via jugular venipuncture with an 18-G 1.5-inch needle into a syringe containing heparin (6.0 U/mL) discarding the first 3 mL. The hollow fiber samples (surface area: 0.6 cm2) were placed into blood collection tubes, filled with blood (5 mL), and rocked for 2 h at 37°C on a hematology mixer. The number of platelets deposited on the samples was determined by a lactate dehydrogenase (LDH) assay (10) with an LDH Cytotoxicity Detection Kit (Takara Bio, Otsu, Shiga, Japan). Scanning electron microscopic images were also taken to assess platelet deposition as previously described (11). The percentage of activated ovine platelets in the bulk phase of the blood contacting the surface samples was quantified using annexin V protein (12). Platelet activation levels from the tube with no HFMs were subtracted from the tubes with HFMs. Data are presented as means with standard deviation. Statistical significance (P < 0.05) between sample groups was determined using analysis of variance followed by post hoc Newman–Keuls testing.
Results and Discussion
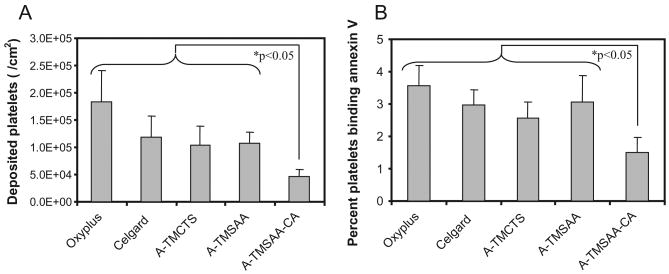
Figure 1 shows the impact of surface modification on platelet deposition as observed by scanning electron microscopy. The CA-modified HFM (A-TMSAA-CA) experienced markedly reduced levels of platelet deposition relative to the HFMs representing intermediate modification steps (A-TMCTS and A-TMSAA) as well as the Celgard base fiber and the Oxyplus control fiber. The quantification of platelet deposition using the LDH assay (Fig. 2A) also indicated that the A-TMSAA-CA fiber surfaces had significantly reduced levels of platelet deposition compared with the other fiber types. As platelets may become activated during the incubation period, but not deposit on the surface, or only transiently deposit on the surface, activated platelets in the bulk phase of blood were also quantified (Fig. 2B). The results generally mirrored the platelet deposition studies with little difference in platelet activation between the unmodified and intermediately modified fibers (Celgard, A-TMCTS, and A-TMSAA). A-TMSAA-CA fibers, however, exhibited significantly reduced levels of platelet activation compared with all other fiber types.
Fig. 1.
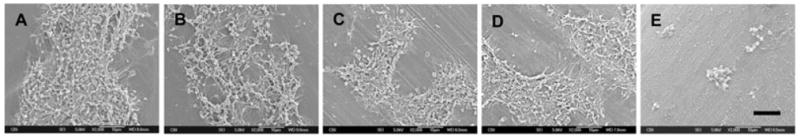
Scanning electron micrographs of HFM surfaces after contact with heparinized ovine blood for 2 h at 37°C. Images were recorded at 2000×. Individual images within a horizontal set represent different experiments (scale bar = 10 μm). (A) Oxyplus; (B) Celgard; (C) A-TMCTS; (D) A-TMSAA; (E) A-TMSAA-CA.
Fig. 2.
Assessment of acute thrombotic deposition and platelet activation. (A) Platelet deposition after contact with ovine blood for 2 h as determined by the lactate dehydrogenase assay (n = 5); (B) Quantification of activated platelets in the bulk phase of ovine blood after sample contact with blood under continuous rocking. Platelet activation was quantified by flow cytometric measurement of annexin V binding (n = 5).
Skinned asymmetric and composite symmetric microporous hollow fibers are attractive candidates for use in artificial lung applications because the non-porous polymer layer on these fiber surfaces blocks or impedes plasma infiltration into the pores. Siloxane polymers, including TMCTS, are of particular interest as coatings due to their gas permeability. A previous report examining several skinned and composite hollow fiber types (i.e., siloxane coated) showed the dual functionality of these fibers in gas transfer and wetting resistance (13). In this study, we evaluated the thrombogenicity of HFMs in which a siloxane polymer was generated on the surface of commercial Celgard HFMs (A-TMCTS), as well as on HFMs where amine groups were introduced onto the siloxane surface (A-TMSAA), thus introducing the capacity for further modification with protein (CA) attachment. Liu et al. (14) demonstrated that albumin-coated surfaces exhibited significant inhibition of platelet adhesion. The albumin mechanism is likely related to its lack of cell adhesion peptide sequences while occupying potential protein adsorption sites. Our findings of reduced acute platelet deposition with our CA attached surface may be due to a similar effect of the attached CA. It seems unlikely that the enzymatic action of CA would be related to the reduced platelet deposition observed in this report.
Conclusion
In our previous study, we covalently immobilized CA to the surface of HFMs and demonstrated facilitated diffusion of CO2, improving the rate of CO2 removal by as much as 75% (9). We believe that the impact of CA-modified fibers on both enhanced hemocompatibility and gas exchange rate may contribute significantly to the development of next-generation artificial lungs or respiratory assist devices.
References
- 1.Federspiel WJ, Henchir KA. Lung, artificial: basic principles and current applications. In: Wnek GE, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. New York, London: Marcel Dekker, Taylor & Francis; 2004. pp. 910–21. [Google Scholar]
- 2.Hattler BG, Federspiel WJ. Gas exchange in the venous system: support for the failing lung. In: Vaslef SN, Anderson RW, editors. Tissue Engineering Intelligence Unit; 7: The Artificial Lung. Georgetown, TX: Landes Bioscience; 2002. pp. 133–74. [Google Scholar]
- 3.Hu CZ, Dolence EK, Osaki S, et al. Hydrocyclosiloxane membrane prepared by plasma polymerization process. USP 5463010. 1995
- 4.Chan CM, Ko TM, Hiraoka H. Polymer surface modification by plasmas and photons. Surf Sci Rep. 1996;24:1–54. [Google Scholar]
- 5.Calderon JC, Harsch A, Gross GW, et al. Stability of plasma-polymerized allylamine films with sterilization by autoclaving. J Biomed Mater Res. 1998;42:597–603. doi: 10.1002/(sici)1097-4636(19981215)42:4<597::aid-jbm16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Hirvela ER. Advances in the management of acute respiratory distress syndrome: protective ventilation. Arch Surg. 2000;135:126–35. doi: 10.1001/archsurg.135.2.126. [DOI] [PubMed] [Google Scholar]
- 7.Deslauriers J, Awad JA. Is extracorporeal CO2 removal an option in the treatment of adult respiratory distress syndrome? Ann Thorac Surg. 1997;64:1581–2. doi: 10.1016/s0003-4975(97)01173-9. [DOI] [PubMed] [Google Scholar]
- 8.Conrad SA, Zwischenberger JB, Grier LR, et al. Total extra-corporeal arteriovenous carbon dioxide removal in acute respiratory failure: a phase I clinical study. Intensive Care Med. 2001;27:1340–51. doi: 10.1007/s001340100993. [DOI] [PubMed] [Google Scholar]
- 9.Kaar JL, Oh HI, Russell AJ, et al. Towards improved artificial lungs through biocatalysis. Biomaterials. 2007;28:3131–9. doi: 10.1016/j.biomaterials.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamada Y, Kulik EA, Ikada Y. Simple method for platelet counting. Biomaterials. 1995;16:259–61. doi: 10.1016/0142-9612(95)92126-q. [DOI] [PubMed] [Google Scholar]
- 11.Ye SH, Johnson CA, Jr, Woolley JR, et al. Covalent surface modification of a titanium alloy with a phosphorylcholine-containing copolymer for reduced thrombogenicity in cardiovascular devices. J Biomed Mater Res A. 2009;91:18–28. doi: 10.1002/jbm.a.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson CA, Jr, Snyder TA, Woolley JR, et al. Flow cytometric assays for quantifying activated ovine platelets. Artif Organs. 2008;32:136–45. doi: 10.1111/j.1525-1594.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eash HJ, Jones HM, Hattler BG, et al. Evaluation of plasma resistant hollow fiber membranes for artificial lungs. ASAIO J. 2004;50:491–7. doi: 10.1097/01.mat.0000138078.04558.fe. [DOI] [PubMed] [Google Scholar]
- 14.Liu TY, Lin WC, Huang LY, et al. Hemocompatibility and anaphylatoxin formation of protein-immobilizing polyacry-lonitrile hemodialysis membrane. Biomaterials. 2005;26:1437–44. doi: 10.1016/j.biomaterials.2004.04.039. [DOI] [PubMed] [Google Scholar]