Abstract
We have previously shown that the spinal cord is capable of learning a sensorimotor task in the absence of supraspinal input. Given the action of BDNF on hippocampal learning, the current studies examined the role of BDNF in spinal learning. BDNF is a strong synaptic facilitator and, in association with other molecular signals (e.g., CREB, CaMKII and synapsin I), important for learning. Spinally transected rats given shock to one hind leg when the leg extended beyond a selected threshold exhibited a progressive increase in flexion duration that minimized shock exposure, a simple form of instrumental learning. Instrumental learning resulted in elevated mRNA levels of BDNF, CaMKII, CREB, and synapsin I in the lumbar spinal cord region. The increases in BDNF, CREB, and CaMKII were proportional to the learning performance. Prior work has shown that instrumental training facilitates learning when subjects are tested on the contralateral leg with a higher response criterion. Pretreatment with the BDNF inhibitor TrkB-IgG blocked this facilitatory effect, as did the CaMKII inhibitor AIP. Intrathecal administration of BDNF facilitated learning when subjects were tested with a high response criterion. The findings indicate that instrumental training enables learning and elevates BDNF mRNA levels within the lumbar spinal cord. BDNF is both necessary, and sufficient, to produce the enabling effect.
Keywords: Neurotrophin, synaptic plasticity, exercise, memory, CaMKII
Introduction
Learning and memory are concepts usually associated with the brain. Yet, the types of synaptic modifications associated with learning and memory appear intrinsic to most neuronal networks. Indeed, there is ample evidence that the spinal cord has the circuitry necessary for motor learning in animals ranging from the lamprey to the cat (Edgerton, 2004, Grau et al., 2006). It has been assumed that most of the recovery in locomotor ability after full spinal cord transection is associated with central pattern generation within the lumbar spinal cord. However, recent evidence indicates that acquisition of stepping and/or standing after a complete low lumbar spinal transection requires an ability to interpret complex sensory input derived from the repetitive execution of a given motor task (Edgerton, 2004). These findings suggest that a “motor learning process” involving activity-dependent mechanisms may enable the recovery of full weight-bearing stepping in the absence of any suprasegmental input.
Several lines of evidence indicate that brain-derived neurotrophic factor (BDNF) is a powerful modulator of neuronal excitability and synaptic transmission, and plays a role in hippocampal-dependent learning and memory. Long-term potentiation (LTP), an electrophysiological correlate of learning, induces the expression of BDNF in the hippocampus (Patterson et al., 1992). The presence of BDNF seems necessary for proper LTP, as animals with a genetic deletion of the BDNF gene fail to exhibit LTP (Linnarsson et al., 1997) while exogenous application of BDNF restores LTP in these animals (Patterson et al., 1996). In spinal cord motoneurons, BDNF induces facilitation of monosynaptic excitatory postsynaptic potentials (EPSPs) (Mendell et al., 2001, Mendell and Arvanian, 2002). BDNF delivered to the injured spinal cord has been shown to stimulate hindlimb stepping (Jakeman et al., 1998).
BDNF modulates molecules important for synaptic transmission such as synapsin I, calcium/calmodulin activated protein kinase II (CaMKII), and the gene transcription factor cAMP-response element binding protein (CREB). Synapsin I is a vesicle-associated phosphoprotein, whose synthesis (Wang et al., 1995) and phosphorylation (Jovanovic et al., 1996) are regulated by BDNF. Synapsin I contributes to synaptic-plasticity by modulating transmitter release (Baekelandt et al., 1994, Melloni et al., 1994, Wang et al., 1995) the formation and maintenance of the presynaptic structure (Takei et al., 1995)and axonal elongation (Akagi et al., 1996). CREB is one of the best-described stimulus-induced transcription factors involved in adaptive responses (Finkbeiner, 2000) and long-term memory (Bading, 2000) and is under the regulatory action of BDNF (Nibuya et al., 1996, Yin and Tully, 1996, Kogan et al., 1997, Tully, 1997). CaMKII, which is also under regulatory control of BDNF (Blanquet and Lamour, 1997), has a critical role in synaptic plasticity and learning and memory. Both CAMKII and CREB have been described as molecular memory switches (Yin et al., 1995, Lisman et al., 2002) and have a well-established connection to the BDNF system. CAMKII may impact learning and memory, in part, by affecting BDNF and CREB expression.
Prior work has shown that plasticity within the spinal cord can be studied using a simple instrumental learning paradigm. Spinally transected rats given shock to a hind leg when the leg is extended beyond a critical level learn to maintain the shocked leg in a flexed position that minimizes net shock exposure (Grau et al., 1998, Grau and Hook, 2006). This learning is not observed in rats that are experimentally coupled (yoked) to subjects given controllable shock (master). Yoked rats receive the same pattern of stimulation but independent of the limb position. Training with controllable shock leads to a form of positive transfer that enables learning. This effect is especially evident when subjects are tested with a high response criterion that impedes learning in untreated subjects (Crown et al., 2002a). In contrast, uncontrollable shock disables learning for up to 48 hrs (Crown et al., 2002b). These learning effects can be blocked by cutting the sciatic nerve, anesthetizing spinal neurons with lidocaine, or intrathecal (i.t.) treatment with a NMDA receptor antagonist (Crown et al., 2002a, Joynes et al., 2004, Ferguson et al., 2006). Here we report that instrumental learning increases BDNF, CaMKII, CREB and synapsin 1 mRNA levels within the lumbar spinal cord. Pretreatment with either a BDNF or CaMKII inhibitor prevented the facilitatory effect and i.t. administration of BDNF fostered learning.
MATERIALS AND METHODS
Animal Subjects
Adult male Sprague-Dawley rats (Harlan, Houston, TX), 90–100 days old, were individually-housed, maintained on a 12-hr light-dark cycle, and given ad libitum access to food and water. The experiments adhered to the NIH guidelines for the care and use of animals and were approved by the University Laboratory Animal Care Committee at Texas A&M University. All experiments employed a group size (n) of 6.
Spinal Transection
Surgery was performed under pentobarbital anesthesia (50 mg/kg, i.p.). Subjects were placed in a stereotaxic instrument and a small gauze “pillow” was placed under its chest. After the second thoracic vertebra (T2) was localized by touch, an anterior–posterior incision was made, the tissue rostral to T2 was cleared away, and the cord was transected by cauterization. Oxycel (Parke-Davis, Morristown, NJ) was placed in the transection site and the wound was closed with Michelle clips. After surgery, subjects were given an intraperitoneal injection of 2.5 ml of 0.9% (wt/vol) saline. Rats were maintained in a temperature-controlled environment (approximately 25.5 °C) during recovery and testing. Bladders were expressed twice daily and additional saline injections were given to maintain hydration. To prevent injury to the hind limbs during recovery, the rat’s rear legs were maintained in a normal flexed position by a piece of porous tape (1.3 cm wide; Orthaletic, Johnson & Johnson, Arlington, TX) gently wrapped once around the rat’s body. Transections were confirmed by (a) inspecting the cord during the operation, (b) observing the behavior of the subjects after they had recovered to ensure that they exhibited paralysis below the level of the forepaws and did not vocalize to the leg shock, and (c) examining the transection site during tissue processing.
Behavioral Procedures
Experimental subjects (N=18) were subdivided into three groups: master, yoked, and unshocked. Master subjects received 30 min of instrumental training with the normal response criterion. Subjects in this group received shock to the tibialis anterior muscle when the leg was in an extended position. Each yoked subject was coupled to a master rat and received shock whenever its master partner was shocked. Unshocked subjects were placed in the apparatus, but did not receive any shock.
Instrumental training and testing was conducted as described (Grau et al., 1998). Briefly, prior to testing, the leg was shaved and marked for placement of the shock leads. A wire electrode was then inserted over the tibia 1.5 cm above the ankle and the rats were placed in restraining tubes. Next the contact electrode used to monitor leg position was taped to the paw. To minimize lateral leg movements, a 20-cm piece of porous tape (Orthaletic, 1.3 cm) was wrapped around the leg and taped to a bar extending across the apparatus directly under the front panel of restraining tube. The tape was adjusted so that it was taut enough to slightly extend the knee. One lead from the shock generator was attached to the stainless steel wire inserted over the tibia. The shock generator was set to deliver a 0.1 mA shock and the region over the second mark was probed to find a site that elicited a vigorous flexion response. The pin was then inserted perpendicular to the body into the tibialis anterior muscle. Shock intensity was then adjusted to elicit a flexion response of 0.4 N using the strain gauge described above. To set the criterion for learning, three short (0.15-s) shock pulses were applied and the level of the salt solution was adjusted so that the tip of the rod was submerged either 4 mm (normal response criterion) or 8 mm (high response criterion) below the surface.
Behavioral Testing Apparatus
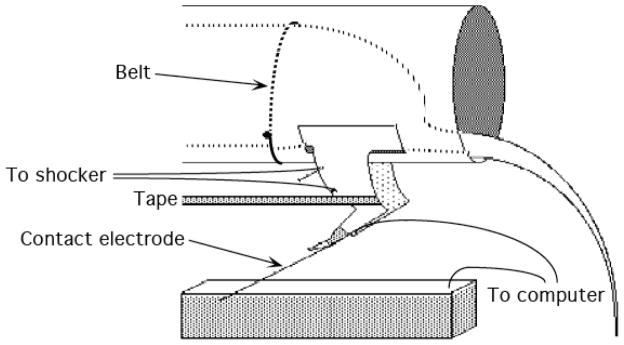
(Fig. 1). During behavioral testing, animals were suspended in Plexiglas tubes containing slots that allowed the hind limbs to hang freely. Subjects were secured in the tube with an insulated wire ‘belt’ that was gently wrapped around the subject and passed through holes on the sides of the tube. Leg position was monitored using a contact electrode constructed from a 7-cm 0.45 mm stainless steel rod that was taped to the foot. The last 2.5 cm of the electrode was insulated from the foot with heat shrink tubing. A fine wire (0.01 sq mm [36 AWG]) was attached to the end of the rod. This wire (20 cm) extended from the rear of the foot and was connected to a digital input monitored by a Macintosh computer. The rod was taped to the plantar surface of the rat’s foot with approximately 8 cm of porous tape (Orthaletic, 1.3 cm, Johnson and Johnson, Arlington, TX) with the end positioned directly in front of the plantar protuberance. A plastic rectangular dish (11.5 [w] × 19 [l] × 5 [d]) containing a NaCl solution was placed approximately 7.5 cm below the restraining tube. A drop of soap was added to the solution to reduce surface tension. A ground wire was connected to a 1 mm stainless steel rod that was placed in the solution. When the contact electrode attached to the rat’s paw touched the solution, it completed the circuit monitored by the computer. The state of this circuit was sampled at 30 Hz. Electrical stimulation was provided by a BRS/LVE shock generator (Model SG-903; Laural, MD). This device delivers a constant current AC (60 Hz) shock that elicits a strong flexion response with no signs of tissue damage.
Figure 1.
Schematic illustration of the instrumental learning apparatus and set up. The instrumental learning performance was examined in rats that received a full transection at the T2 level of the spinal cord. Animals were suspended in Plexiglas tubes with an insulated wire ‘belt’ allowing the hind limbs to hang freely. Leg position was monitored using a contact electrode constructed from a 7-cm 0.45 mm stainless steel rod that was taped to the foot. A fine wire extended from the rear of the foot and was connected to a digital input monitored by a Macintosh computer. A plastic rectangular dish (11.5 [w] × 19 [l] × 5 [d]) containing a NaCl solution was placed approximately 7.5 cm below the restraining tube. A ground wire was connected to a 1 mm stainless steel rod that was placed in the solution. When the contact electrode attached to the rat’s paw touched the solution, it completed the circuit monitored by the computer. The state of this circuit was sampled at 30 Hz. An electrical shock generator (Model SG-903; Laural, MD) delivered a constant current AC (60 Hz) shock that elicited a strong flexion response with no signs of tissue damage. The duration of the flexion response to a shock was recorded to obtain a measure of performance over time.
Flexion force was measured by attaching a monofilament plastic line (“4 lb test” Stren, Dupont) to the rat’s foot immediately behind the plantar protuberance. The strain gauge had previously been calibrated by determining the relationship between voltage and force in Newtons. The data revealed a linear relation that allowed us to convert voltage to force. Shock intensity was adjusted to produce a flexion force of a fixed value. The strain gauge was then removed from the rat’s foot. For additional details, see (Grau et al., 1998).
Behavioral Assessment
In all experiments, our primary measure of learning was response duration, which was calculated using the following formula: response durationi = (60 – time in solutioni)/(flexion numberi + 1), where i is the current training bin. Elsewhere, we have shown how this measure of learning helps to discount some alternative (non-instrumental) accounts of our behavioral effects (Grau et al., 1998).
BDNF function inhibition
To evaluate a possible action of BDNF on learning or the enabling effect, transected rats (N=24) were pretreated with microbeads coated with the BDNF inhibitor TrkB-IgG or saline. The beads were microinjected into the L4-L5 spinal tissue at the time of surgery, as described below. The next day, all subjects were prepared for instrumental training on both hind legs. One leg (counterbalanced across subjects) was designated the pretained leg and had the solution level adjusted to the usual depth (4 mm). A higher response criterion was imposed on the opposite leg by increasing the contact electrode depth to 8 mm. Half of the subjects in each drug condition then received 30 min of controllable shock on the pretrained leg (Pretrn) at the usual (4 mm) depth. This training regimen was identical to that described for the master group above. The remaining subjects received no pretraining (No Pretrn). At the end of this pretraining period, all subjects were tested for 30 min on the opposite leg with the high (8 mm) response criterion.
Preparation of microbeads
Recombinant human TrkB-IgG chimera (R & D System, Inc., Minneapolis, MN) that comprises the intracellular domains of human TrkB and the Fc domain of IgG, was used to block BDNF action (Shelton et al., 1995). We used fluorescent latex microbeads (Lumaflour Corp., Naple, Fl) as the vehicle for drug insertion into the spinal cord. Microbeads were prepared by the methods described previously (Riddle et al., 1997, Lom and Cohen-Cory, 1999). Briefly, these consisted of coating the microbeads with the drug via passive absorbency by incubating overnight at 4 °C with a 1 : 5 mix of microbeads to TrkB-IgG (5 ug / ul in PBS with BSA), or saline as control vehicle. Next morning the solution was centrifuged at 14,000 g for 30 min and the microbeads were resuspended in sterile water at a 10% concentration.
Injection of microbeads
After the spinal cord was transected, an incision was made over T13, the tissue cleared, and a laminectory was performed. We had previously established that, in 90–100 days old rats, this would expose a region of spinal cord tissue between L4 and L5. Prior work has shown that instrumental learning depends on neurons within this region (Liu et al., 2005). A 2 uL solution containing microbeads was injected using a stereotaxic device bilaterally ± 0.6 mm off midline and at a depth of 1.5 mm. The injections were made using a 1.0 uL Hamilton syringe and the solution was slowly infused over a period of 1 min. Half the subjects received microbeads that had TrkB-IgG adhered to their surface. The remaining subjects served as injection controls and received the beads with saline. The location of fluorescent beads microinjected into the lumbar region was determined by fluorescence microscopy using an Olympus BX60 fluorescent microscope. Animals were deeply anesthetized (100 mg/kg of pentobarbital, i.p.) and perfused (intracardially) with 4% paraformaldehyde. A 1-cm long segment of the spinal cord that included the L4-L5 region was taken and embedded in paraffin prior to sectioning. The tissue was sectioned coronally in 20 um-thick sections and every 10th slice was preserved for staining. All sections were stained with luxol fast blue.
BDNF infusion
To assess whether pretreatment with BDNF would foster instrumental learning, spinally transected rats (N=36) were fitted with an intrathecal cannula to infuse recombinant BDNF. Intrathecal cannulas were made using a 25-cm section of polyeurethane tubing (PE-10, VWR International, Bristol, CT). The catheter was inserted into the subarachanoid space on the dorsal surface of the cord and threaded 9 cm down the vertebral column. The exposed end of the tubing was secured externally to the skin with cyanoacrylate and the wire was gently removed. A day after surgery, subjects received an injection of BDNF (Sigma Aldrich, St. Louis, MO) dissolved in 10 μL of CSF+0.1% BSA using a Hamilton syringe. Independent groups received BDNF at a dose of 0.005, 0.01, 0.02, or 0.04 μg/μl. A control group received the vehicle alone. In all cases, the injection was followed by a 20 μl saline flush and was given over a period of 2 min. Subjects were then placed in the test apparatus and, 20 min later, prepared for instrumental testing as described above. Thirty min after the injection, subjects were tested for 30 min with controllable leg-shock using a high (8 mm) response criterion. A sixth group (Pretrained) was pretreated with the vehicle and then given 30 min of training with the usual (4 mm) response criterion. These subjects were then tested on the contralateral leg with the high response criterion.
CaMKII Inhibition
To evaluate whether inhibiting CaMKII affects learning or the enabling effect, spinally transected rats (N=24) were fitted with an intrathecal cannula to infuse a CaMKII inhibitor. Twenty-four hours following surgery, subjects received infusions of saline or the selective CaMKII inhibitor AIP (500 nmol; EMD Biosciences, La Jolla, CA) dissolved in 10 μL of 0.9% saline using a Hamilton syringe, followed by a 20 μL saline flush. To establish the effective dose, additional subjects (N=12) were pretreated with 5 or 50 nmol of AIP. The injection procedure was conducted over a 2 min period. Subjects were then placed in the instrumental apparatus and prepared for instrumental training testing as described earlier. Half the subjects in each drug condition received 30 min of instrumental training (Pretrn) with a moderate (4 mm) response criterion. The remaining subjects served as the unshocked controls (No Pretrn). At the end of this pretraining period, all subjects were tested for 30 min on the opposite leg with the high (8 mm) response criterion.
Intrathecal Injections
For the infusion of recombinant BDNF and CaMKII inhibitor, an intrathecal cannula, consisting of 25 cm length of polyethylene tubing (PE-10, VWR International, Bristol, CT) fitted with a stainless steel wire (0.09 mm diameter; Small Parts Inc., Miami Lakes, FL) was inserted into the subarachanoid space on the dorsal surface of the cord at the time of spinal transection. The cannula was inserted 9 cm down the vertebral column, and the exposed end of the tubing was secured externally to the skin with cyanoacrylate, as described by Yaksh and Rudy (1976). The wound caudal to the exposed tubing was closed with Michel Clips (Fine Science Tools, Foster City, CA) and the stainless steel wire was carefully removed. Drugs were later administered through the cannula using a Hamilton syringe.
Biochemical Analyses
Tissue Extraction
Rats given 30 min of training with controllable shock (master), uncontrollable shock (yoked), or nothing (unshocked), were sacrificed no later than 2 min after training by decapitation and the spinal cords were removed for RT-PCR. Briefly, an anterior posterior incision was made down the length of the rat’s back, the vertebral column was exposed by rongeurs, and bone cutters were used to remove the vertebrae corresponding to the lumbar and sacral spinal segments. Spinal segments L4-S1 were removed for analysis, as these regions are known to play an important role in this form of spinal learning (Liu et al., 2005). The spinal cord was then quickly removed and the tissue was immediately placed on dry ice and frozen for subsequent processing.
Taqman RT-PCR
Total RNA was isolated using RNA STAT-60 kit (TEL-TEST, Inc., Friendswood, TX) as per the manufacturer’s protocol. The mRNAs for BDNF, Synapsin, CaMKII, and CREB were measured by TaqMan real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) using an ABI PRISM 7700 Sequence detection system (Applied Biosystems). The technique is based on the ability to directly detect the RT-PCR product with no downstream processing. This is accomplished with the monitoring of the increase in fluorescence of a dye-labeled DNA probe specific for each factor under study plus a probe specific for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene used as an endogenous control for the assay. Total RNA (100 ng) was converted into cDNA using TaqMan EZ RT-PCR Core reagents (Applied Biosystems). The sequences of probes, forward and reverse primers, designed by Integrated DNA Technologies (Coralville, IA) were: i) BDNF: probe, (5′-AGTCATTTGCGCACAACTTTAAAAGTCTGCATT-3′); forward, (5′-GGACATATCCATGACCAGAAAGAAA-3′); reverse, (5′-GCAACAAACCACAACATTATCGAG-3′) ; synapsin I: probe, (5′-CATGGCACGTAATGGAGACTACCGCA-3′); forward, (5′-CCGCCAGCTGCCTTC-3′); reverse, (5′-TGCAGCCCAATGACCAAA-3′) ; CREB: probe, (5′-CATGGCACGTAATGGAGACTACCGCA-3′); forward, (5′-CCGCCAGCATGCCTTC-3′); reverse, (5′-TGCAGCCCAATGACCAAA-3′); CaMKII: probe, (5′-CGCAGACGCCAATAGCCGCACGA-3′); forward: (5′-GTGGTAGACACCATCCTAGTGAAG-3′); reverse: (5′-TTCTCGTCCAACTGCCAACTC-3′). An oligonucleotide probe specific for the rat GAPDH gene was used as an endogenous control to standardize the amount of sample RNA. The RT-reaction conditions were 2 min at 50 °C as the initial step to activate uracil glycosylase (UNG), followed by 30 min at 60°C as the reverse transcription and completed by a UNG-deactivation at 95 °C for 5 min. The 40 cycles of the two-step PCR-reaction conditions were 20 s at 94 °C and 1 min at 62 °C.
Statistical Analyses
GAPDH was employed as internal standards for real-time RT-PCR. For quantification of TaqMan RT-PCR results, fluorescent signal intensities were plotted against the number of PCR cycles on a semilogarithmic scale. The amplification cycle at which the first significant increase of fluorescence occurred was designated as the threshold cycle (CT). The CT value of each sample then was compared to those of the internal standard. This process is fully automated and carried out with ABI sequence detector software version 1.6.3 (Applied Biosystem, Foster City, CA). Taqman EZ RT-PCR values for GAPDH were substrated from BDNF, synapsin I, CREB, or CaMKII values. The resulting corrected values were used to make comparisons across the different experimental groups. The mean mRNA levels were computed for the control and experimental rats. Linear regression analysis was performed on the individual samples to evaluate the association between variables. The results were expressed as mean percent of unshocked values for graphic clarity and represent the mean ± standard error of the mean (SE) of 6 independent determinations. In all experiments, our treatment effects were assessed using an analysis of variance (ANOVA). Post hoc comparisons were made using Duncan’s New Multiple Range Test. In all cases, the criterion for statistical significance was set at a p < .05.
RESULTS
Instrumental Training
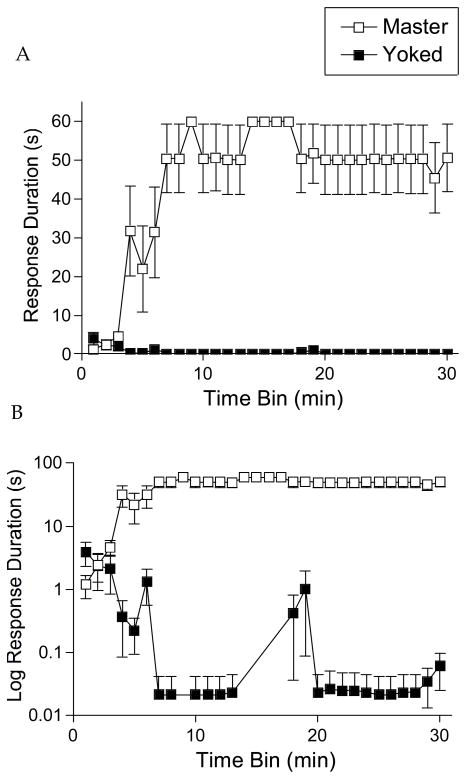
Shock was applied using subcutaneous electrodes to elicit a flexion of the ankle joint, i.e., a decrease of the angle between the tibia and the foot. As in prior studies, e.g. (Grau et al., 1998), master rats learned to hold the leg in a flexed position, yielding an increase in response duration over time (Figure 2). Yoked subjects, that receive the same amount of shock independent of leg position, did not exhibit an increase in response duration, our index of instrumental learning. Instead, they appeared to habituate to the shock and, as a result, exhibited a progressive decrease in response duration as function of training (evident when the data were plotted on a log scale; Figure 2, bottom panel). A mixed analysis of variance (ANOVA) revealed significant main effects for shock condition, F(1, 10)=87.5, p < .001, and time, F(29, 290)=4.52, p < .001. The time x shock interaction was also significant, F(29, 290)=5.47, p < .001.
Figure 2.
Response duration as a function of time for master and yoked rats. Only response contingent (controllable) shock produced a progressive increase in flexion duration over the course of training as illustrated for the master group of rats. A) The panel indicates mean response duration in seconds ± the standard error of the mean (SEM). B) The panel plots the same data on a log scale and shows how yoked subjects exhibited a decline in mean response duration over time while master rats maintain a constant duration after learning. Data for the unshocked rats are not presented because these subjects never exhibited a flexion response, and consequently, all subjects in this group had a mean response duration of 0.
As in prior studies e.g., (Grau et al., 1998, Crown et al., 2002a, Joynes et al., 2004), subjects trained with controllable shock appeared to learn within the first 10–15 min of training. In subsequent analyses, we examined whether BDNF expression was related to the acquisition of the instrumental response. This required an index of instrumental performance during the acquisition phase, the period when master rats exhibited a progressive increase in response duration over time. We determined the approximate point at which subjects obtained maximal performance (aka the asymptote of learning) using the procedure described by (Hook et al., 2004). These analyses revealed that, after 9 min of training, individual response durations were highly correlated with terminal performance (r=0.86, p < .01) and that there were no significant improvements across subsequent time bins (all t’s < 3.05, p > .05). Given this, mean performance during the first 10 min of testing was used to compute the relation between gene expression and learning.
While yoked rats did not exhibit an increase in response duration, shock stimulation generally elicited a motor response. As a result, the average number of respones exhibited by yoked (264.3±157.0) and master (371.3±147.5) rats did not differ significantly, F(1, 10) < 1.0, p > .05.
Impact of Instrumental Training on BDNF mRNA
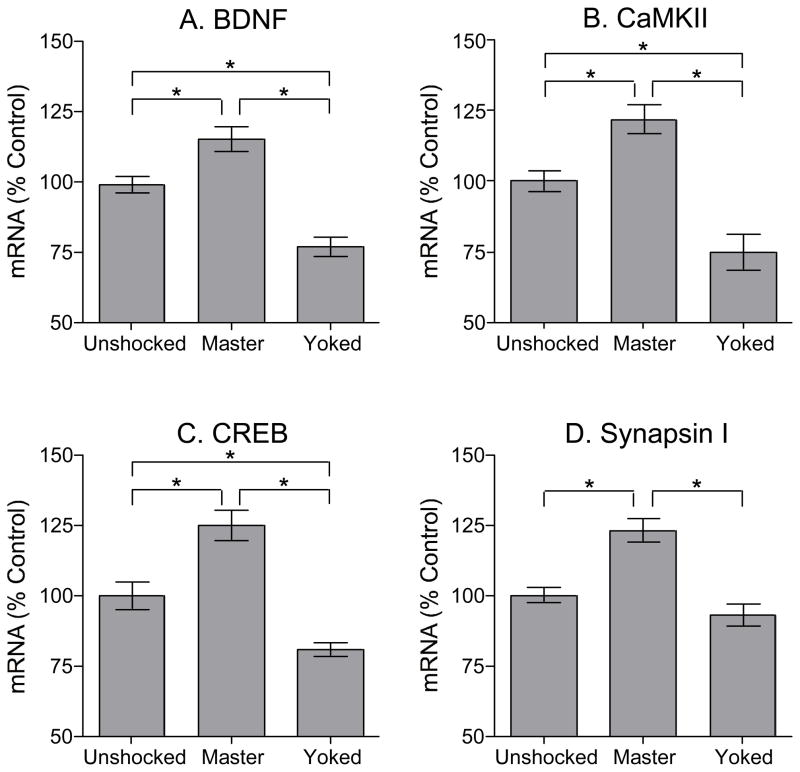
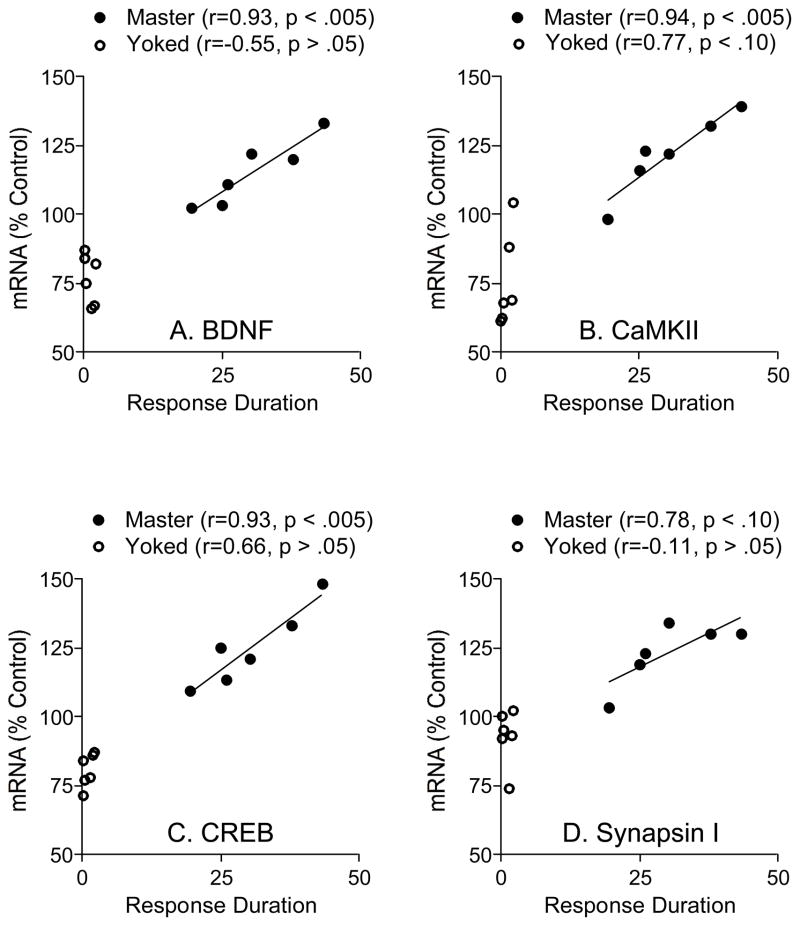
Rats trained with controllable shock (Master) showed significantly higher levels of BDNF mRNA relative to matched yoked controls rats (Figure 3A). Levels of BDNF mRNA in the master group were increased to 114% of unshocked control levels, while BDNF levels in the yoked group were reduced to 76% of unshocked control levels. An ANOVA confirmed that instrumental training had a significant effect on BDNF mRNA levels, F(2, 15)=22.65, p < .0001. Post hoc comparisons showed that all group differences were statistically significant (p < .05). To examine whether BDNF levels were related to learning performance, we computed the correlation between BDNF levels and learning performance for subjects in the Master and Yoked groups (Figure 4A). Mean response duration over the first 10 min of testing was calculated for each subject and plotted against the levels of BDNF mRNA from the lumbar spinal cord. Results showed that BDNF mRNA levels in the Master group were well predicted by learning performance (r=0.93, p < .005, n=6). BDNF expression and training performance were not well correlated within the yoked group (r=−0.55, p > .05, n=6). Interestingly, the low values observed in yoked subjects could be predicted by extrapolating the function derived from an analysis of the Master group. As a result, the combined data yielded a robust correlation (r=0.95, p < .005, n=12). The results suggest that BDNF is involved in spinal cord learning as the levels of BDNF mRNA within the lumbar region of the spinal cord are directly related to instrumental performance.
Figure 3.
Effects of training on BDNF, CaMKII, CREB, and synapsin I mRNA (mean ±SEM). A) Levels of BDNF mRNA were elevated in the group of rats that learned to flex the leg (master) while BDNF was decreased in the yoked group. mRNA levels of CaMKII (B), CREB (C), and synapsin I (D) followed a similar pattern to that of BDNF. *P<0.05 (ANOVA)
Figure 4.
Correlation between mean response duration during the first 10 min of training and mRNA levels for BDNF (A), CaMKII (B), CREB (C), and synapsin I (D). There was a significant positive correlation for all the molecular systems examined except synapsin I.
We also examined whether BDNF mRNA expression was related to response number. Because master rats that exhibited an increase in response duration generally performed fewer responses, there was a negative correlation between response number and BDNF mRNA expression in the master group, but this effect was not statistically significant (r=−0.57, p > .05, n=6). The opposite relation was observed in yoked rats, but again, this effect was not statistically significant (r=0.60, p > .05, n=6).
Impact of Instrumental Training on CamKII, CREB, and Synapsin 1 mRNA
As previously discussed, CaMKII is activated by BDNF binding to its cognate TrkB receptor (Blanquet and Lamour, 1997), and has a critical role on synaptic plasticity and learning and memory. We measured the levels of CaMKII in the same tissue used for BDNF measurements and found a pattern of expression similar to that reported above for BDNF (Figures 3B and 4B).
The transcription factor CREB has been linked to learning/memory and is regulated by BDNF (Finkbeiner et al., 1997, Tully, 1997). Instrumental training induced CREB expression within the spinal cord (Figure 3C) and again, this effect was well predicted by instrumental performance in the master subjects (Figure 4C).
BDNF regulates the synthesis (Wang et al., 1995) and phosphorylation (Jovanovic et al., 1996) of synapsyn I with resultant effects on neurotransmitter release (Baekelandt et al., 1994, Melloni et al., 1994, Wang et al., 1995) and the formation and maintenance of the presynaptic structure (Takei et al., 1995). Master rats exhibited an increase in synapsin I mRNA (123%) and this effect was correlated with instrumental performance (Figures 3D and 4D). Exposure to uncontrollable shock (yoked) had little effect on synapsin 1 levels.
Statistical analyses confirmed that instrumental training had a significant impact on CaMKII, CREB, and syanpsin 1 mRNA levels, all Fs > 16.07, p < .001. For CaMKII and CREB, post hoc comparisons confirmed that all group differences were statisticallly significant (p < .05). For synapsin 1, the master group differed from both the unshocked and yoked groups, but the latter two did not differ. Analyses of the correlation between instrumental training and mRNA levels revealed a robust effect in master rats for CaMKII and CREB (both rs > 0.93, p < .005, n=6), but not synapsin 1 (r=0.78, p < .10, n = 6). In all cases, performance in the yoked groups was not well-correlated with mRNA levels (all rs < 0.77, p > .05, n=6). As noted for BDNF, the values observed in yoked subjects were well predicted by the function derived from subjects in the master condition. As a result, in all cases, combining the data from the master and yoked groups yielded a significant correlation (all rs > 0.90, p < .01, n=12).
Effect of BDNF Inhibition on Instrumental Learning and Positive Transfer
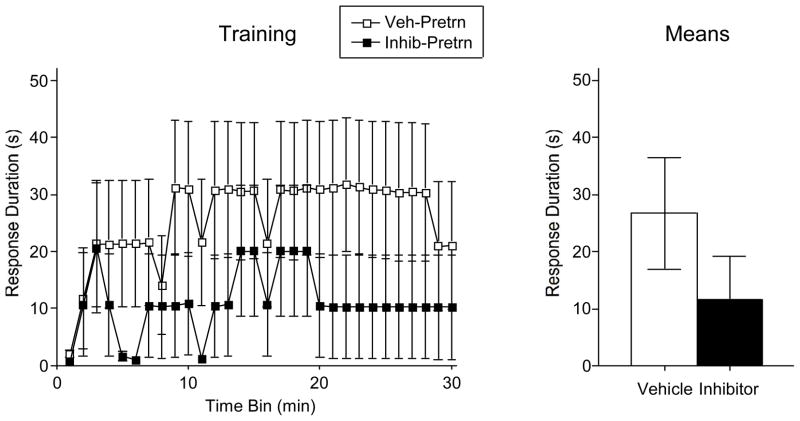
Spinally transected rats (N=24) had fluorescent beads coated with the BDNF inhibitor TrkB-IgG or saline microinjected into the lumbar spinal cord. (L3-L5). Half of the vehicle and Trk-B IgG treated rats were pretrained the next day using the usual (4 mm) response criterion. While it appears that TrkB-IgG had some disruptive effect (Figure 5), neither the main effect of drug treatment, nor its interaction with time bin, approached statistical significance, both Fs < 1.25, p > .05. Because the effect of drug treatment appeared most robust during the last 10 min of training, a separate analysis was performed on these data. Again, a potential effect of TrkB-IgG did not reach statistical significance on instrumental performance, F(1, 10)=1.57, p > .05.
Figure 5.
Effects of BDNF on the acquisition of the flexion reflex. Although the BDNF inhibitor trkB IgG resulted in a reduction in the training performance (A) and the mean performance (B), this effect did not reach statistical significance. The error bars indicate the SEM.
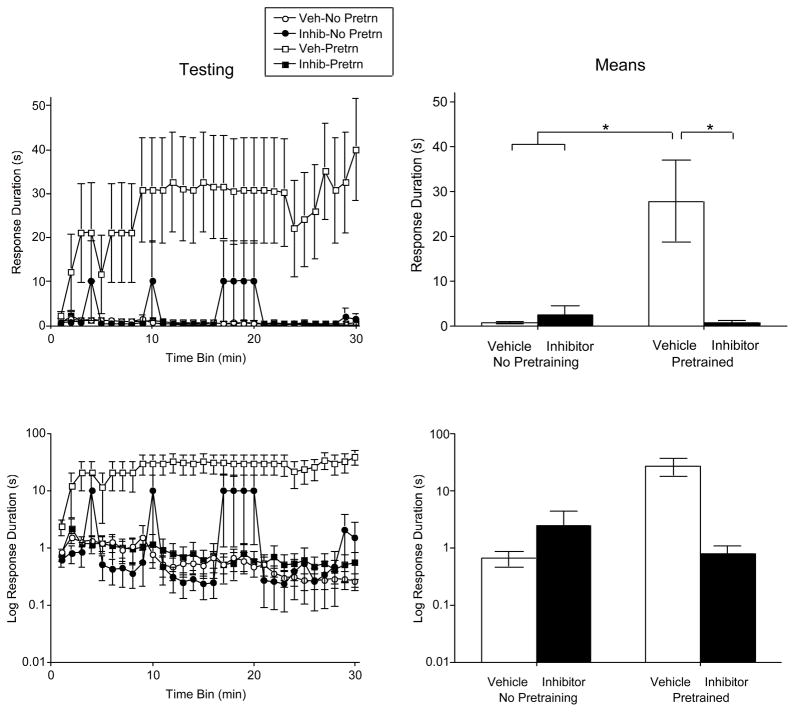
When subjects were tested using a higher response criterion, rats that had not been pretrained, failed to learn (Figure 6). Vehicle treated pretrained rats did learn (positive transfer), and this effect was blocked by TrkB-IgG. An ANOVA confirmed that drug, pretraining, and the drug x pretaining interaction, were all statistically significant, all Fs > 6.06, p < .05. The drug x pretaining interaction indicates that test performance depends on both TrkB-IgG treatment and whether subjects received pretraining. No other differences approached significance, all Fs < 1.25, p > .05. Post hoc comparison of the group means confirmed that the vehicle treated pretrained group differed from the other 3 (p < .05). No other group difference approached significance (p > .05).
Figure 6.
Impact of pretreatment with the BDNF inhibitor TrkB-IgG on learning when subjects were tested with a high response criterion. Performance over time is illustrated to the left and the mean values are indicated to the right. The bottom panels plot the same data on a log scale. The error bars indicated the SEM. Vehicle treated pretrained rats were able to acquire the instrumental response when tested on the contralateral leg with a higher response criterion. This beneficial effect of pretraining was blocked by the BDNF inhibitor.
It could be argued that subjects treated with TrkB-IgG did poorly in testing because they exhibited inferior learning during training. To address this possibility, we examined the relationship between initial learning and test performance using an analysis of covariance (ANCOVA). We focused on the pretrained groups and asked whether TrkB-IgG treatment had a significant impact after the variance attributable to differences in pretraining performance were controlled for using an ANCOVA. Pretraining performance (mean response duration) was marginally related to test performance, F(1, 9)=4.93, p < .055. More importantly, drug treatment had a significant effect on mean test performance above and beyond the differences observed during the pretraining period, F(1, 9)=14.0, p < .005. Controlling for differences in pretraining performance also revealed a significant drug x time interaction, F(29, 261)=2.25, p < .001.
To further evaluate the potential effect of TrkB-IgG on instrumental performance in the absence of pretraining, we performed a separate analysis on the data collected from the non pretrained controls tested with a high response criterion. From both the raw data (Figure 6, top panels) and log values (bottom panels), there is no indication that the inhibitor disrupted performance in the group that did not receive pretraining, F(1, 10) < 1.0, p > .05.
We also looked at whether TrkB-IgG affected performance of the flexion response at the start of testing. The shock intensity (mean±SE) required to elicit a flexion force of 0.4 N ranged from 0.53 (±0.05) to 0.65 (±0.02) across groups. Initial flexion durationsranged from 0.137 (±0.01) to 0.140 (±0.1). Neither difference approached statistical signficance, all Fs < 1.13, p > .05.
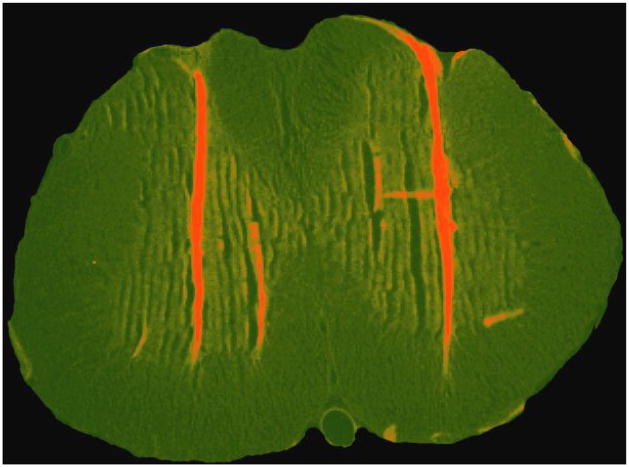
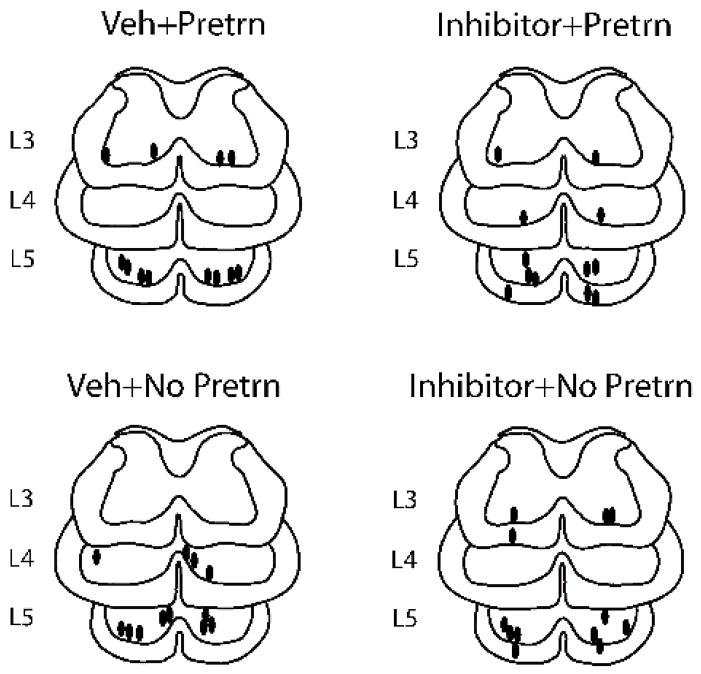
Histological analyses revealed a dense column of beads deposited along the injection tracts (Figure 7). Figure 8 indicates the locus of the injection tips for subjects in each group. It is clear that the bilateral injections generally penetrated to the edge of the ventral gray matter and that most injections were within the L5 region.
Figure 7.
Fluorescent microscopy shows that beads microinjected into the L3-L5 region were localized along the injection tracts.
Figure 8.
Locus of the injection tips for subjects that had micro beads coated with TrkB-IgG (inhibitor) or saline (veh) microinjected into the lumbar spinal cord. The majority of injections penetrated to the ventral rim of the central gray within the L5 region.
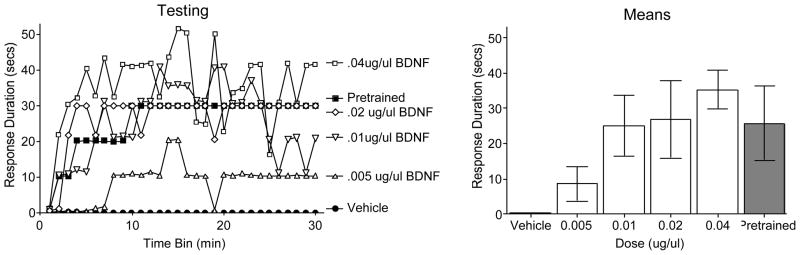
Impact of BDNF Treatment on Instrumental Learning
Spinally transected rats were fitted with an i.t. cannula and tested with a high response criterion the next day. As in prior studies, vehicle treated untrained rats failed to learn (Figure 9). Rats that received instrumental training with a moderate criterion were able to learn when tested with a high criterion on the contralateral leg. Pretreatment with BDNF also enabled learning and did so in a dose-dependent manner. An ANOVA confirmed that drug treatment had a significant effect, F(4, 25)=3.47, p < .05. Post hoc comparisons showed that rats given 0.01 μg/μl of BDNF, or more, were significantly different from the vehicle treated controls (p < .05). In addition, rats given 0.005 μg/μl of BDNF differed from those treated with 0.04 μg/μl. No other drug comparisons were statistically significant. An additional ANOVA was conducted to compare the pretrained group to the untrained groups given either the vehicle or 0.04 μg/μl of BDNF. The ANOVA confirmed that there was a main effect of treatment, F(2, 15)=5.97, p < .05. Post hoc comparisons showed that the pretrained and BDNF treated groups (0.1–0.04 ug/ul) differed from the vehicle treated untrained group (p < .05). No other differences were statistically significant.
Figure 9.
Impact of i.t. BDNF on instrumental learning using a high response criterion. Performance over time is illustrated to the left and the mean values (± SEM) are given to the right. Pretreatment with BDNF fostered learning. The magnitude of this effect was comparable to the benefit of prior instrumental training (Pretrained).
To examine whether BDNF treatment generally enhanced behavioral reactivity, we examined the shock intensity required to elicit a 0.4N flexion response and the duration of the first flexion response (at the start of testing). Shock intensities (mean ± SE) ranged from 0.67 (± 0.04) to 0.70 (± 0.03). Initial flexion duration ranged 0.06 (± 0.02) to 0.12 (± 0.01). Neither difference approached statistical significance, both Fs < 1.0, p > .05.
Effect of CaMKII Inhibition on Instrumental Learning and Positive Transfer
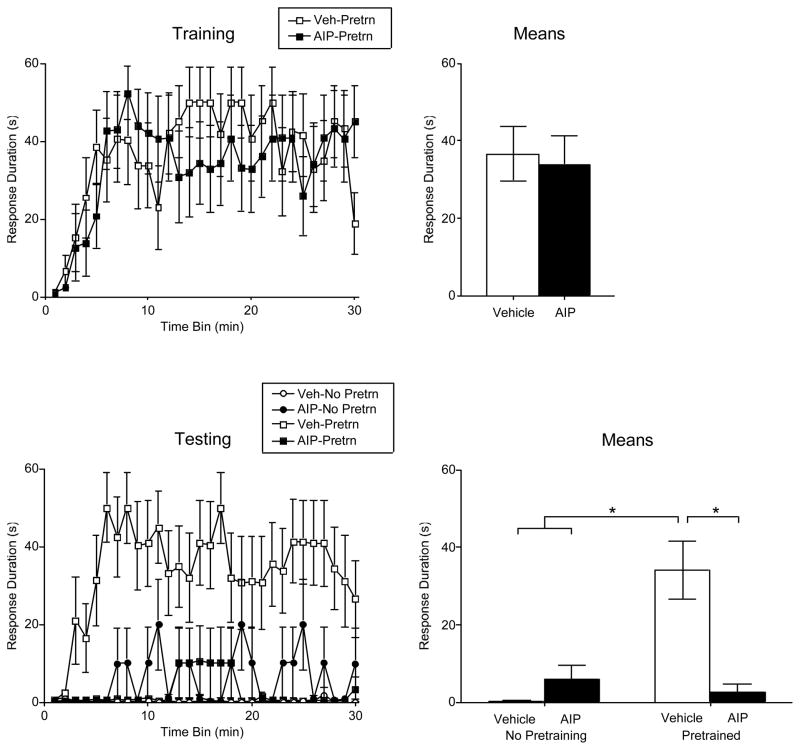
Spinally transected rats were fitted with an i.t. cannula and, 24 hrs later, given the CaMKII inhibitor AIP (500 nmol) or its vehicle. Half the subjects in each drug condition then received pretraining (PreTrn) with the usual (4 mm) response criterion. Vehicle treated rats exhibited a progressive increase in response duration (Figure 10). Learning was unaffected by drug treatment, both Fs < 1.10, p > .05.
Figure 10.
Impact of pretreatment with the CaMKII inhibitor AIP. Drug treatment had no effect on acquisition when subjects were trained with a moderate (4 mm) response criterion (upper panels). Vehicle treated pretrained rats learned when tested on the contralateral leg with a high (8 mm) response criterion (bottom panels). This positive transfer effect was blocked by AIP. Performance over time is illustrated to the left and the means (± SEM) are given to the right.
When subjects were tested on the contralateral leg with a high (8 mm) response criterion, rats that were not pretrained failed to learn (Figure 10). Pretrained subjects that received the vehicle exhibited an increase in response duration and this positive transfer was blocked by pretreatment with AIP. An ANOVA confirmed that the drug, pretraining, and drug x pretraining interaction, were all statistically significant, all Fs > 7.43, p < .05. In addition, the change in response duration observed over time depended on both drug treatment and pretraining condition, F(29, 580) =1.94, p < .005. Post hoc comparisons confirmed that the vehicle treated pretrained group differed from the other groups (p < .05). No other group differences were significant (p > .05).
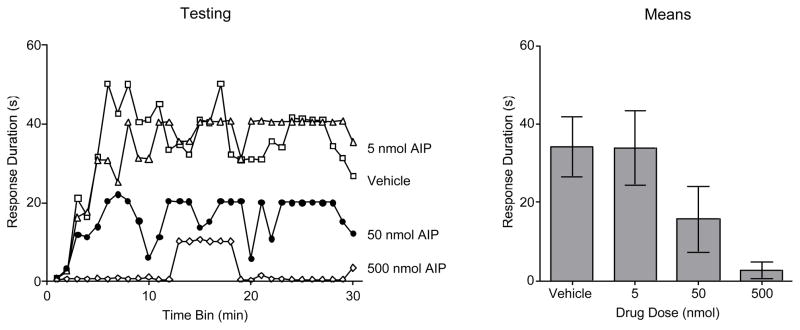
To determine the effective dose, additional subjects were treated with intermediate (5 or 50 nmol) doses of AIP and pretreained with a moderate (4 mm) response criterion. When compared to other pretrained groups, there was no indication that AIP treatment affected training performance, both Fs < 1.0, p > .05 (data not shown). Subjects were then tested on the contralateral leg with a high (8 mm) response criterion. AIP dose-dependently attenuated the positive transfer observed in vehicle treated rats (Figure 1). An ANOVA confirmed that drug treatment had a significant effect, F(1, 20)=3.47, p < .05.
Again, we also evaluated whether our experimental treatments affected the shock intensity needed to elicit a flexion response of 0.4 N or initial flexion duration at the start of testing. Shock intensities (mean + SE) ranged from 0.44 (+ 0.05) to 0.64 (+ 0.07). Initial flexion duration ranged from 0.11 (± 0.01) to 0.14 (± 0.01). In both cases, the group differences were not statistically significant, all Fs < 2.35, p > .05.
DISCUSSION
We have used a quantitative instrumental learning paradigm to determine the involvement of the BDNF systems on the mechanisms of learning in the spinal cord. Instrumental training was conducted after a complete spinal cord transection that was performed to eliminate any influence from suprasegmental centers on the motor behavior. Therefore, any new motor learning would be related to molecular adaptations in the neural circuits remaining in the spinal cord below the lesion. Animals that learned the task showed an increase in BDNF mRNA in the spinal cord lumbar enlargement related to the trained leg. BDNF increased proportionally to the learning performance, and this was accompanied by an increase in molecular systems associated to the action of BDNF on synaptic plasticity and learning and memory. As in prior studies (Crown et al., 2002a), instrumental training enabled learning when subjects were tested with a high response criterion. Pretreatment with TrkB-IgG did not have a statistical significant effect on instrumental learning, but blocked the facilitatory effect. Conversely, pretreatment with BDNF fostered learning when subjects were tested with a high response criterion. The CaMKII inhibitor AIP also blocked the facilitatory effect. The results suggest that instrumental training induces BDNF mRNA expression and that the beneficial effect of instrumental training on subsequent learning depends on BDNF and CaMKII. BDNF appears to be both necessary, and sufficient, to induce the enabling effect.
BDNF Fosters Instrumental Learning
The day after the injection of the BDNF inhibitor trkB-IgG, independent groups of rats were trained with instrumental learning on one hindlimb, then tested on the opposite hindlimb with a more difficult criterion for learning. During initial training, rats that received controllable shock exhibited an increase in flexion duration indicative of learning. Pretreatment with the BDNF inhibitor reduced the duration of the flexion response, however, this reduction did not reach statistical significance. These results suggest several case scenarios. One possibility is that, although initial instrumental learning elevates levels of BDNF mRNA, the initial learning may not require BDNF. It is, however, important to consider the fact that pretreatment with BDNF inhibitor promoted a reducing trend in the flexion response. This suggests that a larger number of subjects or a higher dose of the BDNF inhibitor dose could have led to a larger reduction in the flexion response reaching statistical significance. Another possibility is that the increase in BDNF resultant from the training could, nonetheless, foster subsequent learning when subjects were tested with a more difficult response criterion. We have previously shown that increasing the response criterion caused untrained rats to fail, while trained rats exhibited the positive transfer effect and acquired the instrumental response (Crown & Grau, 2001). Pretreatment with TrkB-IgG blocked this transfer effect, suggesting that instrumental training promotes learning and that BDNF facilitates this positive transfer effect. In conformity with these findings, pretreatment with BDNF fostered learning when subjects were tested with a difficult response criterion. These results suggest that BDNF contributes to the long-term benefit of instrumental training, a benefit that might generally enhance adaptive plasticity or neuronal excitability.
It is important to consider that BDNF can indirectly support learning mechanisms by increasing neuronal excitability and facilitating synaptic events in neural circuits associated with locomotion. Indeed, it has been shown that BDNF can affect the excitability of spinal locomotor networks (Jakeman et al., 1998) and facilitate synaptic transmission (Mendell et al., 2001, Mendell and Arvanian, 2002). The finding that levels of BDNF, and BDNF-related molecular systems, increased in proportion to the learning performance may indicate that expression of these systems is a resultant effect of neuronal excitability associated with learning.
The enhancement of BDNF expression and release by controllable stimulation may illustrate a mechanism by which learning can benefit spinal cord plasticity or neuronal health. We have shown that exposure to uncontrollable stimulation, such as that received by yoked subjects, impairs subsequent instrumental learning (Grau et al., 1998, Crown et al., 2002b). Prior training with controllable stimulation blocks the induction of this learning deficit (Crown and Grau, 2001). Uncontrollable stimulation also impairs recovery after a contusion injury (Grau et al., 2004). Importantly, controllable stimulation has no adverse effect on recovery. In both cases, controllable stimulation may have a protective effect because it promotes the expression and release of BDNF. Indeed, a key feature of both functional electrical stimulation (Creasey et al., 2004) and locomotor training (Edgerton, 2004) is that they provide a form of response-contingent stimulation and, therefore, provide an opportunity for instrumental learning (Grau et al., 2006, Grau and Hook, 2006). In both cases, the long-term benefit of training may hinge, in part, on an up-regulation of BDNF function.
Mechanisms of Action
To explore the mechanisms involved in the BDNF-mediated enhancement of learning, we also assessed the expression of other key factors linked to BDNF and synaptic plasticity. The modulations of BDNF, CaMKII, and CREB mRNAs were associated closely with levels of learning performance during the training period. These results are in general agreements with several studies showing the involvement of these molecules in synaptic plasticity and learning and memory mechanisms.
CREB is one of the best-characterized transcription factors in the brain and can be modulated by BDNF (Shaywitz and Greenberg, 1999). CREB is phosphorylated by BDNF at the transcription regulatory site, and CREB can feed-back on BDNF by regulating its gene transcription through a calcium-dependent mechanism (Finkbeiner, 2000). CREB is required for various forms of memory (Yin and Tully, 1996, Tully, 1997, Silva et al., 1998).
Several lines of evidence indicate that CaMKII is critical to learning and memory. Pharmacological and genetic manipulations have demonstrated the importance of CaMKII for memory processes (Silva et al., 1992a, Silva et al., 1992b, Giese et al., 1998). Long-term potentiation (LTP), an activity dependent strengthening of synapses believed to be an electrophysiological correlate of learning and memory, induces the activation of CaMKII. Genetic deletion of the major isoform of CaMKII results in impaired hippocampal LTP and learning deficits (Cho et al., 1998). As CaMKII lies downstream to BDNF action, activated by BDNF binding to its cognate TrkB receptor (Blanquet and Lamour, 1997), CaMKII may provide the signal transduction pathway through which BDNF affects synaptic and cognitive plasticity. Supporting this, we found the CaMKII inhibitor AIP blocked the enabling effect in a dose-dependent fashion.
Although levels of synapsin I mRNA were elevated in the master rats during the training phase, the learning performance was only weakly correlated with levels of synapsin I mRNA. These results may be consistent with a more preponderant action of synapsin I on synaptic function rather than on learning encoding events per se. As previously discussed, synapsin I activation under the action of BDNF results in neurotransmitter release (Jovanovic et al., 1996, Jovanovic et al., 2000). The fact that synapsin I is under regulatory control of BDNF (Wang et al., 1995, Jovanovic et al., 1996, Causing et al., 1997) suggests that increases in synapsin I as a results of learning can impact synaptic growth and/or function using a mechanism associated with BDNF.
Implications for Functional Recovery After Injury
There are several indications that synaptic plasticity is an intrinsic property of spinal cord cells as much as in the rest of the CNS. Molecular mechanisms involved with learning are highly conserved from invertebrates to mammals. The recurrent collateral tree and subsequent synaptic strength that are reduced after motor neuron axotomy, attains its original strength after about 3 months (Havton and Kellerth, 1990b). This recovery in recurrent inhibition is attributed to a strengthening of synaptic transmission from axon collaterals to Renshaw cells (Havton and Kellerth, 1990a). Moreover, LTP has been shown to be induced in the ventral root reflexes in the isolated neonatal rat spinal cord preparation (Lozier and Kendig, 1995). The capacity of repetitive locomotor activity to promote functional restoration after CNS injury is well recognized. It seems that the outcome of recovery is task specific such that rehabilitative strategies that simulate the action of walking are particularly effective in promoting the recovery of stepping. This implies that locomotor learning can be an intrinsic component of the functional recovery process. The present demonstration that motor training can involve BDNF-mediated synaptic plasticity illustrates a viable mechanism for how spinal cord learning can benefit spinal cord plasticity. According to this view, BDNF either by supporting learning directly or neuronal excitability associated with learning can benefit spinal cord events that are critical components of the functional recovery process.
Figure 11.
Impact of the CaMKII inhibitor AIP on test performance over a range of doses. Subjects were pretreated with the vehicle or AIP (5, 50, or 500 nmol) and trained with a moderate (4 mm) response criterion. They were then tested on the contralateral leg with a high (8 mm) response criterion. Vehicle treated rats learned and this positive transfer was blocked by AIP in a dose-dependent fashion. Mean performance (± SEM) is illustrated to the right.
Acknowledgments
This work was supported by NIH awards NS41548 (JWG.), NS45804 (FGP), and NS16333 (VRE)
Abbreviations
- BDNF
brain-derived neurotrofic factor
- LTP
Long-term potentiation
- EPSPs
excitatory postsynaptic potentials
- CaMKII
calcium/calmodulin activated protein kinase II
- CREB
cAMP-response element binding protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- UNG
uracil glycosylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagi S, Mizoguchi A, Sobue K, Nakamura H, Ide C. Localization of synapsin I in normal fibers and regenerating axonal sprouts of the rat sciatic nerve. Histochem Cell Biol. 1996;105:365–373. doi: 10.1007/BF01463657. [DOI] [PubMed] [Google Scholar]
- Bading H. Transcription-dependent neuronal plasticity: the nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. doi: 10.1046/j.1432-1327.2000.01565.x. [DOI] [PubMed] [Google Scholar]
- Baekelandt V, Arckens L, Annaert W, Eysel UT, Orban GA, Vandesande F. Alterations in GAP-43 and synapsin immunoreactivity provide evidence for synaptic reorganization in adult cat dorsal lateral geniculate nucleus following retinal lesions. Eur J Neurosci. 1994;6:754–765. doi: 10.1111/j.1460-9568.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Blanquet PR, Lamour Y. Brain-derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J Biol Chem. 1997;272:24133–24136. doi: 10.1074/jbc.272.39.24133. [DOI] [PubMed] [Google Scholar]
- Causing CG, Gloster A, Aloyz R, Bamji SX, Chang E, Fawcett J, Kuchel G, Miller FD. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta- mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Ho CH, Triolo RJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. Clinical applications of electrical stimulation after spinal cord injury. J Spinal Cord Med. 2004;27:365–375. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord II. Evidence for central mediation. Physiology & Behavior. 2002a;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behavioral Neuroscience. 2002b;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. J Neurophysiol. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJT, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal circuitry after injury. Ann Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behav Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hook MA. Spinal neurons exhibit a surprising capacity to learn and a hidden vulnerability when freed from the brain’s control. Curr Neurol Neurosci Rep. 2006;6:177–180. doi: 10.1007/s11910-006-0001-3. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Havton L, Kellerth JO. Elimination of intramedullary axon collaterals of cat spinal alpha-motoneurons following peripheral nerve injury. Exp Brain Res. 1990a;79:65–74. doi: 10.1007/BF00228873. [DOI] [PubMed] [Google Scholar]
- Havton L, Kellerth JO. Plasticity of recurrent inhibitory reflexes in cat spinal motoneurons following peripheral nerve injury. Exp Brain Res. 1990b;79:75–82. doi: 10.1007/BF00228874. [DOI] [PubMed] [Google Scholar]
- Hook MA, Ferguson AR, Garcia G, Washburn SN, Koehly LM, Grau JW. Monitoring recovery after injury: procedures for deriving the optimal test window. J Neurotrauma. 2004;21:109–118. doi: 10.1089/089771504772695995. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Stow YL, Sihra S, Sanghera JS, Pelecch SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate sinapsyn I-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts the acquisition and maintenance of an acquired flexion response. Behav Brain Res. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:7–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu GT, Ferguson AR, Crown ED, Bopp AC, Miranda RC, Grau JW. Instrumental learning within the rat spinal cord: localization of the essential neural circuit. Behav Neurosci. 2005;119:538–547. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. Journal of Neuroscience. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier AP, Kendig JJ. Long-Term Potentiation in an Isolated Peripheral Nerve-Spinal Cord Preparation. Journal of Neurophysiology. 1995;74:1001–1009. doi: 10.1152/jn.1995.74.3.1001. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr, Apostolides PJ, Hamos JE, DeGennaro LJ. Dynamics of synapsin I gene expression during the establishment and restoration of functional synapses in the rat hippocampus. Neuroscience. 1994;58:683–703. doi: 10.1016/0306-4522(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Arvanian VL. Diversity of neurotrophin action in the postnatal spinal cord. Brain Research - Brain Research Reviews. 2002;40:230–239. doi: 10.1016/s0165-0173(02)00205-9. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Munson JB, Arvanian VL. Neurotrophins and synaptic plasticity in the mammalian spinal cord [Review] Journal of Physiology-London. 2001;533:91–97. doi: 10.1111/j.1469-7793.2001.0091b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Katz LC, Lo DC. Focal Delivery of Neurotrophins Into the Central Nervous System Using Fluorescent Latex Microspheres. Biotechniques. 1997;23:928. doi: 10.2144/97235rr02. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T, Hirokawa N. Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol. 1995;131:1789–1800. doi: 10.1083/jcb.131.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T. Regulation of gene expression and its role in long-term memory and synaptic plasticity. Proc Natl Acad Sci U S A. 1997;94:4239–4241. doi: 10.1073/pnas.94.9.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]