Abstract
We have assessed potential mechanisms associated with the deleterious effects of TBI on the integrity of plasma membranes in the hippocampus, together with consequences for behavioral function. In addition, we have investigated the efficacy of a dietary intervention based on a pyrazole curcumin derivative with demonstrated bioactivity and brain absorption, to re-establish membrane integrity. We report that moderate fluid percussion injury (FPI) increases levels of 4-Hydroxynonenal (HNE), an intermediary for the harmful effects of lipid peroxidation on neurons. A more direct action of FPI on membrane homeostasis was evidenced by a reduction in calcium-independent phospholipase A2 (iPLA2) important for metabolism of membrane phospholipids such as DHA, and an increase in the fatty acid transport protein (FATP) involved in translocation of long-chain fatty acids across the membrane. A potential association between membrane disruption and neuronal function was suggested by reduced levels of the NR2B subunit of the transmembrane NMDA receptor, in association with changes in iPLA2 and syntaxin-3 (STX-3, involved in the action of membrane DHA on synaptic membrane expansion). In addition, changes in iPLA2, 4-HNE, and STX-3 were proportional to reduced performance in a spatial learning task. In turn, the dietary supplementation with the curcumin derivative counteracted all the effects of FPI, effectively restoring parameters of membrane homeostasis. Results show the potential of the curcumin derivative to promote membrane homeostasis following TBI, which may foster a new line of non-invasive therapeutic treatments for TBI patients by endogenous up-regulation of molecules important for neural repair and plasticity.
Keywords: rat, membrane damage, curcumin, 4-hydoxynonenal, cognition
Introduction
Traumatic brain injury (TBI) likely leads to disruption of plasma membrane function resulting in deficits in neuronal signaling and behavior, even on the absence of major cell death. The pathology of TBI involves an increase in excitatory neurotransmitter efflux (Biegon, 2004), ionic imbalance, ATP depletion, proteolysis, and oxidative stress (Ansari, et al., 2008); and all of these processes are somehow associated with the membrane dysfunction. For example, an increase in oxidative stress has been known to result in cellular membrane damage following TBI (Merenda, et al., 2008). The brain is believed to be particularly vulnerable to oxidative membrane damage based on its high contents of polyunsaturated fatty acids (PUFA), high consumption of oxygen, and relatively low antioxidant defenses compared to other organs. Beyond the initial damage to membranes, reaction of free radicals with double bonds of phospholipids produces peroxides that give rise to α,β-unsaturated aldehydes such as 4-hydroxynonenal (4-HNE). In turn, 4-HNE binds to proteins, particularly at cysteine, histidine, or lysine residues (Subramaniam, et al., 1997), and can cause a number of deleterious effects in cells including inhibition of DNA synthesis, disturbance in calcium homeostasis, and inhibition of mitochondrial respiration.
Phospholipase A2 (PLA2) forms an expanding super family of esterases that specifically cleave the acyl ester bond at the sn-2 position of membrane phospholipids to produce a free fatty acid and lysophospholipid (Farooqui and Horrocks, 2006). In particular, the calcium-independent PLA2 (iPLA2) type (Murakami, et al., 1997, Schaloske and Dennis, 2006, Six and Dennis, 2000) seems to play an important role in long-term potentiation and depression believed to underlie certain aspects of learning and memory (Fitzpatrick and Baudry, 1994, Wolf, et al., 1995). Fatty acid transport proteins (FATPs) are recognized for their role in facilitating translocation of long-chain fatty acids across the plasma membrane (Abumrad, et al., 1999). Changes in FATP4 expression and FATP4 polymorphisms have been linked to markers of insulin resistance and obesity in humans (Doege and Stahl, 2006).
Syntaxin 3 (STX-3) is ideally positioned in the presynaptic plasma membrane to sense local transient increases in PUFA (Darios and Davletov, 2006), and plays a crucial role in the docking and fusion of vesicles during neurotransmitter release (McMahon and Sudhof, 1995). The growth-associated protein 43 (GAP-43) has been shown to provide a general indication for the effects of diet and exercise on axonal growth and synaptic plasticity (Chytrova, et al., 2009). The NR2B subunit of the NMDA receptor, which is deeply implicated in synaptic plasticity and learning and memory (Loftis and Janowsky, 2003) has also been shown to be susceptible to dietary supplementations of PUFAs in rodents (Calon, et al., 2005, Dyall, et al., 2007).
Curcumin, a polyphenolic compound abundant in the Indian spice turmeric, has been found to have neuroprotective effects in animal models of neurodegenerative conditions such as Alzheimer’s disease (Begum, et al., 2008, Lim, et al., 2001, Yang, et al., 2005), focal cerebral ischemia (Thiyagarajan and Sharma, 2004, Zhao, et al., 2008), and lately TBI (Sharma, et al., 2009, Wu, et al., 2006). In this study, we have used a curcumin derivative with the demonstrated ability to penetrate the brain and maintain high biological activity (Liu, et al., 2008, Maher, et al., 2008). The current study aims to provide important information to better understand the mechanisms involved with the pervasive effects of TBI on membrane homeostasis, in ways that can explain how a loss in membrane homeostasis can compromise synaptic plasticity and cognitive abilities. We have investigated molecular systems that can relate cell membrane function with synaptic plasticity in the hippocampus. Neurocognitive deficits observed in TBI patients have been related to hippocampal dysfunction. Results show that our novel curcumin derivative has a great capacity to counteract specific aspects of TBI pathology on membrane function, synaptic plasticity, and behavior.
Materials and Methods
The experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were continually monitored and all procedures were approved by the UCLA Chancellor’s Animal Research Committee. The suffering and number of animals used were minimized.
Experimental design and tissue preparation
Male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) approximately 2 months old were housed in cages (two rats per cage) and maintained in environmentally controlled rooms (22–24°C) with a 12 h light/dark cycle. After acclimatization for 1 week on standard rat chow, a moderate fluid percussion injury (FPI) was performed in approximately half of the rats, another half of rats received identical surgery procedure except the injury step (sham). After surgery, one set of rats was randomly assigned to regular diet or curcumin derivative supplemented diet for two weeks. The regular diet provided in powder form (TestDiet Inc., Richmond, IN) containing a standard vitamin and mineral mix with all essential nutrients and rats were fed ad libitum in a large bowl. A dose of 500 ppm curcumin mixed freshly with powdered regular diet was provided daily in a large bowl. Previous studies from our lab have shown the efficacy of 500 ppm dose of curcumin in the diet against TBI-induced oxidative damage and alterations in synaptic plasticity (Wu, et al., 2006). The rats were trained in Morris water maze starting at day 7 after FPI for five consecutive days. A probe trial was performed 72 hours after the last acquisition trial. Rats were killed by decapitation, and their brains were rapidly dissected, frozen on dry ice, and stored at −70°C until use for analyses. By the end of the experiment, there were 4 experimental groups which were used for cognitive testing and western blotting (n = 6 per group): I) regular diet plus sham (RD-sham); II) regular diet plus FPI (RD-FPI); III) curcumin derivative plus sham (CD-sham); IV) curcumin derivative plus FPI (CD-FPI). A separate cohort of animals (n = 5 per group) was used for immunohistochemistry, which were not tested in the water maze. There were no differences among the groups on body weight and food intake at the end of experiments.
We utilized a pyrazole curcumin derivative (CD, provided by Dr. David Schubert, Salk Institute, La Jolla, CA), which has demonstrated capacity to enter the brain and to maintain high biological activity (Liu, et al., 2008, Liu and Schubert, 1998). CNB001 is a hybrid molecule composed of curcumin attached to a steroid related compound, cyclohexyl bisphenol A which has neuroprotective capacity in vitro (Liu, et al., 2008, Liu and Schubert, 1998). In turn, CD has been shown to be neuroprotective under conditions of excitotoxicity, oxidative stress, and glucose starvation (Liu, et al., 2008). CD activates CaMKII by a cAMP-independent mechanism, facilitates the induction of hippocampal LTP, and has been shown to be orally active in a rat object recognition test for memory (Maher, et al., 2008).
Fluid percussion injury
The injury was performed as previously described (Wu, et al., 2003). In brief, with the aid of a microscope (Wild, Heerburg, Switzerland), a 3.0-mm diameter craniotomy was made 3.0 mm posterior to bregma and 6.0 mm lateral (left) to the midline with a high-speed drill (Dremel, Racine, WI). A plastic injury cap was placed over the craniotomy with silicone-adhesive and dental cement. When the dental cement hardened, the cap was filled with 0.9% saline solution. Anesthesia was discontinued, and the injury cap was attached to the fluid percussion device. A moderate fluid percussion pulse (2.7 atm) was administered at the first sign of hind-limb withdrawal to a paw pinch. Sham animals underwent an identical preparation with the exception of the lesion. Immediately upon responding to a paw pinch, anesthesia was restored, and the skull was sutured. Neomycin was applied on the suture, and the rats were placed in a heated recovery chamber for approximately an hour before being returned to their cages.
Cognitive assessment using Morris Water Maze (MWM)
Briefly, the rats were trained in the water maze starting at day 7 after FPI for two consecutive trials per day for 5 days. The rats were placed into the tank facing the wall from one of the equally spaced start locations that were randomly changed every trial. Each trial lasted until the rat found the platform or for a max of 1 min. If the rat failed to find the platform in the allocated time, it was gently placed on the platform. At the end of each trial, the rats were allowed to rest on the platform for 1 min, and the escape latencies were recorded. The escape latency to find the platform during acquisition trial gives a measure of speed of learning. In order to assess spatial memory retention, spatial probe trial was performed at 72 h after the last trial by removing the platform from the pool. The rats were allowed to swim for 1 min in the pool where escape platform was unavailable, and the percentage of time spent in each zone was calculated. The time spent in the target quadrant zone indicated the degree of memory consolidation which had taken place after learning.
Tissue preparation and protein determination
The entire hippocampus on the FPI-treated side of the brain was collected upon decapitation for protein determination. Tissue was collected into 1.5-mL Eppendorf tubes, immediately frozen on dry ice and stored at −70°C. Hippocampi ipsilateral to the craniotomy were homogenized in a freshly prepared lysis buffer (NaCl, 137 mm; Tris-HCl pH 8.0, 20 mm; NP-40, 1%; glycerol, 10%; phenylmethylsulphonyl fluoride, 1 mm; aprotinin, 10 µg / mL; leupeptin, 1 µg / mL; and sodium vanadate, 0.5 mm). Homogenates were centrifuged at 12,000 g for 20 min to remove insoluble material. The supernatants were collected into clean 1.5-mL tubes, frozen on dry ice and stored at −70°C. The total protein concentration of hippocampal homogenates was determined with a MicroBCA kit (Pierce, Rockford, IL, USA), using BSA as a standard.
Western blot protein analysis
Total proteins from hippocampal tissue were extracted as described above. Equal amounts (25 µg) of protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA). Nonspecific binding sites were blocked with 5% nonfat milk in TBS buffer with 0.1% Tween-20 (pH 7.6). Membranes were incubated with the following primary antibodies: anti-iPLA2 (1: 1,000; Santa Cruz Biotechnology, California), anti-FATP (1:1,000; Santa Cruz Biotechnology, California), anti-Syntaxin-3 (1:1,000; Santa Cruz Biotechnology, California), anti-NR2B (1:1,000; Santa Cruz Biotechnology, California), anti-GAP-43(1:1,000; Santa Cruz Biotechnology, California), anti-4-HNE (1:100; Santa Cruz Biotechnology, California), and anti-actin (1 : 2,000; Santa Cruz Biotechnology, California) followed by antigoat or antirabbit IgG horseradish peroxidase conjugate. After rinsing with buffer (0.1% Tween-20 in TBS), the immunocomplexes were visualized by chemiluminescence using the Amersham ECL Plus Western Blotting Detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) according to the manufacturer’s instructions. The film signals were digitally scanned using an HP Scanner (HP Scanjet 3970) and quantified with NIH Image software, normalized for actin levels.
4-HNE Immunohistochemistry
Serial coronal sections (25 µm) were cut on a cryostat, collected in Phosphate buffered saline (PBS) and processed for immunohistochemistry, as previously described (Gomez-Pinilla, et al., 2001). A 1: 1,000 dilution was used for the goat polyclonal anti-4-HNE (Santa Cruz Biotechnology, California). Immunohistochemistry controls were performed with omission of the primary antibody, and no staining was observed in cell structures.
Statistical analysis
Actin was employed as internal standard for Western blot. The regular diet-fed rats with sham surgery were regarded as experimental controls for comparisons with other experimental groups. For Western blot, the values were expressed as a ratio of actin value and then converted to percent of RD/sham group as presented in bar figures and represented the mean ± SEM. All statistical analyses were performed by commercial software SPSS 16.0. A level of 5% probability was considered significant. Data are shown as the mean ± SEM. MWM and biochemical data were analyzed by two-way ANOVA (diet vs. injury), post-hoc analyses were conducted using Bonferroni’s comparisons, and interaction effects were further analyzed. Analysis of correlation (linear regression) was performed to evaluate the association between specific proteins and Morris water maze performance.
Results
Traumatic brain injury promotes lipid peroxidation
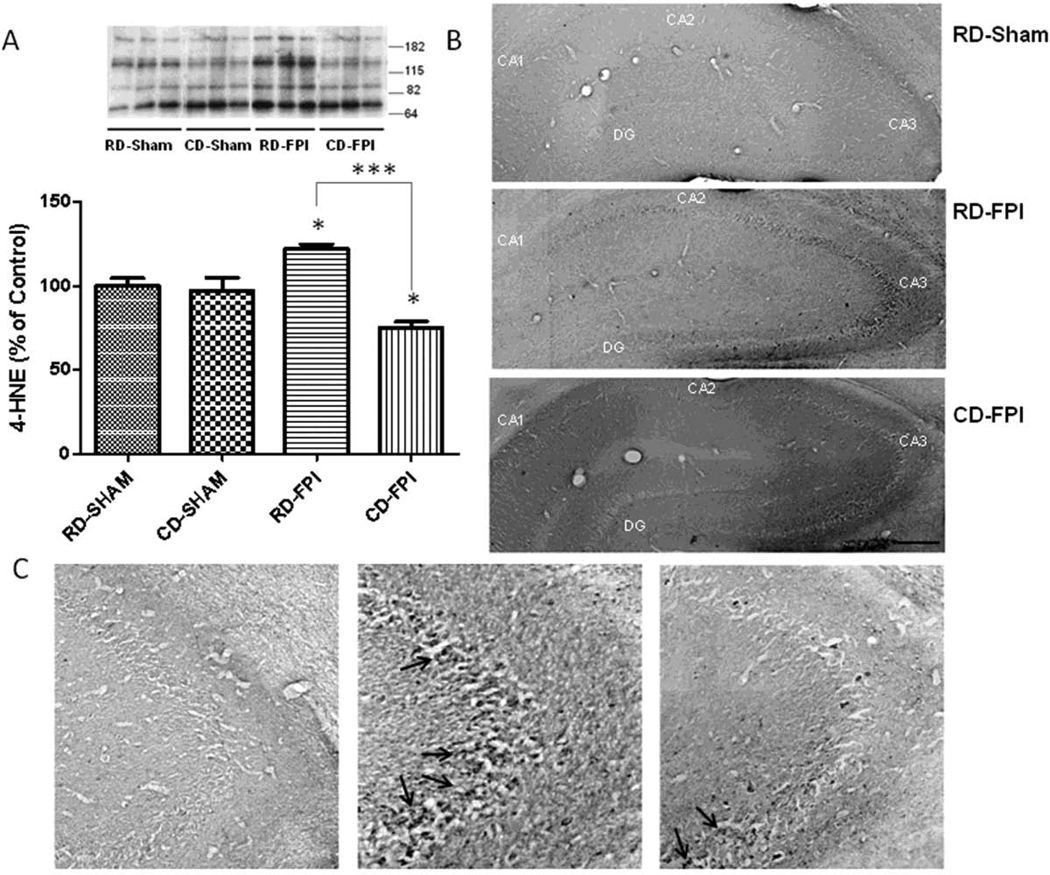
Free radical attack to unsaturated fatty acids has been shown to result in 4-HNE (Subramaniam, et al., 1997). In the present study, the levels of 4-HNE were changed significantly with injury in RD-FPI rats (p<0.05) as compared to RD-Sham rats (Fig. 1A). Dietary supplementation with curcumin derivative after FPI significantly decreased the levels of 4-HNE in CD-FPI rats (p<0.001) compared to rats fed regular diet (RD-FPI) (Fig. 1A). Qualitative HNE-immunoreactivity (HNE-ir) was examined in sham and injured brains form both regular diet and curcumin derivative supplemented diet groups (Fig. 1B–C). Weak 4-HNEir was observed in the sham control group. The nature of HNE-ir was diffuse, showing presence of numerous cell bodies and pyramidal neurons containing HNE-ir in the CA1 and CA3 layers of hippocampus (Fig. 1C). FPI resulted in a qualitative increase in 4-HNE-ir in hippocampus of RD-FPI animals, while the curcumin derivative supplementation attenuated the effects of the FPI as seen in the CD-FPI rats.
Figure 1.
(A) Effects of FPI on lipid peroxidation as evidenced by changes in 4-HNE in the hippocampus. Western blot analysis revealed that the levels of 4-HNE were significantly increased after FPI, whereas dietary curcumin derivative supplementation significantly reduced the levels of 4-HNE in FPI rats. Representative Western blot for 4-HNE from different groups in hippocampus are depicted, multiple bands were recognized. For quantification purpose, multiple bands of HNE-bound proteins were grouped and quantified using NIH Image software as described elsewhere (Wu, et al., 2004a, Wu, et al., 2004b). The values were converted to percent of RD sham group (mean ± SEM). ANOVA followed by post-hoc tests with Bonferroni’s comparisons, *p < 0.05; ***p < 0.001. (B) Immunostaining showed 4-HNE labeling throughout the hippocampal formation but with preferential distribution in pyramidal layer as shown in the high magnifications photographs (arrows, C). FPI increased 4-HNE immunostaining but dietary curcumin derivative counteracted the effect of FPI. Scale bars (B, C) = 400 µm and 50 µm respectively. RD: regular diet; FPI: fluid percussion injury.
TBI disrupts proteins involved in membrane turnover
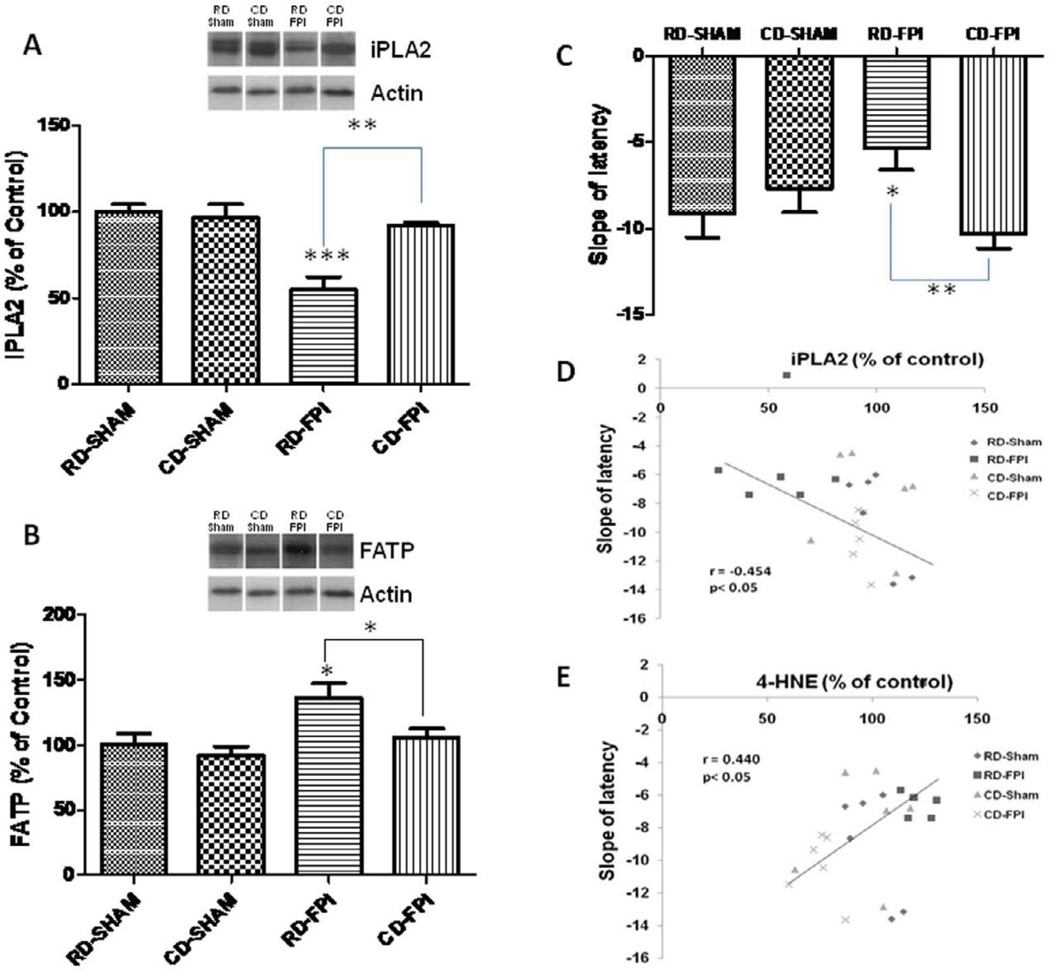
With regards to iPLA2, there were significant effects of diet (F1,20 = 8.53; p<0.01) and injury (F1,20 = 17.72; p<0.001), as well as a significant interaction between diet and injury effects (F1,20 = 12.16; p<0.002). We found that FPI decreased (p<0.001; Fig. 2A) the levels of iPLA2 in RD-FPI rats compared to RD-sham rats. Dietary supplementation with curcumin derivative after FPI restored (p<0.01) the levels of iPLA2 in CD-FPI rats compared to RD-FPI rats. The levels of iPLA2 were not significantly different in CD-sham rats compared to RD-sham rats (Fig. 2A). Although the levels of FATP changed significantly with diet (F1,20 = 4.92; p<0.038) and injury (F1,20 = 8.05; p<0.01), there was no interaction effect between diet and injury for FATP levels. FPI increased the levels of FATP in RD-FPI rats (p<0.05) compared to RD-sham rats. Treatment with curcumin derivative after FPI significantly decreased the levels of FATP in CD-FPI rats (p<0.023) compared to RD-FPI rats (Fig. 2B).
Figure 2.
(A–B) Effects of FPI and curcumin derivative treatment on molecular systems important for maintenance of plasma membrane homeostasis, and cognitive abilities. FPI decreased iPLA2 levels (A) while increased FATP levels (B) in the hippocampus. Representative Western blots for iPLA2 and FATP from all experimental groups in hippocampus are shown here. Dietary curcumin derivative supplementation significantly enhanced the levels of iPLA2 after FPI. (C) Slope of escape latency (the learning speed) to find the platform in training trial during Morris water maze test for each group of animals. FPI significantly decreased the learning speed in regular diet animals but the dietary curcumin derivative supplementation significantly improved the learning speed after FPI. (D) Correlation of slope of latency with the hippocampal levels of iPLA2. Slope of latency changed in inverse proportion to iPLA2 levels. The values were converted to percent of RD sham group (mean ± SEM). *p < 0.05, **p< 0.01, ***p< 0.001, ANOVA followed by post-hoc tests with Bonferroni’s comparisons. RD, regular diet; FPI, fluid percussion injury
Cognitive deficits are associated to alterations in membrane related proteins after TBI
The results of Morris water maze assessments show that animals from all the groups learned to find the submerged platform but there were differences in the speed of learning (Fig. 1D). There were no significant differences in the probe trial between different groups. This moderate injury may not have severe effects in terms of memory consolidation as reflected in results of probe trial. We calculated the slope of the escape latency, which gives a measure of the learning speed - the steeper the slope the faster the learning (Chytrova, et al., 2009). The speed of learning was significantly decreased in RD/FPI rats and the curcumin derivative supplements counteracted the effects of FPI to the control levels (Fig. 2C). We found that the slope of the latency varied in proportion to the expression of the membrane associated protein iPLA2 (r= − 0.454; p<0.05; Fig. 2D), which suggests that learning performance may rely on levels of select membrane proteins. We also find that the levels of 4-HNE were correlated with the slope of escape latency (r=0.440; p<0.05, data not shown) representing that increased membrane lipid peroxidation may contribute to decrease in speed of learning and cognitive deficits as seen in the water maze task.
TBI reduces expression of membrane related proteins involved in synaptic plasticity and cognition
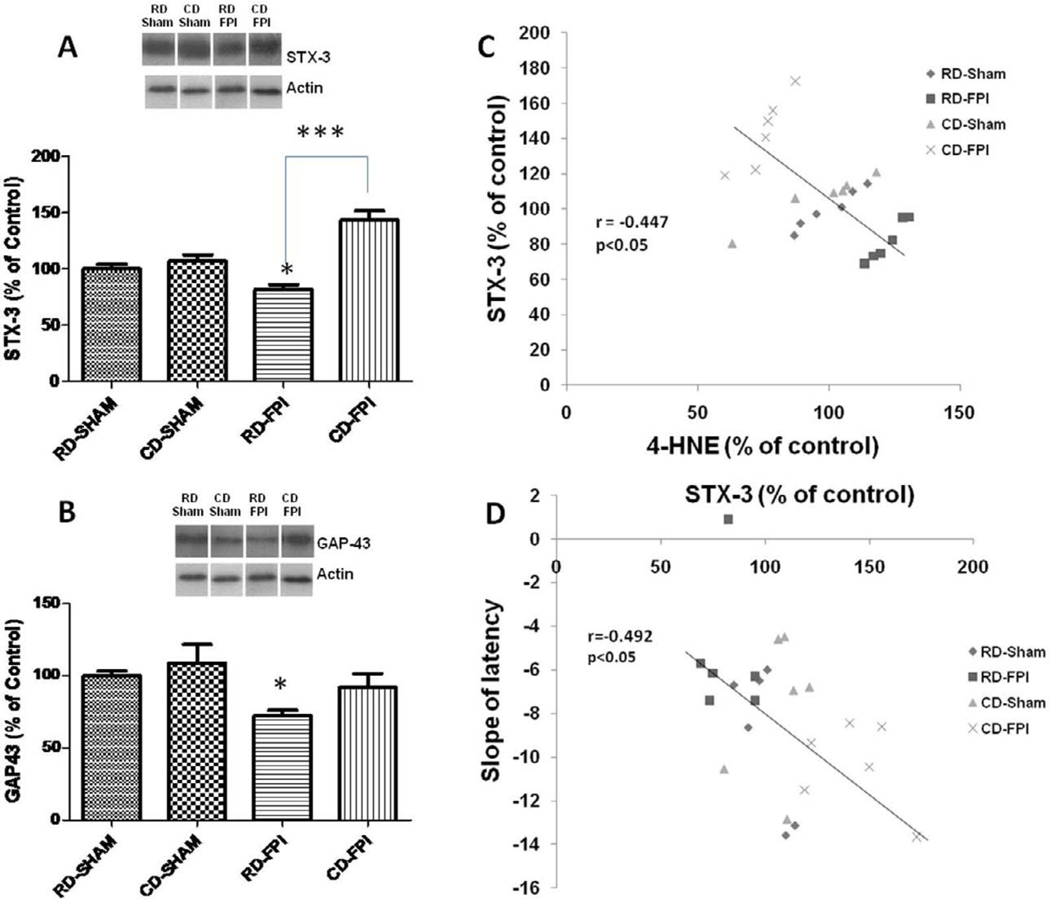
There was a significant effect of diet on STX-3 (F1,20 = 32.6; p<0.001), and there was a significant interaction between diet and injury effects (F1,20 = 20.95; p<0.001) on the levels of STX-3. Bonferroni’s comparisons showed that FPI significantly decreased the levels of STX-3 in RD-FPI rats (p<0.05) compared to RD-Sham rats (Fig. 3A). Curcumin derivative supplementation after FPI significantly restored the levels of STX-3 in CD-FPI rats (p<0.001) compared to RD-FPI rats (Fig. 3A). We found that the slope of latency (speed of learning) varied in inverse proportion to levels of STX-3 (r= −0.492; p<0.05; Fig. 3B), suggesting an association between learning performance and the expression of STX-3. The levels of STX-3 changed in inverse proportion to 4-HNE levels (r = 0.447; p = 0.028) (Fig. 3C), indicating that lipid peroxidation may influence levels of STX-3 with implications for synaptic plasticity. The levels of GAP-43 were significantly decreased after FPI in RD-FPI rats (p<0.05) compared to RD-Sham rats, and the dietary curcumin derivative supplementation normalized GAP-43 levels in the CD-FPI rats (Fig. 3D).
Figure 3.
Effects of FPI and curcumin derivative treatments on molecular systems important for plasma membrane expansion such as syntaxin-3 and GAP-43. (A) FPI decreased the levels of STX-3 whereas dietary curcumin derivative supplementation significantly counteracted these effects. Representative Western blots for STX-3 from all experimental groups in hippocampus are shown here. (B) Levels of the lipid peroxidation marker 4-HNE changed in inverse proportion to STX-3 levels. (C) The levels of STX-3 changed in inverse proportion to the slope of latency. (D) FPI decreased the levels of GAP-43 in regular diet group (RD/FPI), dietary curcumin derivative supplementation significantly counteracted the FPI effects (CD/FPI). The values were converted to percent of RD sham group (mean ± SEM). *p < 0.05, ***p< 0.001, ANOVA followed by post-hoc tests with Bonferroni’s comparisons. RD, regular diet; FPI, fluid percussion injury
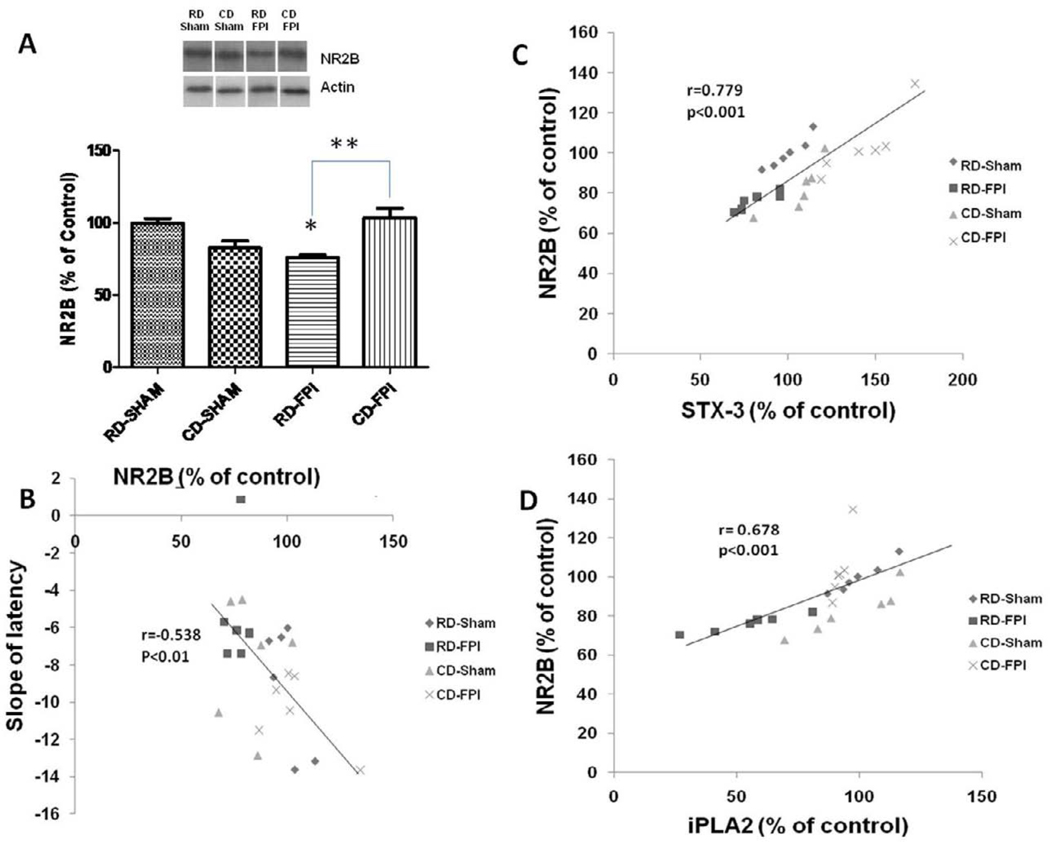
Membrane disruption may be associated with dysfunction of membrane receptors
There were significant interactive effects of diet and injury (F1,20 = 24.16; p<0.001) on the levels of NR2B. The levels of NR2B were significantly decreased after FPI in RD-FPI rats (p<0.001) compared to RD-Sham rats (Fig. 4A). Dietary supplementation with curcumin derivative significantly increased the levels of NR2B levels in CD-FPI rats (p<0.001) compared to RD-FPI rats. We found that slope of latency changed accordingly to levels of NR2B (r= −0.538; p<0.01; Fig. 4B), which corroborates previous findings about an association between NR2B and learning performance in water maze task. More importantly, we find that the levels of NR2B proteins were positively correlated with the levels of iPLA2 (r=0.678; p=0.001, Fig. 4C) and STX-3 (r=0.779; p=0.001). The relationships between NR2B levels with both iPLA2 and STX-3 further suggest a potential influence of membrane homeostasis on levels of NR2B protein, with potential implications for the modulation of synaptic plasticity (Fig. 4D).
Figure 4.
Effects of FPI and curcumin derivative treatment on levels of NR2B, and their associations with cognitive abilities and membrane homeostasis. (A) The levels of NR2B were significantly decreased after FPI, and dietary curcumin derivative supplementation counteracted these effects. Representative Western blots for NR2B from different groups in hippocampus. (B) The levels of NR2B changed in inverse proportion to the slope of latency. (C–D) The levels of NR2B changed in direct proportion to iPLA2 levels and STX-3 levels. The values were converted to percent of RD sham group (mean ± SEM). *p< 0.05, **p< 0.01. ANOVA followed by post-hoc tests with Bonferroni’s comparisons. RD, regular diet; FPI, fluid percussion injury
Discussion
Our results show that TBI potentiates pathways that can lead to disruption of membrane homeostasis, and this may have detrimental consequences for neuronal function. We have found that TBI damaged plasma membranes as evidenced by an increase in the levels of the lipid peroxidation marker 4-hydroxynonenal (4-HNE). More specific effects of TBI on the stability of plasma membranes were reflected in changes in specific molecules that regulate phospholipid metabolism such as iPLA2 and FATP. These events seem to be associated with reductions in structural components of synaptic membranes such as STX-3 and GAP-43, which are also required for synaptic plasticity, growth and repair. Our results also showed that TBI reduced levels of the NR2B subunit of the transmembrane NMDA receptor, and that these reductions were associated with the decrease in iPLA2. These results seem to suggest that TBI triggers a vicious cycle of events in which lipid peroxidation can disrupt the plasma membrane integrity and normal cellular function. For example, membrane dysfunction may be expressed as failure in neuronal signaling through membrane embedded receptors. Interestingly, changes at the molecular levels (4HNE, iPLA2, STX-3) were associated with reduced performance in a spatial learning and memory task that is under the scope of action of NMDA receptors. Even more interestingly, our results show that a pyrazole curcumin derivative with demonstrated bioactivity and brain absorption (Liu, et al., 2008, Maher, et al., 2008) supplemented in the diet counteracted all the assessed effects of FPI. Results indicate that this dietary supplementation stabilizes pathways important for membrane homeostasis, thereby normalizing synaptic plasticity and cognitive abilities.
TBI induces lipid peroxidation and loss of plasma membrane homeostasis
Our results showed that FPI elevated levels of 4-HNE in the hippocampus providing a strong indication for the effects of TBI on lipid peroxidation. Peroxidation of membrane bound arachidonic acid results in the generation of 4-HNE, which produces alterations in the function of key membrane proteins including glucose transporter, glutamate transporter, and sodium potassium ATPases (Lauderback, et al., 2001, Mark, et al., 1997). Inhibition of sodium potassium ATPase by 4-HNE can result in the depolarization of neuronal membranes leading to the opening of NMDA receptor channels and the influx of additional calcium ions into the cell with harmful consequences for neurons (Kadoya, et al., 2003). 4-HNE also increases the permeability of the blood brain barrier (Mertsch, et al., 2001) during excitotoxicity. 4-HNE blocks neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin, which may contribute to the cytoskeletal changes in neurons undergoing neurodegeneration (Farooqui, et al., 2004, Neely, et al., 1999). 4-HNE also decreased cellular ATP levels and mitochondrial function by impairing glucose transport across the membrane (Keller, et al., 1997a, Keller, et al., 1997b). According to our results, an increase in 4-HNE after TBI was associated with reductions in the membrane-bound STX-3 and the NR2B subunit of the NMDA receptor. The overall evidence seems to indicate that membrane lipid peroxidation is a decisive step by which TBI disrupts membrane function, and as discussed below this can have crucial consequences for synaptic plasticity and behavior.
The Influence of Membrane homeostasis on neuronal signaling and cognitive function
iPLA2 plays a crucial role in controlling membrane homeostasis and our results show that iPLA2 levels are reduced after experimental TBI. A growing line of evidence indicates that normal iPLA2 levels are important for learning and memory as iPLA2 blocking in laboratory animals has been shown to result in impaired learning and memory (Holscher, et al., 1995, Holscher and Rose, 1994, Sato, et al., 2007). Moreover, it has been found that selective inhibition of iPLA2-VIB prevents LTP in the CA1 field of rat hippocampal slices (Martel, et al., 2006). These results suggest that a loss of membrane stability may be an underlying cause for the effects of TBI in reducing learning and memory performance. Indeed, according to our results, learning performances in the MWM were associated with levels of hippocampal iPLA2. In addition, TBI promoted an increase in the fatty acid transporter protein FATP, which has been shown to facilitate translocation of long-chain fatty acids across the plasma membrane. The increase in FATP may reflect an elevated potential of the TBI brain for the translocation and breakdown of fatty acids.
Given the importance of membrane stability for proper function of embedded signaling transduction receptors, it is likely that the effects of TBI on membrane metabolism can heavily compromise neuronal signaling events. According to our studies, FPI reduced iPLA2 levels in proportion to levels of NR2B, which may provide a general indication for how the effects of TBI on membrane function may compromise receptor function and signaling. In accordance to this view, loss of membrane integrity after TBI, as revealed by elevated lipid peroxidation and abnormal levels of iPLA2 and FATP, may compromise function of embedded receptors such as the NR2B subunit of the NMDA receptor. NR2B is a critical component of molecular signaling pathways regulating synaptic plasticity, and learning and memory (Loftis and Janowsky, 2003, Muller, et al., 2009, Nakazawa, et al., 2004). In addition, NR2B is abundantly expressed in growth cones of growing neurites and is a critical component of molecular signaling pathways regulating LTP, LTD, synaptic growth and plasticity (Loftis and Janowsky, 2003, Muller, et al., 2009, Nakazawa, et al., 2004). Our current findings showing that TBI reduces hippocampal levels of NR2B in conjunction with iPLA2 levels and a learning impairment seem to indicate that abnormal NR2B levels may be associated with a loss in membrane stability.
The possibility that the function of NR2B relies on membrane stability is supported by studies showing that hippocampal levels of NR2B and learning and memory performance (Chytrova, et al., 2009) are modulated by dietary DHA (Calon, et al., 2005, Dyall, et al., 2007). DHA is an important component of the phospholipid composition of the membrane and its insufficiency in the diet has been shown to reduce plasma membrane fluidity (Hashimoto, et al., 2006, Lund, et al., 1999, Suzuki, et al., 1998). In turn, STX-3 is a membrane-bound synaptic protein and its function is influenced by DHA, i.e., DHA stimulates the action of STX-3 on membrane expansion (Darios and Davletov, 2006). Therefore, our results showing that TBI affected lipid peroxidation, as well levels of iPLA2, STX-3, and NR2B, suggest a potential mechanism by which TBI can damage the membrane with functional implications. It is also possible that our results showing that FPI reduced STX-3 and GAP-43 levels may also be expressed in the reduction of membrane expansion or neurite growth. It has been shown that the actions of STX-3 (Darios and Davletov, 2006) and GAP-43 (Hocquemiller, et al., 2010) are important for neuronal growth. In addition, the action of membrane iPLA2 has been associated with neuronal growth and differentiation in development and in response to neuronal injury (Forlenza, et al., 2007).
Curcumin derivative improves membrane homeostasis, neuronal signaling and cognitive deficits after TBI
The polyphenolic curcumin when provided before the injury has been shown protect the brain against the effects of experimental TBI (Wu, et al., 2006). The current study describes the efficacy of a curcumin derivative to counteract the effects of TBI when supplied after the injury onset. The curcumin derivative CNB001 has rapid absorption into the blood and the brain, and has high biological activity (Liu, et al., 2008, Maher, et al., 2008). As discussed above, it is likely that membrane lipid peroxidation plays a major role on the cascade of events leading to loss of neuronal signaling (Fig. 5). Free radical formation is a sub-product of dysfunctional energy metabolism, and we have shown that curcumin can restore several parameters associated with control of mitochondrial energy metabolism after TBI (Sharma, et al., 2009). Therefore, it is likely that curcumin can play a major action on the stabilization of membrane homeostasis by restoring mitochondrial oxidative function. A recent study suggested a potential link between membrane homeostasis and energy management, as it showed that genetic ablation of iPLA2γ results in defects in mitochondrial lipid metabolism and function (Mancuso, et al., 2007). Our results demonstrate that the treatment with curcumin derivative normalized all the effects of FPI, including lipid peroxidation, and levels of iPLA2, STX-3, and NR2B. It is also notable that curcumin derivative treatment attenuated the effects of FPI on learning capacity. We hypothesize that restoring membrane properties may be a crucial step by which the curcumin derivative can support neuronal signaling through NR2B and other receptors. The flexibility of the membrane is crucial for the function of embedded receptors and signal transduction events, and the current studies represent the first step in characterizing the changes that may lead to disruption of cell membrane homeostasis following TBI. In addition, these studies show the feasibility of a non-invasive dietary therapy using curcumin supplementation to counteract the effects of TBI on the disruption of membrane homeostasis.
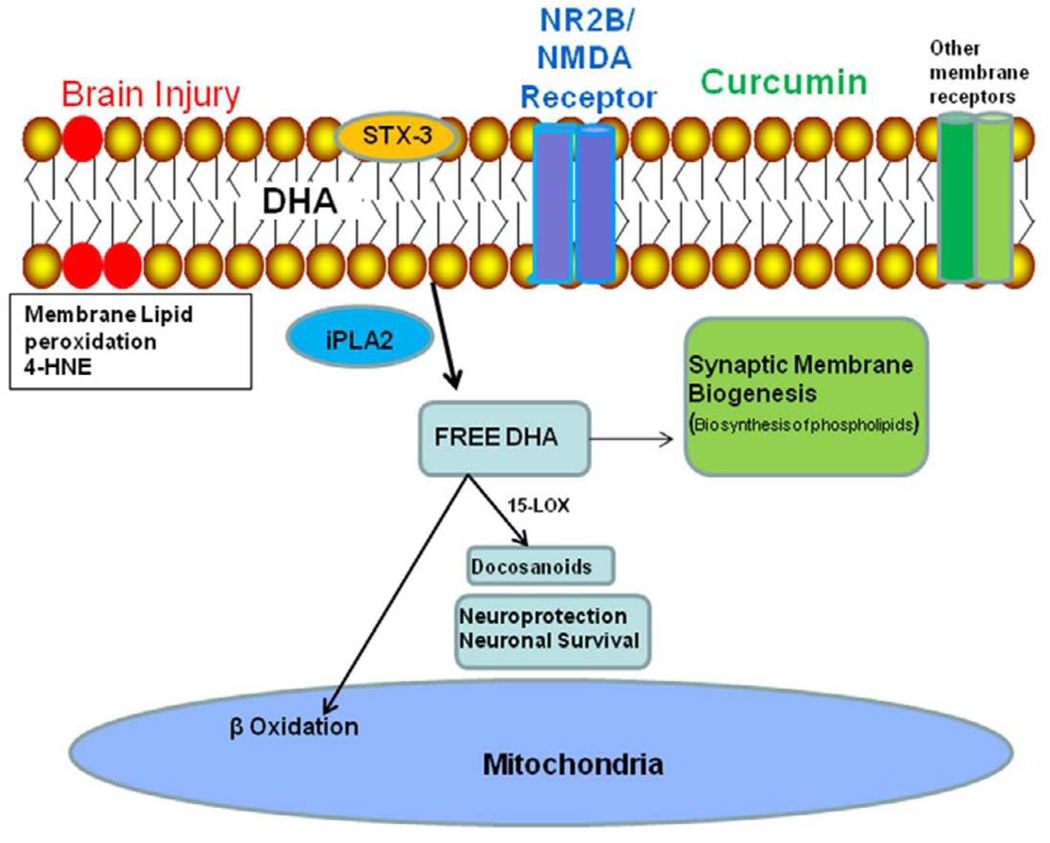
Figure 5.
Possible events by which TBI can disrupt plasma membrane homeostasis. TBI increases free radical formation in the mitochondria, thereby promoting oxidation of membrane lipids, as detected by the lipid peroxidation marker 4-HNE. These events may lead to membrane dysfunction as evidenced by reduced levels of iPLA2. These events may lower membrane function and expansion capacity as evidenced by reduced levels of STX-3 and GAP-43. The loss of membrane function and flexibility may compromise performance of membrane embedded receptors such as the subunit NR2B of the NMDA receptor. These alterations can result in abnormal neuronal signaling, which can reduce learning capacity and other functions that rely on synaptic plasticity and neuronal excitability. The curcumin derivative curcumin derivative administration had a comprehensive effect on most of these alterations. The observed effects of curcumin derivative after TBI may be primarily due to its ability to counteract lipid peroxidation, thereby, preserving membrane homeostasis, and reducing TBI induced cognitive deficits.
Acknowledgements
This work was supported by National Institute of Health awards RC1 NS068473 and R01 NS50465. We thank Dr. David Schubert from Salk Institute for providing the curcumin derivative used in this study.
Abbreviations
- TBI
traumatic brain injury
- FPI
fluid percussion injury
- RD
regular diet
- iPLA2
independent phospholipase A2
- 4-HNE
4-Hydroxynonenal
- FATP
fatty acid transport proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. doi: 10.1016/s1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 2.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biegon A. Cannabinoids as neuroprotective agents in traumatic brain injury. Curr Pharm Des. 2004;10:2177–2183. doi: 10.2174/1381612043384196. [DOI] [PubMed] [Google Scholar]
- 5.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 6.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2009 doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 8.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 2006;21:259–268. doi: 10.1152/physiol.00014.2006. [DOI] [PubMed] [Google Scholar]
- 9.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, the ugly. Neuroscientist. 2006;12:245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 11.Farooqui AA, Ong WY, Horrocks LA. Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res. 2004;29:1961–1977. doi: 10.1007/s11064-004-6871-3. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick JS, Baudry M. Blockade of long-term depression in neonatal hippocampal slices by a phospholipase A2 inhibitor. Brain Res Dev Brain Res. 1994;78:81–86. doi: 10.1016/0165-3806(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 13.Forlenza OV, Mendes CT, Marie SK, Gattaz WF. Inhibition of phospholipase A2 reduces neurite outgrowth and neuronal viability. Prostaglandins Leukot Essent Fatty Acids. 2007;76:47–55. doi: 10.1016/j.plefa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 16.Hocquemiller M, Vitry S, Bigou S, Bruyere J, Ausseil J, Heard JM. GAP43 overexpression and enhanced neurite outgrowth in mucopolysaccharidosis type IIIB cortical neuron cultures. J Neurosci Res. 2010;88:202–213. doi: 10.1002/jnr.22190. [DOI] [PubMed] [Google Scholar]
- 17.Holscher C, Canevari L, Richter-Levin G. Inhibitors of PLA2 and NO synthase cooperate in producing amnesia of a spatial task. Neuroreport. 1995;6:730–732. doi: 10.1097/00001756-199503270-00006. [DOI] [PubMed] [Google Scholar]
- 18.Holscher C, Rose SP. Inhibitors of phospholipase A2 produce amnesia for a passive avoidance task in the chick. Behav Neural Biol. 1994;61:225–232. doi: 10.1016/s0163-1047(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 19.Kadoya A, Miyake H, Ohyashiki T. Contribution of lipid dynamics on the inhibition of bovine brain synaptosomal Na+-K+-ATPase activity induced by 4-hydroxy-2-nonenal. Biol Pharm Bull. 2003;26:787–793. doi: 10.1248/bpb.26.787. [DOI] [PubMed] [Google Scholar]
- 20.Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997a;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 21.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997b;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 22.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 23.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Dargusch R, Maher P, Schubert D. A broadly neuroprotective derivative of curcumin. J Neurochem. 2008;105:1336–1345. doi: 10.1111/j.1471-4159.2008.05236.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Schubert D. Steroid hormones block amyloid fibril-induced 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) formazan exocytosis: relationship to neurotoxicity. J Neurochem. 1998;71:2322–2329. doi: 10.1046/j.1471-4159.1998.71062322.x. [DOI] [PubMed] [Google Scholar]
- 26.Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 27.Lund EK, Harvey LJ, Ladha S, Clark DC, Johnson IT. Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann Nutr Metab. 1999;43:290–300. doi: 10.1159/000012797. [DOI] [PubMed] [Google Scholar]
- 28.Maher P, Akaishi T, Schubert D, Abe K. A pyrazole derivative of curcumin enhances memory. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, Moon SH, Pietka T, Abumrad NA, Schlesinger PH, Gross RW. Genetic ablation of calcium-independent phospholipase A2gamma leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem. 2007;282:34611–34622. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 31.Martel MA, Patenaude C, Menard C, Alaux S, Cummings BS, Massicotte G. A novel role for calcium-independent phospholipase A in alpha-amino-3-hydroxy-5-methylisoxazole-propionate receptor regulation during long-term potentiation. Eur J Neurosci. 2006;23:505–513. doi: 10.1111/j.1460-9568.2005.04565.x. [DOI] [PubMed] [Google Scholar]
- 32.McMahon HT, Sudhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 33.Merenda A, Gugliotta M, Holloway R, Levasseur JE, Alessandri B, Sun D, Bullock MR. Validation of brain extracellular glycerol as an indicator of cellular membrane damage due to free radical activity after traumatic brain injury. J Neurotrauma. 2008;25:527–537. doi: 10.1089/neu.2007.0359. [DOI] [PubMed] [Google Scholar]
- 34.Mertsch K, Blasig I, Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- 35.Muller T, Albrecht D, Gebhardt C. Both NR2A and NR2B subunits of the NMDA receptor are critical for long-term potentiation and long-term depression in the lateral amygdala of horizontal slices of adult mice. Learn Mem. 2009;16:395–405. doi: 10.1101/lm.1398709. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 37.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 38.Neely MD, Sidell KR, Graham DG, Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Ishida T, Irifune M, Tanaka K, Hirate K, Nakamura N, Nishikawa T. Effect of NC-1900, an active fragment analog of arginine vasopressin, and inhibitors of arachidonic acid metabolism on performance of a passive avoidance task in mice. Eur J Pharmacol. 2007;560:36–41. doi: 10.1016/j.ejphar.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Zhuang Y, Ying Z, Wu A, Gomez-Pinilla F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161:1037–1044. doi: 10.1016/j.neuroscience.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 43.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product-4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Park SJ, Tamura M, Ando S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: a comparison of sardine oil diet with palm oil diet. Mech Ageing Dev. 1998;101:119–128. doi: 10.1016/s0047-6374(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 45.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 46.Wolf MJ, Izumi Y, Zorumski CF, Gross RW. Long-term potentiation requires activation of calcium-independent phospholipase A2. FEBS Lett. 1995;377:358–362. doi: 10.1016/0014-5793(95)01371-7. [DOI] [PubMed] [Google Scholar]
- 47.Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 48.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004a;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 49.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004b;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]