Abstract
Metabolic properties of the four subclasses of human IgG were investigated by performing 47 turnover studies in individuals with normal IgG serum concentrations, as well as in patients with an increased level of one of the subclasses. Studies in 12 subjects with normal IgG serum concentration showed that the average biologic half-life of G1, G2, and G4 was 21 days, while that of G3 was only 7.1 days. Fractional catabolic rates of G1, G2, and G4 were 6.9 to 8% of the intravascular pool per day. G3, however, had a higher fractional catabolic rate, amounting to 16.8% of the intravascular pool per day. Distribution of the subclasses was such that the intravascular compartment contained 51-54% of the total body pools of G1, G2, and G4, but 64% of the total body pool of G3.
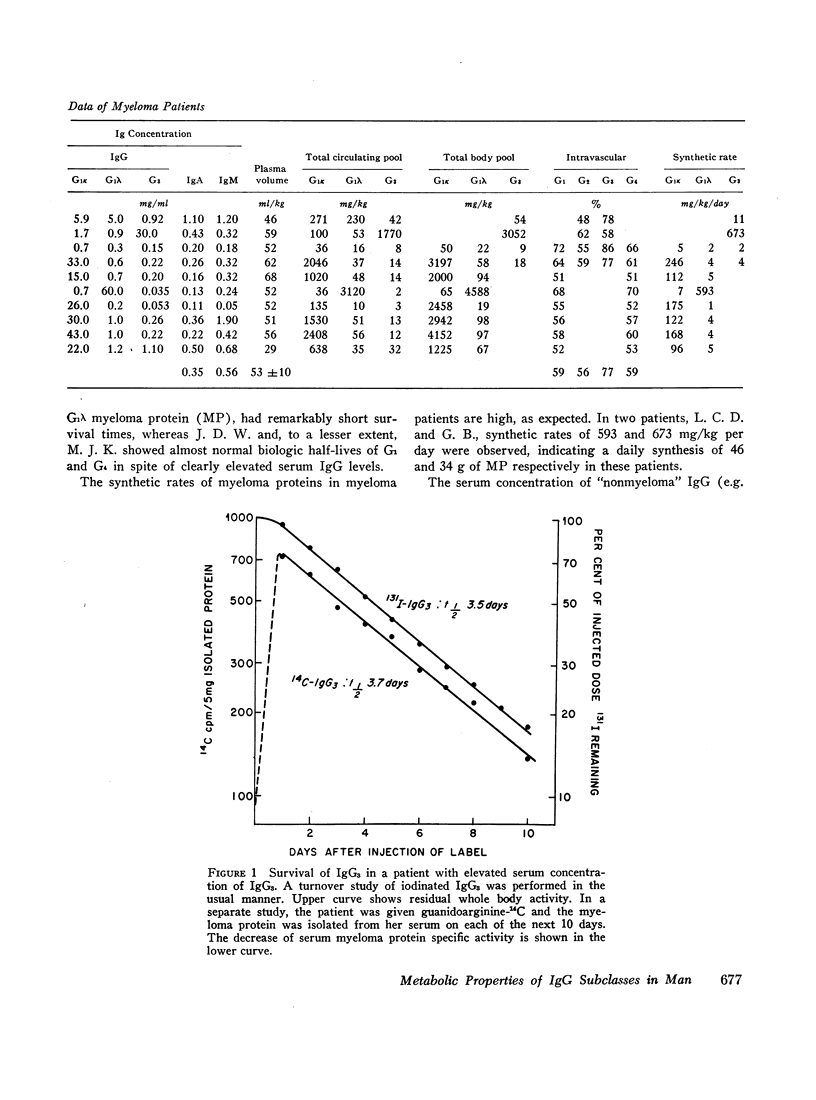
The short survival and high fractional catabolic rate of G3 is an inherent property of these molecules, and is not due to denaturation during isolation and radiolabeling. This was demonstrated by studies of a patient with a serum G3-myeloma protein. The survival of her own protein, separately labeled either in vivo with guanidoarginine-14C or in vitro with 125I, was determined in the patient. Survivals of the in vivo and in vitro labeled proteins were identical.
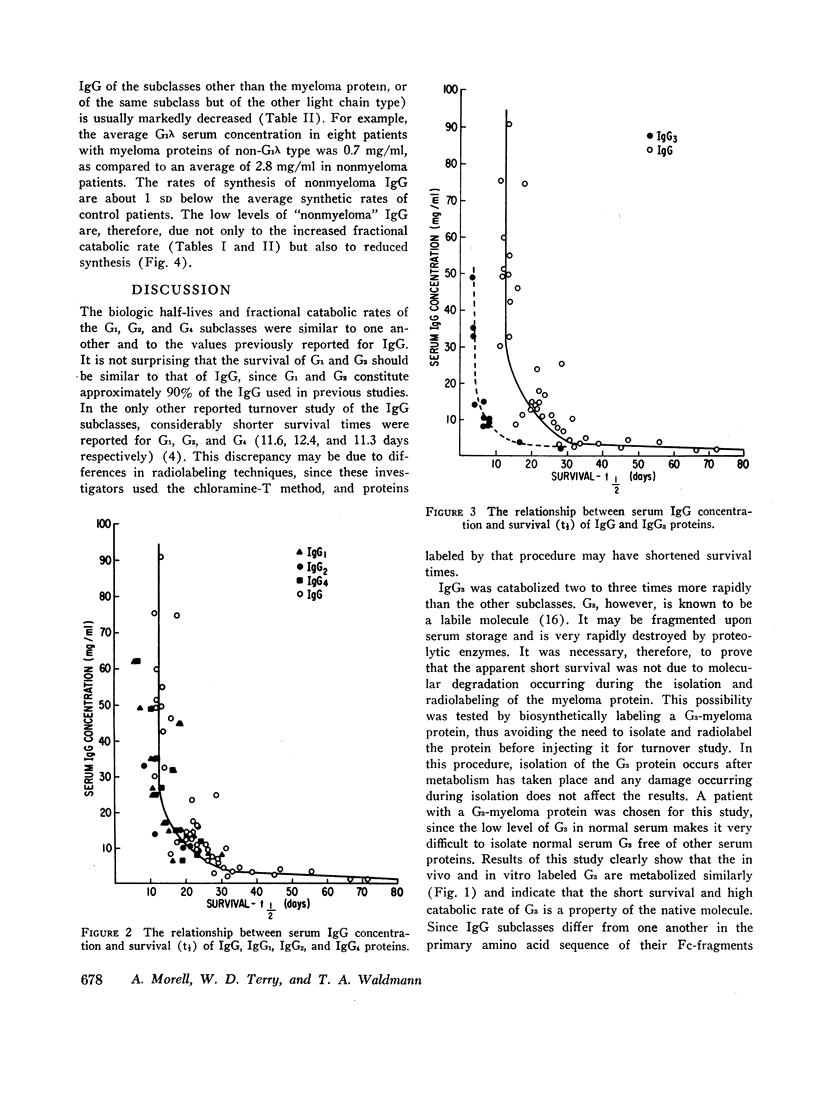
G1 and G3 serum concentrations and synthetic rates were determined. The mean serum concentration of G1 was 6.8 mg/ml and that of G3 was 0.7 mg/ml, while their synthetic rates were 25.4 and 3.4 mg/kg per day respectively. The low serum concentration of IgG2 thus results from a combination of high catabolic and low synthetic rates.
Studies in 10 patients with multiple myeloma showed that an elevated serum concentration of any IgG subclass was associated with shortened biologic half-life and increased fractional catabolic rate of all subclasses. The implications of this concentration-catabolism relationship are discussed. The serum concentration of nonmyeloma IgG was usually low in myeloma patients and the synthesis of nonmyeloma IgG was somewhat decreased, suggesting that low serum concentrations of nonmyeloma IgG result from decreased synthesis, as well as from an increased fractional catabolic rate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKE G., LILJEDAHL S. O., OLHAGEN B., PLANTIN L. O., AHLINDER S. Catabolism and distribution of gamma-globulin. A preliminary study with 131 I-labelled gammaglobulin. Acta Med Scand. 1963 May;173:589–603. [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., MORRIS I. G. A THEORETICAL MODEL OF GAMMA-GLOBULIN CATABOLISM. Nature. 1964 Sep 26;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Ballieux R. E., Tominaga K. T., Putnam F. W. Heavy chain subclasses of human gamma G-globulin. Serum distribution and cellular localization. J Exp Med. 1967 Feb 1;125(2):303–316. doi: 10.1084/jem.125.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- FAHEY J. L., ROBINSON A. G. FACTORS CONTROLLING SERUM GAMMA-GLOBULIN CONCENTRATION. J Exp Med. 1963 Nov 1;118:845–868. doi: 10.1084/jem.118.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., SCOGGINS R., UTZ J. P., SZWED C. F. INFECTION, ANTIBODY RESPONSE AND GAMMA GLOBULIN COMPONENTS IN MULTIPLE MYELOMA AND MACROGLOBULINEMIA. Am J Med. 1963 Nov;35:698–707. doi: 10.1016/0002-9343(63)90140-2. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Pink J. R. Structural studies of immunoglobulin G. Nature. 1969 Jan 11;221(5176):145–148. doi: 10.1038/221145a0. [DOI] [PubMed] [Google Scholar]
- LIPPINCOTT S. W., KORMAN S., FONG C., STICKLEY E., WOLINS W., HUGHES W. L. Turnover of labeled normal gamma globulin in multiple myeloma. J Clin Invest. 1960 Apr;39:565–572. doi: 10.1172/JCI104069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mann D., Granger H., Fahey J. L. Use of insoluble antibody for quantitative determination of small amounts of immunoglobulin. J Immunol. 1969 Mar;102(3):618–624. [PubMed] [Google Scholar]
- McKelvey E. M., Fahey J. L. Immunoglobulin changes in disease: quantitation on the basis of heavy polypeptide chains, IgG (gammaG), IgA (gammaA), and IgM (gammaM), and of light polypeptide chains, type K (I) and type L (II). J Clin Invest. 1965 Nov;44(11):1778–1787. doi: 10.1172/JCI105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W. The C-terminal sequences of the heavy chains of human immunoglobulin G myeloma proteins of differing isotopes and allotypes. Biochem J. 1967 Dec;105(3):1019–1028. doi: 10.1042/bj1051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL S., FAHEY J. L. RELATIONSHIP BETWEEN GAMMA-GLOBULIN METABOLISM AND LOW SERUM GAMMA-GLOBULIN IN GERMFREE MICE. J Immunol. 1964 Jul;93:81–87. [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L. Clinical and experimental metabolism of normal 6.6s gamma-globulin in normal subjects and in patients with macroglobulinemia and multiple myeloma. J Lab Clin Med. 1963 Jul;62:1–17. [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Fishkin B. G., Grey H. M. Catabolism of human gammaG-immunoglobulins of different heavy chain subclasses. I. Catabolism of gammaG-myeloma proteins in man. J Clin Invest. 1968 Oct;47(10):2323–2330. doi: 10.1172/JCI105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Grey H. M. Catabolism of human gamma-G immunoglobulins of different heavy chain subclasses. II. Catabolism of gamma-G myeloma proteins in heterologous species. J Immunol. 1968 Oct;101(4):711–716. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Kunkel H. G., Litwin S. D. Studies of the Vi (gamma-2c) subgroup of gamma-globulin. A relationship between concentration and genetic type among normal individuals. J Exp Med. 1967 Jan 1;125(1):177–190. doi: 10.1084/jem.125.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]



