Abstract
PEPFAR, national governments, and other stakeholders are investing unprecedented resources to provide HIV treatment in developing countries. This study reports empirical data on costs and cost trends in a large sample of HIV treatment sites.
In 2006–2007, we conducted cost analyses at 43 PEPFAR-supported outpatient clinics providing free comprehensive HIV treatment in Botswana, Ethiopia, Nigeria, Uganda, and Vietnam.
We collected data on HIV treatment costs over consecutive 6-month periods from scale-up of dedicated HIV treatment services at each site. The study included all patients receiving HIV treatment and care at study sites (62,512 ART and 44,394 pre-ART patients). Outcomes were costs per-patient and total program costs, subdivided by major cost categories.
Median annual economic costs were $202 (2009 USD) for pre-ART patients and $880 for ART patients. Excluding ARVs, per-patient ART costs were $298. Care for newly initiated ART patients cost 15–20% more than for established patients. Per-patient costs dropped rapidly as sites matured, with per-patient ART costs dropping 46.8% between first and second 6-month periods after the beginning of scale-up, and an additional 29.5% the following year. PEPFAR provided 79.4% of funding for service delivery, and national governments provided 15.2%.
Treatment costs vary widely between sites, and high early costs drop rapidly as sites mature. Treatment costs vary between countries and respond to changes in ARV regimen costs and the package of services. While cost reductions may allow near-term program growth, programs need to weigh the trade-off between improving services for current patients and expanding coverage to new patients.
Keywords: AIDS, HIV, Antiretroviral Therapy, Cost, Economics, Developing Countries, Resource-Limited Settings
Introduction
In 2008 alone over US$13.7 billion was spent on global HIV control [1], yet less than half of those who might benefit from antiretroviral therapy (ART) currently receive treatment [2]. As HIV treatment programs pursue universal access goals [3], careful budgeting is needed to maintain access to quality services over the long term.
Resource needs projections for HIV treatment have been conducted at global [1,4] and country level [5,6], yet these efforts are hampered by a lack of data. Some data on service delivery costs have recently become available [7–12]. These studies generally adhere to methodological guidelines [13,14], yet differences in key areas—study perspective, inclusion/exclusion of administrative overheads, and treatment of capital investments—make comparisons difficult. Moreover, the studies report data from a single or limited number of sites. It is unclear to what extent the wide range in costs reported by these studies—$292–$2,830 (2009 USD) for ART and $131–$457 for non-ART care—is due to real differences in clinical practice, price differentials, or differences in costing methodology. A recent South African study [12] represents a promising exception to this trend. Using a standardized methodology in four sites, the investigators found a much tighter range of per-patient costs, from US$756 to $1,126 for the first 12 months on ART.
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) supports HIV control in a number of high-prevalence countries. The PEPFAR ART Costing Project was initiated with the objective of estimating HIV treatment costs in a sample of PEPFAR-supported countries and programs. Data were collected to estimate per-patient treatment costs for pre-ART and ART patients, how costs are distributed across sources of support, input types, and program activities, and how costs change as sites grow and mature. Economic theory suggests the possibility of falling per-patient costs due to economies of scale [15], and there is evidence of such a trend among HIV prevention programs [16]. Data on time-trends in HIV treatment costs are scarce [17], though one study has shown decreasing per-patient costs of HIV treatment over time [9]. As budgetary constraints increasingly limit program growth, the extent to which programs can reduce per-patient costs may determine whether universal access goals can be achieved.
Empirical data on per-patient treatment costs will also provide inputs for modeled analyses estimating costs and cost-effectiveness of competing programmatic approaches [18–22], providing a link between the minimal costing data collected through routine reporting systems and the intensive data requirements of cost-effectiveness analyses [23]. This paper reports the data on HIV treatment costs collected by the PEPFAR ART Costing Project.
Methods
Population and Setting
The study included 45 HIV treatment sites across five countries: Botswana, Ethiopia, Nigeria, Uganda and Vietnam, chosen to reflect the range of the PEPFAR country programs. Sites were out-patient clinics providing free treatment for HIV-infected individuals and receiving direct or indirect PEPFAR support. In each country a sample of nine sites was purposively selected by local treatment program experts to reflect the range of publicly-funded outpatient HIV treatment sites in the country, considering location, program size (number of ART patients), and type of administration. The sample was restricted to sites that had been operating for >12 months in order to reveal time trends, however some younger sites (n=3) were included in the sample when necessary to better reflect current service delivery for a particular country. Of the original 45-site sample, two sites (in Uganda) were subsequently excluded from final analyses due to lack of adequate patient volume data. Final analyses included all patients receiving HIV treatment at 43 study sites. By the end of the evaluation, a total of 106,906 individuals were currently receiving HIV treatment through the study sites, comprising 62,512 patients receiving ART and 44,394 receiving pre-ART care.
Intervention
Comprehensive HIV treatment is comprised of antiretroviral therapy and supportive care. The service mix included multiple discrete health interventions and was expected to vary across programs. Two main patient types were identified: ART and pre-ART patients. In general, ART patients received a standardized antiretroviral (ARV) regimen and regular clinical and laboratory monitoring to assess treatment response. Patients transitioned to alternate first- or second-line ARV regimens, as indicated by treatment failure or adverse reactions, or based on drug availability. Supportive care could include prophylaxis and treatment of opportunistic infections and other conditions; nutritional support; adherence interventions; and other clinic- or community-based health interventions. Pre-ART patients generally received supportive care similar to ART patients, as well as regular clinical and laboratory monitoring, though potentially at a different frequency than ART patients. Pre-ART patients transitioned onto ART according to disease progression and site capacity for additional ART patients. ART patient costs were subdivided according to whether patients were adult (>15 years old) or pediatric (0–15 years old), and whether they were newly initiated (≤6 months on ART) or established (>6 months on ART).
Perspective and Costing Methods
The study adopted a programmatic perspective, considering all site-level costs of outpatient ART and supportive care. In addition to direct service provision costs, the study included site administration and management costs, as these can contribute substantially to total costs [24]. Medical costs incurred offsite were excluded, as were patient time and travel costs and higher-level program costs incurred by central government and donor management.
Analyses calculated both economic costs and financial costs. Economic costs approximate the opportunity cost of resources devoted to an intervention, useful information for long-term resource allocation decisions. For economic costs, investments (renovation/construction, equipment, training, and ARV buffer stock) were annualized over their useful life (30 years for renovation/construction, five years for equipment, two years for training, and perpetuity for buffer stock) using a 3% discount rate [13,14]. Results were robust to changes in useful life and discount rate values. Financial costs provide information on ‘real-time’ expenditures, with the cost of each investment included in the time period when the expenditure occurred, and are useful for shorter-term fiscal planning. In both economic and financial cost analyses, donated resources were valued at market prices, to capture the opportunity cost of all program contributions. For this reason the issue of donations and subsidies, usually considered part of the distinction between financial and economic costs, did not arise.
Overheads were allocated by direct allocation [13], and the opportunity cost of existing infrastructure was estimated as the equivalent rental cost. ARV buffer stock costs were calculated from the average number of months of ARV drugs in stock held (typically 6–12 months per ART patient), growth in ART patient volume, regimens distributions, and prevailing ARV prices.
Cost data were labeled using three categorizations. ‘Input Type’ categories comprised recurrent costs, including personnel, dispensed ARV drugs, other drugs, laboratory supplies, other supplies, building use, utilities, travel, and contracted services (such as contracted security); as well as investments, including renovation/construction, equipment, training, and ARV buffer stock. ‘Programmatic Activity’ categories included clinical care, laboratory services, training and supervision, supply chain management, M&E (monitoring and evaluation) and HMIS (health management information systems), and general administration/operations. ‘Source of Support’ categories included PEPFAR, national government, and other sources. As the study identified sources of funding at the site-level, it was not possible to identify donor funding channeled through government budgets (including Global Fund or World Bank funding). This funding is included in the government category.
Data Collection
Data were collected at each site and its supporting organizations (i.e., training institutions, procurement agents) between April 2006 and March 2007. Data were collected on all services that met three criteria: (a) the service was primarily a health intervention, (b) the primary recipient of the service was the HIV-infected individual, and (c) the service was administered by the site. Data were collected through retrospective record review, including accounting records, prescribing logs, equipment inventories, and routine reports. Key informant interviews were conducted to identify program activities to which resources were devoted and develop a comprehensive description of HIV treatment at the site. In Botswana, data on ARV usage could not be validated, and for this reason results reporting ARV costs exclude Botswana sites.
Data were organized into 6-month periods, starting from the scale-up of dedicated HIV treatment services at each site. Cost data were collected in original currency, converted to US dollars using prevailing inter-bank exchange rates, and inflated to constant 2009 dollars using the medical care component of the U.S. consumer price index. Routine reporting data were used to calculate the total patient-years of treatment by patient type and time period, then combined with cost data to estimate annualized per-patient costs. Unless specified, results are presented for the most recent 6-month period at each site. For time trends over multiple periods, results are calculated as the median per-patient costs (Figure 2) or total costs (Figure 3) across all sites at the start of the evaluation, adjusted for the average percentage change in costs between each subsequent period. The duration for which data were available varied by site, from 6 to 36 months (median = 20 months). The percentage change in cost between periods was calculated for all sites with data available in that period. Analyses were conducted using Stata SE 9™ (StataCorp, College Station, TX).
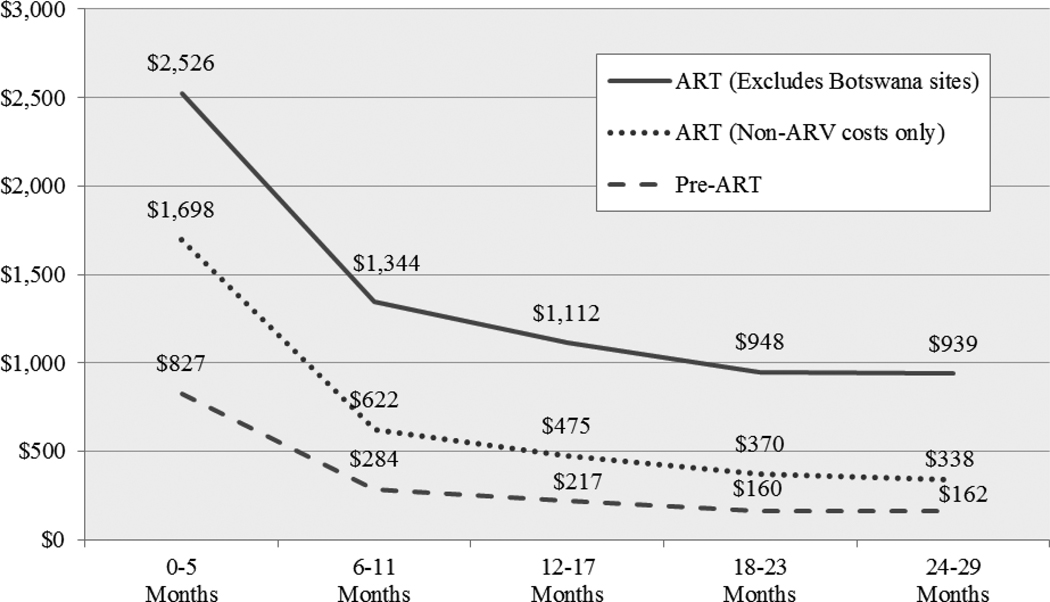
Figure Two.
Change in Median Per-Patient Financial Costs in Successive 6-Month Periods, from Start of HIV Treatment Scale-Up in Each Site through 2006–07 (2009 USD)
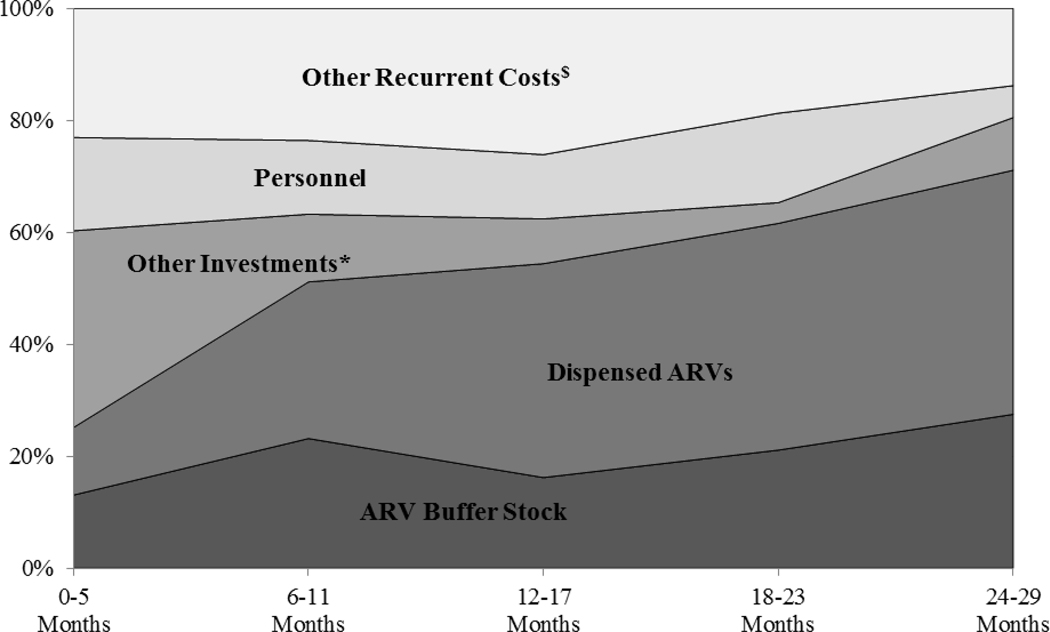
Figure Three.
Distribution of Total Financial Costs in Successive 6-Month Periods, from Start of HIV Treatment Scale-Up in Each Site through 2006–07δ.
* Other Investments include construction/renovation, equipment and training.
$ Other Recurrent Costs include non-ARV medications, laboratory supplies, other supplies, building rental, travel expenses, utilities and contracted services.
δ Figure excludes Botswana sites.
Results
Characteristics of HIV Treatment Sites and Patients
Of the 43 sites in the analysis, 7 were primary health centers, 15 were secondary centers, and 21 were tertiary sites. Thirty-six sites were government-run facilities and 7 were administered by non-profit organizations. Three sites were in rural areas, 9 in peri-urban areas, and 31 in urban areas. The mean number of patients per site was 680 on ART and 494 on pre-ART at the beginning of the evaluation, rising to 1,454 and 1,032, respectively by the end of the evaluation, with an mean scale-up rate of 39 ART and 28 pre-ART patients per site per month. Most sites had a majority adult population, with pediatric patients representing 7.1% of all ART patients. Two dedicated pediatric sites were included in the study, treating a mean of 95.7% pediatric patients. In total, the costing included 62,512 ART patients and 44,394 pre-ART patients by the end of the evaluation, representing 54,519 and 38,581 patient-years of ART and pre-ART treatment, respectively.
Total Costs Per Patient
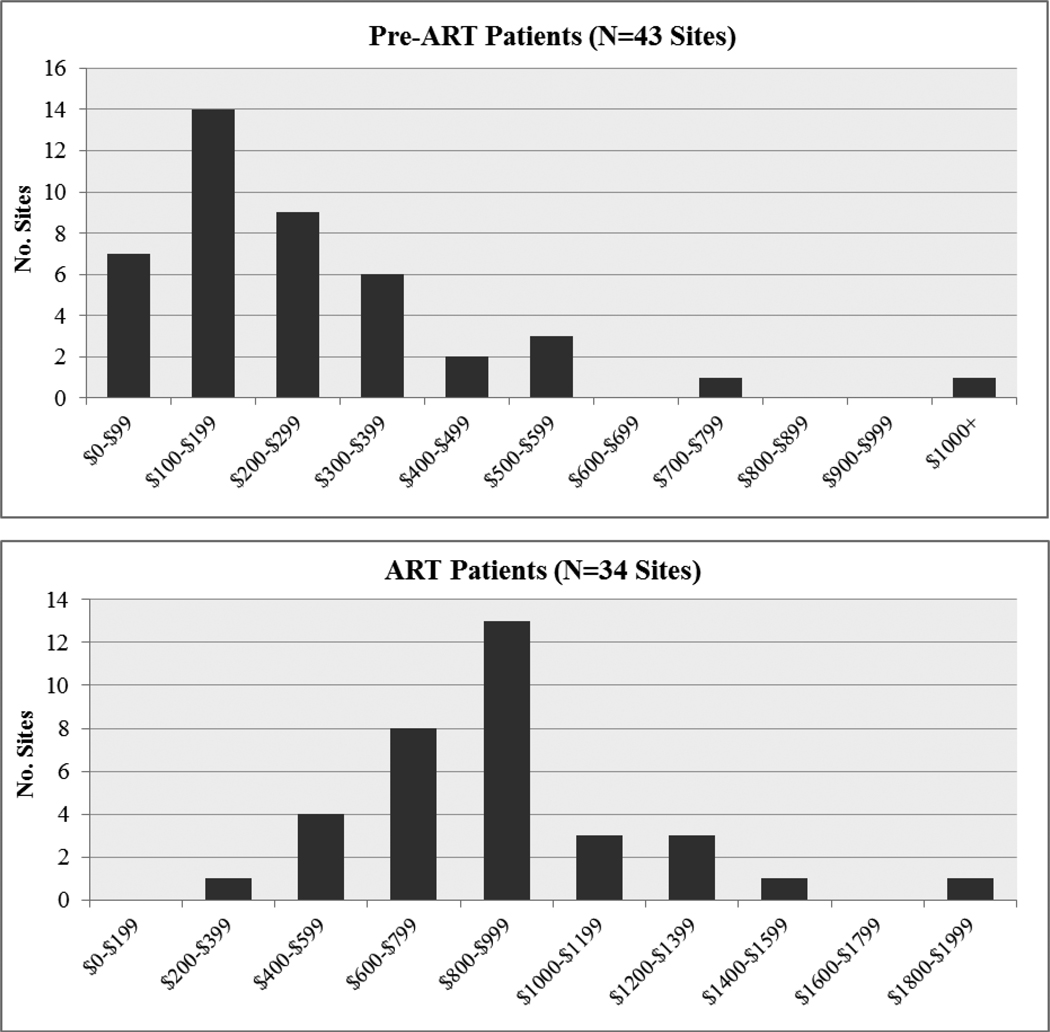
Table One presents summary data on total annual economic costs of HIV treatment for different patient types. ARVs represented the major contributor to total costs. ARV costs are sensitive to price levels and changes in preferred regimens, and for this reason results are reported inclusive and exclusive of ARV costs. Median annual costs were $202 for pre-ART patients and $880 for ART patients. Excluding ARVS, median annual costs for ART patients were $298 (or $287 excluding the Botswana sites). Countries with higher input prices (Nigeria, Botswana) tended to have higher per-patient costs. Annual per-patient costs varied widely between sites, especially when ARV costs are removed. The distribution of costs across sites was positively skewed (Figure One) with annual costs clustering around $100–$199 for pre-ART patients and $600–999 for ART patients, with a small number of sites reporting much higher costs. Per-patient costs varied across countries, and also between patient types, with newly initiated ART patients (first 6 months of ART) costing 15–20% more than established ART patients (or approximately 50% more when ARVs are excluded), due to more frequent clinical and laboratory follow-up during initial months on ART. Average per-patient costs were higher for pediatric patients compared to adults, though these patients generally represented a small fraction of total patients. In the two dedicated pediatric sites, the average annual economic cost per patient was $823 and $698 for newly initiated and established pediatric ART patients, respectively.
Table One.
Annualized Per-Patient Costs for each Patient Type, by Country and Overall in 2006–07 (Economic Costs, 2009 USD).
| Pre-ART | All ART | Newly Initiated Adult ART |
Established Adult ART |
Newly Initiated Pediatric ART |
Established Pediatric ART |
|
|---|---|---|---|---|---|---|
| Total Per Patient Costs, Including ARVs | ||||||
| BY COUNTRY (median): | ||||||
| Botswana* | $195 | ---- | ---- | ---- | ---- | ---- |
| Ethiopia | $150 | $682 | $781 | $643 | $1,011 | $982 |
| Nigeria | $259 | $988 | $969 | $861 | $1,771 | $1,564 |
| Uganda | $142 | $843 | $967 | $947 | $374 | $454 |
| Vietnam | $172 | $936 | $961 | $924 | $844 | $710 |
| OVERALL (excluding Botswana for ART results): | ||||||
| Median | $202 | $880 | $914 | $834 | $886 | $792 |
| Mean | $267 | $896 | $968 | $863 | $1,053 | $978 |
| Max | $1,466 | $1,979 | $2,007 | $1,949 | $3,121 | $3,088 |
| Min | $26 | $303 | $345 | $317 | $192 | $151 |
| Interquartile Range | $197 | $351 | $527 | $341 | $693 | $573 |
| Total Per Patient Costs, Excluding ARVs | ||||||
| BY COUNTRY (median): | ||||||
| Botswana | $195 | $360 | $732 | $335 | $732 | $343 |
| Ethiopia | $150 | $210 | $270 | $166 | $270 | $187 |
| Nigeria | $259 | $407 | $462 | $339 | $462 | $369 |
| Uganda | $142 | $185 | $202 | $182 | $202 | $186 |
| Vietnam | $172 | $280 | $278 | $141 | $242 | $200 |
| OVERALL: | ||||||
| Median | $202 | $298 | $361 | $235 | $369 | $252 |
| Mean | $268 | $382 | $508 | $325 | $521 | $334 |
| Max | $1,468 | $1,973 | $3,301 | $1,685 | $3,301 | $1,685 |
| Min | $26 | $40 | $61 | $35 | $66 | $35 |
| Interquartile Range | $197 | $249 | $377 | $216 | $409 | $274 |
Data on ARV costs for Botswana could not be validated; therefore cost estimates for ART that include ARV costs exclude Botswana data.
Figure One.
Distribution of Annualized Per-Patient Costs for ART and Pre-ART Patients Across HIV Treatment Sites in 2006–07 (Economic Costs, 2009 USD)*.
For both ART and Pre-ART patients, the high cost outlier was a site with comparatively low patient volume that was undergoing rapid expansion, having added 76% to its existing ART patient volume during the period.
*ART distribution graph excludes Botswana sites.
Distribution of Costs
Table Two shows the distribution of economic costs across input types and program activities, for both pre-ART and ART patients. Recurrent costs comprise the majority of all costs, 87.2% for pre-ART patients and 95.1% for ART patients. Personnel and laboratory supplies are the largest input type categories for pre-ART patients, while ARVs are the largest component for ART patients, followed by personnel and laboratory supplies. Contracted services were variable across sites and included a range of different activities, the most common of these being tests out-sourced to external laboratories.
Table Two.
Mean Distribution of Per-Patient Costs by Input Type, Programmatic Activity, and Source of Support in 2006–07 (Last Period Economic Costs)
| Pre-ART | ART* | |
|---|---|---|
| Distribution of Costs Across Input Types | ||
| Personnel | 31.1 % | 9.2 % |
| Dispensed ARV Drugs | N/A | 64.7 % |
| Other Drugs | 8.3 % | 3.3 % |
| Laboratory Supplies | 15.9 % | 5.8 % |
| Other Supplies | 5.7 % | 1.8 % |
| Building Use | 7.5 % | 4.5 % |
| Travel | 1.5 % | 0.5 % |
| Utilities | 4.4 % | 1.6 % |
| Contracted Services | 12.6 % | 3.7 % |
| ALL RECURRENT COSTS | 87.2 % | 95.1 % |
| Equipment | 7.3 % | 2.3 % |
| Training | 4.7 % | 1.4 % |
| Renovation/Construction | 0.8 % | 0.2 % |
| ARV Buffer Stock | N/A | 1.0 % |
| ALL INVESTMENTS | 12.8 % | 4.9 % |
| Distribution of Costs Across Programmatic Activities | ||
| Training and Supervision | 6.7 % | 1.7 % |
| Clinical Care (excl. ARVs) | 41.9 % | 17.3 % |
| Clinical Care (ARVs) | N/A | 60.6 % |
| Laboratory Services | 37.8 % | 11.9 % |
| Supply Chain Mgmt (excl. ARVs) | N/A | 4.0 % |
| Supply Chain Mgmt (ARVs) | N/A | 1.0 % |
| M&E and HMIS | 3.8 % | 0.7 % |
| General Admin/Operations | 9.8 % | 2.8 % |
Excludes Botswana sites.
The second part of Table Two shows the distribution of costs across program activities. Clinical care and laboratory services together represent the majority of all costs for both pre-ART and ART patients (79.7% and 90.7%, respectively), however other activities, taken together, represent non-trivial additions to total service delivery costs.
Time Trends in Per-Patient Costs
While earlier figures and tables presented economic costs, Figure Two presents financial costs (including the cost of donated resources), showing the time trends in average annual financial costs for ART and pre-ART patients from the start of program scale-up at each site. As the figure illustrates, per-patient costs drop rapidly over the first year, with a 65.6% reduction in per-patient costs for pre-ART patients and a 46.8% reduction for ART patients between first and second 6-month periods. Ongoing minor reductions are still evident after this first year, with pre-ART and ART per-patient costs dropping an average of 17.1% and 11.3%, respectively, in each successive 6 month period from months 6–11 to months 24–29. Excluding ARV costs, per-patient ART costs dropped 63.4% between first and second 6-month periods, and 18.4% in each successive six month period thereafter. Cost reductions were most pronounced for investment costs, which dropped by an average 61.8% and 37.8% in each successive 6-month period for pre-ART and ART patients respectively. Recurrent cost also declined with time, though at a lower rates than investments. This pattern—of large reductions in early periods followed by ongoing minor reductions in later periods—was seen in most individual cost categories, and was also observed when sites were disaggregated into primary, secondary and tertiary sites.
Total Site-level Costs
Although per-patient financial costs decreased over time, total site-level financial costs continued to rise due to rapidly growing patient populations. Total site financial costs averaged $712,564 in the first 6-months of scale-up, and increased by an average of 28.5% in each successive 6-month period. The distribution across cost categories also changed, as shown in Figure 3, with recurrent costs representing an increasingly larger share of total costs after the start-up phase. This was particularly true of ARV expenses, which grew from 25.2% of all spending in months 0–5 to 71.1% by months 24–29, with buffer stock expenditures representing a primary driver of financial costs due to the rapid scale-up of patient rolls.
In the four countries excluding Botswana, PEPFAR contributed an average of 79.4% of all site support, with national governments contributing 15.2%, and other funders the remaining 5.4%. It was not possible to calculate the distribution of costs across sources of support for Botswana given the difficulties confirming ARV costs, however, it is clear that Botswana is atypical amongst Sub-Saharan African countries, providing the large majority of HIV treatment funding in national-level assessments [25]. Funders contributed in different ways, with PEPFAR mainly supporting ARVs, equipment, and personnel (53.0%, 8.1%, and 7.7% of total PEPFAR support, respectively). In contrast, national governments mainly supporting personnel, buildings, and equipment (33.6%, 21.4%, and 17.2% of total national government support, respectively), and other donors mainly supported equipment, ARVs, and renovation/construction (19.1%, 16.0%, and 13.2% of total other support, respectively). It should be noted that, as the sample was limited to sites receiving PEPFAR support and higher-level central support costs were excluded, this breakdown across sources of support may not be representative of total national HIV treatment spending.
Discussion
This study provides a detailed description of HIV treatment costs at PEPFAR-supported sites. In particular, the study revealed progressive reductions in per-patient financial costs as sites matured. For investment expenditures, it would be expected that per-patient financial costs drop as sites mature—much of the site infrastructure and equipment must be present before patients are enrolled, and expansion in patient numbers must be preceded by an expansion of clinic capacity. Less apparent is why recurrent costs drop as sites mature, but a similar rationale—the need to develop capacity before bringing on additional patients—also applies to a number of recurrent costs, such as personnel. Additionally, programs likely experience economies of scale as patient numbers increase, and the accumulation of program experience may improve efficiency. Reductions in per-patient costs bode well for program financial sustainability, suggesting that financial resources needed to support programs over the long-term may be less than suggested by the expenditures required over the start-up period.
Another notable finding from this study is the wide range in per-patient costs between sites. While this variation may reflect price differentials and different stages of program development, differences in the package of services provided to patients may also be a factor. Taken as a whole, the health improvements provided by HIV treatment are well understood. Less well understood is the incremental value provided by individual components of the package of care. Given the varied service mix provided to patients, future program improvements may be possible through identifying and promoting the most cost-effective elements of the care package.
In focusing on programmatic costs, this study did not consider savings attributable to HIV treatment that result from less frequent illness and hospitalization. These cost-savings may be considerable, and the net costs of treatment programs would be lower if these costs-savings were considered [18]. The beneficiaries of reduced health care usage and greater personal productivity are the broader health care system and the patient, respectively. While these gains do not reduce the funding required for HIV treatment programs, they represent beneficial spillovers for the wider health system. Additionally, the study did not consider the costs of higher-level management and administration. While not part of service delivery, these activities are important for supporting site development and should not be ignored in resource-needs projections.
The field of HIV treatment is evolving rapidly. Annual per-patient ARV regimen costs, averaging $549 (USD 2009) per-patient in this study, were low compared to developed-world prices, but high compared to now prevailing ARV costs, and were the major contributor to HIV treatment costs in mature sites. Major ARV price reductions have occurred over recent years, with first-line regimen prices dropping by 15% per year on average from 2004–2009 [26,27]; at the same time, ART guideline revisions [28] have recommended transition to more expensive regimens, with Tenofovir preferred to Stavudine in adult 1st-line regimens. As a consequence, projections of future HIV treatment costs should consider the long-term trends in ARV prices together with shifts in preferred ARV regimens, in addition to the trends in service provision costs revealed by this study.
While cost reductions should allow for continued program growth in the near term, resource constraints may limit scale-up before universal access targets are reached. Programs need to weigh the trade-offs between focusing resources on improved regimens and services for current patients and extending coverage to those not yet receiving care.
Acknowledgements
The PEPFAR ART Costing Project is a PEPFAR-funded public health evaluation (PHE) study led by the U.S. Centers for Disease Control and Prevention and implemented with ICF-Macro, in collaboration with USAID. JMB, NAM, RB, SF, TVE and RF contributed to protocol development and provided study oversight. JMB, NAM, AAB, SF, and RB contributed to instrument design and fieldwork planning, and participated in fieldwork. AAB, NAM and JMB analyzed the data and drafted the manuscript. JMB, NAM, AAB, RB, SF, TVE and RF reviewed and edited the manuscript. NAM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would also like to acknowledge the time and effort of the PEPFAR country teams in Botswana, Ethiopia, Nigeria, Uganda and Vietnam, as well as collaborators and participants at health facilities and their supporting organizations. For more information on current PEPFAR evaluation activities, please refer to the website http://www.cdc.gov/globalaids/.
Funding sources: This study was conducted with funding from the United States President’s Emergency Plan for AIDS Relief.
Footnotes
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention or USAID.
References
- 1.UNAIDS. What countries need: investments needed for 2010 targets. Geneva: UNAIDS; 2009. [Google Scholar]
- 2.UNAIDS. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector: progress report 2009. Geneva: UNAIDS; 2009. [Google Scholar]
- 3.United Nations. Political declaration on HIV/AIDS: resolution adopted by the General Assembly 60/262. New York: United Nations General Assembly; 2006. [Google Scholar]
- 4.Cleary SM, McIntyre D, Boulle AM. Assessing efficiency and costs of scaling up HIV treatment. AIDS. 2008;22 Suppl 1:S35–S42. doi: 10.1097/01.aids.0000327621.24232.71. [DOI] [PubMed] [Google Scholar]
- 5.Quentin W, Konig H-H, Schmidt J-O, Kalk A. Recurrent costs of HIV/AIDS-related health services in Rwanda: implications for financing. Trop Med Int Health. 2008;13(10):1245–1256. doi: 10.1111/j.1365-3156.2008.02142.x. [DOI] [PubMed] [Google Scholar]
- 6.Over M, Revenga A, Masaki E, Peerapatanapokin W, Gold J, Tangcharoensathien V, et al. The economics of effective AIDS treatment in Thailand. AIDS. 2007;21 Suppl 4:S105–S116. doi: 10.1097/01.aids.0000279713.39675.1c. [DOI] [PubMed] [Google Scholar]
- 7.Bikilla AD, Jerene D, Robberstad B, Lindtjorn B. Cost estimates of HIV care and treatment with and without anti-retroviral therapy at Arba Minch Hospital in southern Ethiopia. Cost Effectiveness and Resource Allocation. 2009;7:6. doi: 10.1186/1478-7547-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harling G, Wood R. The evolving cost of HIV in South Africa: changes in health care cost with duration on antiretroviral therapy for public sector patients. J Acquir Immune Defic Syndr. 2007;45(3):348–354. doi: 10.1097/QAI.0b013e3180691115. [DOI] [PubMed] [Google Scholar]
- 9.Harling G, Bekker L-G, Wood R. Cost of a dedicated ART clinic. S Afr Med J. 2007;97:593–596. [PubMed] [Google Scholar]
- 10.Koenig SP, Riviere C, Leger P, Severe P, Atwood S, Fitzgerald DW, et al. The cost of antiretroviral therapy in Haiti. Cost Effectiveness and Resource Allocation. 2008;6:3. doi: 10.1186/1478-7547-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinson N, Mohapi L, Bakos D, Gray GE, McIntyre JA, Holmes CB. Costs of providing care for HIV-infected adults in an urban HIV clinic in Soweto, South Africa. J Acquir Immune Defic Syndr. 2009;50:327–330. doi: 10.1097/QAI.0b013e3181958546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Tropical Medicine and International Health. 2008;13(8):1005–1015. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 13.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of healthcare programmes: third edition. Oxford: Oxford University Press; 2005. [Google Scholar]
- 14.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Kumaranayake L. The economics of scaling up: cost estimation for HIV/AIDS interventions. AIDS. 2008;22 Suppl 1:S23–S33. doi: 10.1097/01.aids.0000327620.47103.1d. [DOI] [PubMed] [Google Scholar]
- 16.Marseille E, Dandona L, Marshall N. HIV prevention costs and program scale: data from the PANCEA project in five low and middle-income countries. BMC Health Services Research. 2007;7:108. doi: 10.1186/1472-6963-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns B, Tan Torres T. Costs of scaling up health interventions: a systematic review. Health Policy & Planning. 2005;20(1):1–13. doi: 10.1093/heapol/czi001. [DOI] [PubMed] [Google Scholar]
- 18.Badri M, Maartens G, Mandalia S, Bekker L-G, Penrod JR, Platt RW, et al. Cost-Effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med. 2006;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishai B, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21:1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 20.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings — the case of Cote d’Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 21.Wolf LL, Ricketts P, Freedberg KA, Williams-Roberts H, Hirschhorn LR, Allen-Ferdinand K, et al. The cost-effectiveness of antiretroviral therapy for treating HIV disease in the Caribbean. J Acquir Immune Defic Syndr. 2007;46:463–471. doi: 10.1097/qai.0b013e3181594c38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen S, Long L, Fox M, Sanne I. Cost and cost-effectiveness of switching from Stavudine to Tenofovir in first-line antiretroviral regimens in South Africa. J Acquir Immune Defic Syndr. 2008;48:334–344. doi: 10.1097/QAI.0b013e31817ae5ef. [DOI] [PubMed] [Google Scholar]
- 23.Beck EJ, Santas XM, DeLay PR. Why and how to monitor the cost and evaluate the cost-effectiveness of HIV services in countries. AIDS. 2008;22 Suppl 1:S75–S85. doi: 10.1097/01.aids.0000327626.77597.fa. [DOI] [PubMed] [Google Scholar]
- 24.Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions. Cost Effectiveness and Resource Allocation. 2003;1:1. doi: 10.1186/1478-7547-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNAIDS. Report on the global HIV/AIDS epidemic 2008. Geneva: UNAIDS; 2008. [Google Scholar]
- 26.WHO. Transaction prices for antiretroviral medicines and HIV diagnostics from 2004 to September 2008: a summary report from the global price reporting mechanism on antiretroviral drugs, October 2008. Geneva: WHO; 2008. [Google Scholar]
- 27.Holmes CB, Coggin W, Jamieson D, Mihm H, Granich R, Savio P, et al. Use of Generic Antiretroviral Agents and Cost Savings in PEPFAR Treatment Programs. JAMA. 2010;304(3):313–320. doi: 10.1001/jama.2010.993. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Rapid advice: antiretroviral therapy for HIV infection in aduts and adolescents. Geneva: WHO; 2009. [Google Scholar]