Abstract
Background
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) has supported the extension of HIV care and treatment to 2.4 million individuals by September 2009. With increasing resources targeted toward scale-up, it is important to understand the characteristics of current PEPFAR-supported HIV care and treatment sites.
Methods
Forty-five sites in Botswana, Ethiopia, Nigeria, Uganda, and Vietnam were sampled. Data were collected retrospectively from successive 6-month periods of site operations, through reviews of facility records and interviews with site personnel between April 2006 and March 2007. Facility size and scale-up rate, patient characteristics, staffing models, clinical and laboratory monitoring, and intervention mix were compared.
Results
Sites added a median of 293 patients per quarter. By the evaluation’s end, sites supported a median of 1,649 HIV patients, 922 of them receiving antiretroviral therapy (ART). Patients were predominantly adult (97.4%) and the majority (96.5%) were receiving regimens based on nonnucleoside reverse transcriptase inhibitors (NNRTIs). The ratios of physicians to patients dropped substantially as sites matured. ART patients were commonly seen monthly or quarterly for clinical and laboratory monitoring, with CD4 counts being taken at 6-month intervals. One-third of sites provided viral load testing. Cotrimoxazole prophylaxis was the most prevalent supportive service.
Conclusions
HIV treatment sites scaled up rapidly with the influx of resources and technical support through PEPFAR, providing complex health services to progressively expanding patient cohorts. Human resources are stretched thin, and delivery models and intervention mix differ widely between sites. Ongoing research is needed to identify best-practice service delivery models.
Keywords: HIV, Acquired Immunodeficiency Syndrome, Highly Active Antiretroviral Therapy, Workplace
INTRODUCTION
By 2008, the number of people living with HIV/AIDS reached 33.4 million worldwide, with two-thirds of them residing in sub-Saharan Africa.1 The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) represented a major commitment by the U.S. Government (USG) to undertake comprehensive, evidence-based action against the global HIV/AIDS epidemic. The first phase of this initiative was authorized in 2003 and set out ambitious 5-year goals of treating 2 million HIV-positive people with effective combination antiretroviral therapy (ART), caring for 10 million HIV-positive and HIV-affected persons, and preventing 7 million infections. The focus was on 15 priority countries, including 12 in sub-Saharan Africa.2 The number of patients receiving treatment in PEPFAR-supported programs in the 15 focus countries rose from 25,000 in July 2004,3 to 401,000 in September 2005.4 By September 2009, this number exceeded 2.4 million in more than 30 countries.5
In this study we collected data to describe the characteristics of care and treatment services in PEPFAR-supported ART programs. Data were collected as part of a larger study of HIV treatment costs. Existing costing studies about HIV care and treatment vary widely in their description of the intensity and comprehensiveness of services provided to patients receiving HIV therapy. Differences in enrollment criteria, preferred drug regimens, intensity of supportive care, and other clinical characteristics result in different resource needs and potentially influence variations in long-term clinical outcomes. Given the major resources that PEPFAR, local governments, and other international donors are devoting to HIV treatment, our study sought to obtain accurate information about the package of services being purchased and look for areas where programs differed in their approach to providing HIV care and treatment.6
METHODS
Study Population and Sample
We collected data from sites in five countries receiving PEPFAR support: Botswana, Ethiopia, Nigeria, Uganda, and Vietnam. Within each country, nine PEPFAR-supported outpatient HIV treatment clinics providing free ART and pre-ART care services were chosen for inclusion in the study. In each country the site sample was selected by local HIV treatment experts to reflect the range of publicly funded outpatient HIV treatment sites in the country which had been operating for >12 months, considering location, program size (number of ART patients), and type of administration.. Two sites in Uganda were subsequently excluded from the analysis due to insufficient quality data. Of the 43 sites in the analysis, 7 were primary health centers, 15 were secondary centers, and 21 were tertiary sites. Thirty-six sites were government-run facilities and 7 were administered by non-profit organizations. Three sites were in rural areas, 9 in peri-urban areas, and 31 in urban areas.
Data Collection
Data were collected through reviews of health facilities records and reports, data extraction from monitoring and evaluation systems and pharmacy records, and key informant interviews, to develop a comprehensive description of the structure and functioning of the HIV treatment program at each facility. The analysis considered all services that met three criteria: the service was primarily intended as a health intervention (thus excluding such activities as educational programs and income-generation activities); the primary recipient of the service was the HIV-positive individual (as opposed to the patient’s family or the general community); and the service was provided in or administered by the facility. Data were collected between April 2006 and March 2007.
Patient Types
Our study included all HIV-positive individuals enrolled in care or treatment programs at the sampled facilities. The range and frequency of services differed depending on patient characteristics. For example, until their treatment is stabilized, newly initiated ART patients may require more frequent clinical and laboratory monitoring than do established ART patients. To account for the differences, we collected data to characterize care delivery according to patient type. Results were reported separately for five different groups: pre-ART patients, newly initiated pediatric ART patients, established pediatric ART patients, newly initiated adult ART patients, and established adult ART patients. We defined ART patients as those receiving ongoing combination ART for HIV treatment (excluding those receiving antiretroviral drugs [ARVs] temporarily for prevention of mother-to-child HIV transmission) as well as other HIV care or support services provided by the clinics. Pre-ART patients were defined as those patients evaluated but not yet eligible for ART but receiving regular clinical or laboratory monitoring, or both, for disease progression as well as any other HIV care or support services provided by the clinics. Pediatric patients were those aged 0–15 years and adult patients were those aged > 15 years. Newly initiated patients were those in their first 6 months of ART, and established patients were those who had been receiving ART for > 6 months.
Assessment Periods
Our study assessed treatment programs over sequential 6-month periods to reveal changes in the size and composition of the patient population and staff. The key informant interviews conducted to characterize the treatment programs at the selected facilities were recorded for the last period for which this data were collected.
RESULTS
Scale-Up of HIV Treatment at Sites
Patients initiating ART were predominantly adults (97.4%). At most of the sites (37 of 43), pediatric patients represented less than 10% of the total ART patient population. However, for these 37 points of service, the proportion of pediatric ART patients rose over time, from a median of 0.00% of all ART patients 3 months after initiation of PEPFAR support to a median of 2.60% of all ART patients at the end of the evaluation. Two facilities (one in Uganda and one in Vietnam) primarily managed pediatric patients and another four facilities (two in Uganda, one in Botswana, and one in Vietnam) reported proportions of pediatric AIDS patients between 10% and 21% at the end of the last period.
Sites varied in terms of how long they had been providing ART. In some cases, ART only became available as a result of PEPFAR support, whereas other facilities had been providing ART for a number of years before the start of PEPFAR. In all cases, PEPFAR support resulted in rapid scale-up in clinic capacity and infrastructure. Scale-up was marked by expansion and renovation of clinic space, improvement of laboratory facilities, provision of HIV-specific clinical training, and establishment of dedicated administrative and reporting systems. These changes were followed by a rapid scale-up in patient volume, from a median of 241 pre-ART and ART patients per site at the end of the first 3 months of PEPFAR support to a median of 1,649 pre-ART and ART patients per site by the end of the evaluation (Table 1). We reported data for the end of the first quarter, rather than from the start of PEPFAR support, due to the possibility of initial data quality issues as reporting systems were being established.
TABLE 1.
Changes in patient volume at following rapid scale-up in clinic capacity and infrastructure, 43 sites. Patient volume increased from a median of 241 pre-ART and ART patients per site at the end of the first 3 months of the President’s Emergency Plan for AIDS Relief (PEPFAR) support to a median of 1,649 pre-ART and ART patients per site by the end of the evaluation.
| Number of patients at each site after the first 3 months of PEPFAR support:* | |
| Individuals receiving HIV care (ART+pre-ART) | Median (range): 241 (0–7,888) |
| Individuals receiving ART | Median (range): 117 (0–6,496) |
| Pediatric patients as a percentage of total ART patients | Median: (range): 0.0% (0.0% –100.0%) |
| Number of patients at each site at the end of evaluation: | |
| Individuals receiving HIV care (ART+pre-ART) | Median (range): 1,649 (119–9,899) |
| Individuals receiving ART | Median (range): 922 (17–9,315) |
| Pediatric patients as a percentage of total ART patients | Median: (range) 2.6% (0.0%–100.0%) |
| Average quarterly growth of patient volume at each site over evaluation (percentage): | |
| Individuals receiving care (ART+pre-ART) | Median (range): 43.1% (1.3%–345.3%) |
| Individuals receiving ART | Median (range): 55.8% (3.6%–289.6%) |
| Average quarterly growth of patient volume at each site over evaluation (absolute): | |
| Individuals receiving care (ART+pre-ART) | Median (range): 293 (26–1,261) |
| Individuals receiving ART | Median (range): 205 (16–776) |
Some facilities did not enroll patients during the first 3 month of PEPFAR.
Staffing Models
The 43 HIV clinics followed the staffing models adopted by their respective countries. Predominantly, these models focused on physicians providing the majority of pre-ART assessments, initial ART prescriptions, and ongoing follow-up, with other clinic staff playing supporting roles. While the models were similar across facilities, the ratio of physicians to patients (average number of full-time equivalent [FTE] physicians per 1,000 patients [ART and pre-ART]) varied widely among sites (0–25 physicians per 1,000 patients, with a median of 1.29 physicians per 1,000 patients at the end of the evaluation) (Table 2). The ratio changed substantially over the course of the evaluation, as physicians were responsible for progressively greater numbers of patients as sites grew and matured (median of 7.60 physicians per 1,000 patients in the first period compared with 1.29 in the last period). Similar trends were seen in the ratios of clinical staff (physicians, nonphysician clinicians, nurses, counselors, social workers, pharmacists, and other staff with clinical responsibilities) to patients, which declined from a median of 53.69 staff per 1,000 patients in the first period to a median of 11.61 staff by the last period of the evaluation.
TABLE 2.
Staffing models adopted by the 43 HIV clinics focusing on physician-driven care, with other clinic staff playing supporting roles. The ratios of physicians and clinicians to patients varied among sites and changed substantially over the course of the evaluation as patient loads progressively increased.
| Staffing ratios at the end of the first period per facility: | |
| Physician:patient | Median (range): 7.60 (0.12–88.00) |
| Clinical staff:patient | Median (range): 53.69 (0.53–450.98) |
| Staffing ratios at the end of the last period per facility: | |
| Physician:patient | Median (range): 1.29 (0–25.07) |
| Clinical staff:patient | Median (range): 11.61 (2.43–104.18) |
| Initial ART assessment and prescriptions per facility provided by: | |
| Physicians | 42 of 43 (97.7%) |
| Nurses | 1 of 43 (2.3%) |
| Follow-up HIV care and treatment provided by: | |
| Physicians | 40 of 43 (93.0%) |
| Nurses | 3 of 43 (7.0%) |
Average number of full-time equivalents (FTE) per 1,000 patients (ART and pre-ART).
Clinical and Laboratory Monitoring
The schedule of clinic visits for pre-ART patients varied across the 43 sites inclided in the analysis (Table 3). At all sites, clinical staff initially evaluated each new HIV-infected patient for disease status and ART eligibility. Patients who were not initiated on ART immediately were scheduled for return visits at regular intervals—commonly 3 months—for follow-up and repeat assessment. For patients who were initiated on ART, most sites asked them to return for assessment 2 weeks after initiation and then either monthly or two times in the first 6 months. The regularity of follow-up for established ART patients ranged continuing to see patients monthly to having patients come in every 2–4 months to the extreme of 6-month intervals between visits.
TABLE 3.
Number of facilities with scheduled clinic visits for pre-ART patients, newly initiated adult ART patients, and established adult ART patients. Scheduling frequency is indicated by percentage. Data were analyzed for 43 sites.
| Pre-ART patients: | |
| Every month | 11 of 43 (25.58%) |
| Every 3 months | 21 of 43 (48.8%) |
| Every 6 months | 6 of 43 (14.0%) |
| No routine schedule | 5 of 43 (11.6%) |
| Newly initiated adult ART patients* (first 6 months): | |
| At baseline, every month | 26 of 41 (63.4%) |
| At baseline, every 3 months | 14 of 41 (34.1%) |
| At baseline, every 6 months | 1 of 41 (2.4%) |
| Established adult ART patients* (after first 6 months): | |
| Every month (or more often) | 18 of 41 (43.9%) |
| Every 2 months | 4 of 41 (9.8%) |
| Every 3 months | 17 of 41 (41.5%) |
| Every 6 months | 2 of 41 (4.9%) |
One facility in Uganda and one facility in Vietnam are pediatric facilities.
All of the sites conducted regular CD4 count tests to assess the eligibility of pre-ART patients for ART (Table 4). For most patients on ART, repeat CD4 counts were performed either quarterly or every 6 months to assess immunologic recovery and, in many cases, as a proxy measure for treatment success. Viral load testing was only available at all nine facilities in Botswana, three of the nine health facilities in Nigeria, and two of the seven facilities in Uganda. Viral load tests, where available, were performed routinely at baseline and either quarterly or every 6 months. Blood chemistry and hematology tests to identify side effects and monitor treatment were performed at the majority of facilities and often followed the pattern of outpatient follow-up visits.
TABLE 4.
Number of facilities providing for laboratory monitoring of HIV patients and scheduled frequency, by patient type, at 43 sites for which data were analyzed. Services included CD4 count testing, viral load testing, and blood and hematology testing.
| Scheduled CD4 count tests: | |
| Pre-ART patients | |
| Every 3 months | 14 of 43 (32.6%) |
| Every 6 months | 19 of 43 (44.2%) |
| Baseline only, no routine testing | 10 of 43 (23.3%) |
| Newly initiated adult ART patients* (first 6 months): | |
| At baseline, every 3 months | 24 of 41 (58.5%) |
| At baseline, every 6 months | 14 of 41 (34.2%) |
| Baseline only, no routine testing | 3 of 41 (7.3%) |
| Established adult ART patients* (after first 6 months): | |
| Every 3 months | 10 of 41 (24.4%) |
| Every 6 months | 26 of 41 (63.4%) |
| Annually | 1 of 41 (2.4%) |
| Nonroutine testing | 4 of 41 (9.8%) |
| Scheduled blood chemistry and hematology tests: | |
| Pre-ART patients | |
| Every 3 months | 7 of 43 (16.3%) |
| Every 6 months | 13 of 43 (30.2%) |
| At baseline only | 14 of 43 (32.6%) |
| Never | 5 of 43 (11.6%) |
| Nonroutine testing | 4 of 43 (9.3%) |
| Newly initiated adult ART patients* (first 6 months) | |
| At baseline, every 3 months | 25 of 41 (61.0%) |
| At baseline, every 6 months | 11 of 41 (26.8%) |
| At baseline only | 4 of 41 (9.8%) |
| Nonroutine testing | 1 of 41 (2.4%) |
| Established adult ART patients* (after first 6 mo.) | |
| Every 3 months | 14 of 41 (34.2%) |
| Every 6 months | 20 of 41 (48.8%) |
| Never | 2 of 41 (4.9%) |
| Nonroutine testing | 5 of 41 (12.2%) |
| Perform viral load tests: | 14 of 43 (32.6%) |
One facility in Uganda and one facility in Vietnam are pediatric facilities.
Range of Supportive Care Services Provided
Sites differed in terms of the supportive care services provided to ART and pre-ART patients (Table 5). Cotrimoxazole prophylaxis was part of the care package at all but one of the facilities. Most facilities investigated whether patients had active tuberculosis (TB), a majority had TB treatment available onsite (31 of 43), and those that did not referred patients to a separate facility for TB treatment. While most facilities had the ability to manage basic opportunistic infections (such as common clinical presentations of pneumonia or diarrhea), six of 43 reported being unable to treat oral candidiasis. Community-based activities to support ART adherence and retention, which commonly involved clinic staff or volunteers tracking patients in the community when they missed an appointment, were present in 23 of 43 facilities.
Table 5.
Number and type of supportive care services provided to ART and pre-ART patients at 43 sites for which data were analyzed. Cotrimoxazole prophylaxis was provided by the greatest number of facilities. Nearly three-fourths of the sites offered TB treatment onsite, and slightly over one-half had programs in place to follow up patients in the community.
| Follow-up of patients in the community | 23 of 43 (53.5%) |
| TB treatment available on site | 31 of 43 (72.1%) |
| Oral candidiasis/thrush treated | 37 of 43 (86.0%) |
| Cotrimoxazole regularly provided | 42 of 43 (97.7%) |
| Psychosocial support regularly provided | 40 of 43 (93.0%) |
| Pain management regularly provided | 39 of 43 (90.7%) |
| End-of-life care provided | 23 of 43 (53.5%) |
Antiretroviral Drug Regimens
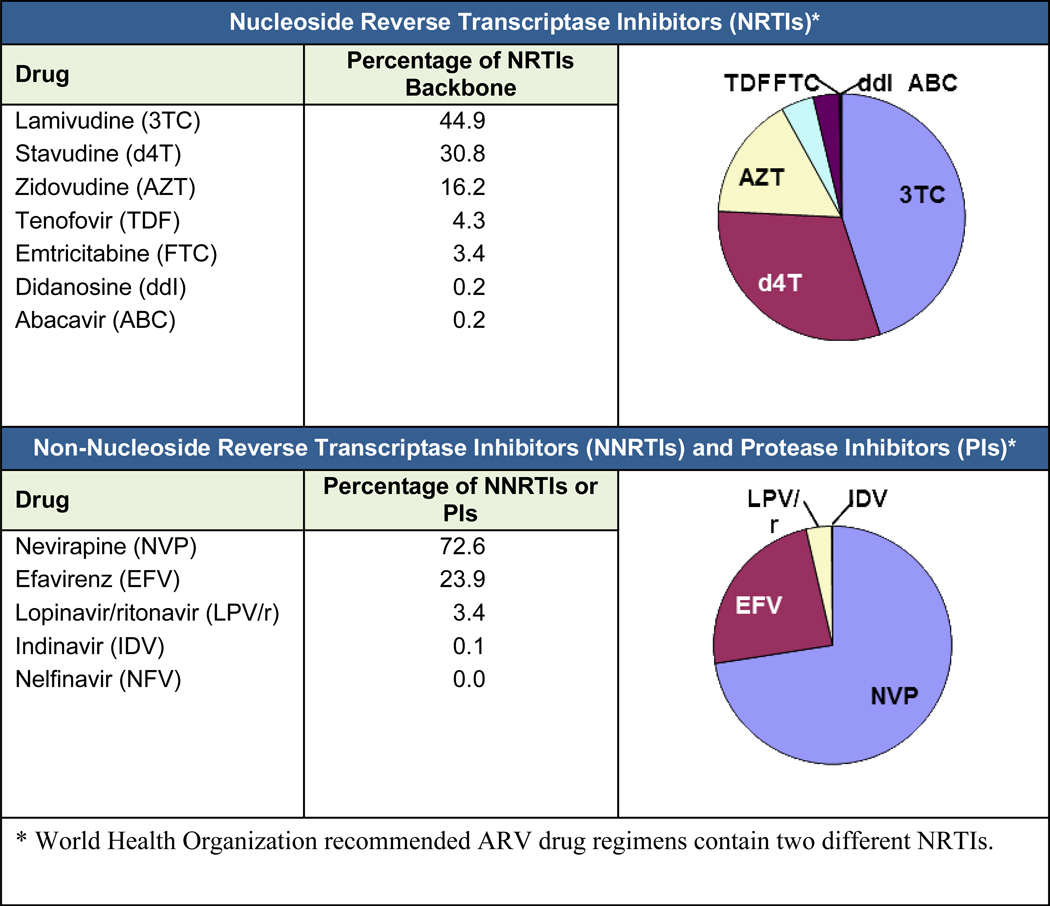
Information about the ARV drug regimens received by adult ART patients was obtained from the ARV dispensing records at study sites. In total, 96.5% of all adult ART patients were receiving a nonnucleoside reverse transcriptase inhibitor (NNRTI) ARV drug regimen during the last period of the evaluation. While individual regimens were not collected as part of the data exercise, it is possible to interpolate the choice of drugs from the distribution of ARVs within each class. The distribution of individual ARVs within each drug class is shown in (Fig. 1).
Figure 1.
Distribution of ARV drugs within each drug class (adult ART patients). Most patients received a nucleoside reverse transcriptase inhibitor (NRTI) backbone of stavudine (d4T) or zidovudine (AZT) in combination with lamivudine (3TC). Only a small percentage of patients received tenofovir (TNF). Most NRTI backbones were complemented by an nonnucleoside reverse transcriptase inhibitor (NNRTI), with nevirapine (NVP) being the most common. Protease inhibitors (PIs) in resource-limited settings are often reserved for second-line therapy and were used for only a small number of patients.
DISCUSSION
The data collected in this study provide a detailed description of the context in with HIV treatment clinic operate and characteristics of the service delivery models they have adopted. Over recent year the sites included in the study have scaled up rapidly with the influx of resources and technical support through PEPFAR and other partners, providing complex health services to progressively expanding patient cohorts.
Nevertheless, major barriers must be overcome to achieve universal access to ART. One of the main constraints is a shortage of health workers. The service delivery models described in this study placed emphasis on physicians providing initial ART assessments and follow-up. As countries decide to add new points of service or increase numbers of patients at existing sites, or both, reliance on a physician-driven model may be unfeasible. In this situation, task shifting—the reallocation of clinical tasks between cadres—is a potential alternative to conventional service delivery models and may provide the best approach for increasing human resources quickly enough to match the continuing scale-up of treatment programs.7
Of the study sites, less than one-third performed viral load testing. Current World Health Organization (WHO) guidelines in resource-limited settings recommend the use of CD4 counts to monitor therapy in programs where viral load testing is not available.8 Not monitoring viral loads raises concerns that CD4 counts can mislead clinicians into changing ARV drug regimens too early (CD4 count decline with suppressed viral load) or too late (CD4 count stable with persistent viremia).9 The optimal strategy for monitoring patients receiving ART needs further study, but our evaluation pointed out the variability in this clinical practice.
Our findings showed variations at sites in terms of the supportive care provided to patients. Certain key services—such as cotrimoxazole prophylaxis—had been adopted at all but one point of service. Other services are provided less commonly. Programs differed in their approaches on the ability to treat TB onsite and the ability to track patients in the community to support adherence and retention. While there are few data to support improved outcomes resulting from colocated TB and HIV services, it is likely that collocation would reduce patient attrition between different components of care. Slightly more than one-half of the sites had community-based activities to support ART adherence. The incremental cost of community-based activities needs to be evaluated to determine if the activities improve patient adherence, increase retention of ART patients, and ultimately improve outcomes.
When examining the ARV drugs that are being used across sites, the patterns clearly reflect the WHO guidelines for ART in resource-limited settings at the time of the study.10 Most patients were receiving a nucleoside reverse transcriptase inhibitor (NRTI) backbone of stavudine (d4T) or zidovudine (AZT) in combination with lamivudine (3TC). Only a small percentage of patients were receiving tenofovir (TNF). Most NRTI backbones were complemented by an NNRTI, with nevirapine (NVP) being the most common. Protease inhibitors, which in resource-limited settings are often reserved for second-line therapy, were used in only a small number of patients.
The study has limitations due the nature of its design. Because the sites were selected purposively from a subsample of countries, the sampled sites may not represent care and treatment services across the PEPFAR initiative. In addition, a subset of the clinical descriptors—such as staffing roles and clinical protocols—were captured only for the last 6-month periods and so we were unable to assess the trends in these characteristics as the treatment programs matured.
These data, collected in 2006–2007 represent a snap-shot of the initial phase of treatment program scale-up and maturation, allowing us to better understand the package of services provided at HIV treatment sites and the service delivery models used to provide them. To be successful, the ongoing efforts to increase ART access must overcome constraints in infrastructure and human and financial resources. The present study revealed the heterogeneity of program designs, highlighting the opportunity for further operational research to identify and promote best practices. Ultimately, through refining the package of services that patients receive and the methods used to provide them, strategies can be identified that maximize the effectiveness and efficiency of treatment programs, and in this way promote the universal access goals for treatment programs.
Acknowledgments
Supported by: U.S. Centers for Disease Control and Prevention, Atlanta, GA
REFERENCES
- 1.Report on the global AIDS epidemic. Geneva: UNAIDS; 2008. Joint United Nations Programme on HIV/AIDS. [Google Scholar]
- 2.Celebrating life: The U.S. President’s Emergency Plan for AIDS Relief: 2009 Report to Congress. Washington, DC: U.S. Department of State; 2009. President’s Emergency Plan for Aids Relief. [Google Scholar]
- 3.Office of the Global AIDS Coordinator. Bringing hope and saving lives: building sustainable HIV/AIDS treatment. Washington, DC: U.S. Department of State; 2004. [Google Scholar]
- 4.Office of the Global AIDS Coordinator. Focusing on Our Future: Prevention, Diagnosis, and Treatment of Pediatric HIV/AIDS. Washington, DC: U.S. Department of State; 2005. [Google Scholar]
- 5.Celebrating life: The U.S. President’s Emergency Plan for AIDS Relief: 2009 Report to Congress. Washington, DC: U.S. Department of State; 2009. President’s Emergency Plan for Aids Relief. [Google Scholar]
- 6.Levy AR, James D, Johnston KM, et al. The direct costs of HIV/AIDS care. Lancet Infect Dis. 2006;6:171–177. doi: 10.1016/S1473-3099(06)70413-3. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Task shifting: global recommendations and guidelines. Geneva: World Health Organization; 2007. [Accessed June 16, 2010]. Available at: http://data.unaids.org/pub/Manual/2007/ttr_taskshifting_en.pdf. [Google Scholar]
- 8.World Health Organization. [Accessed June 16, 2010];Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 Available at: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. [PubMed]
- 9.Moore DM, Mermin J, Awor A, et al. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43:436. doi: 10.1097/01.qai.0000243105.80393.42. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach. Geneva: World Health Organization; 2002. p. 165. [Google Scholar]