Summary
Segregation of homologous chromosomes at the first meiotic division (MI) is facilitated by crossovers and by a physical constraint imposed on sister kinetochores that allows them to make a monopolar attachment to the MI spindle. Recombination failure or premature separation of homologs results in univalent chromosomes at MI, and univalents constrained to form monopolar attachments should be inherently unstable and trigger the spindle assembly checkpoint (SAC) [1]. Although this appears to be the case in the male [2–5], the presence of one or several univalents does not cause cell cycle delay or arrest in the mammalian oocyte [6, 7]. The spindle assembly portion of the SAC appears to function normally in the oocyte [8–10], but two hypotheses have been proposed to explain the surprising lack of response to univalent chromosomes: 1) reduced stringency of the oocyte SAC to aberrant chromosome behavior [7], and 2) the ability of univalents to form bipolar attachments that satisfy SAC requirements [6]. Results of the present study of Mlh1 mutant mice demonstrate that metaphase alignment is not a prerequisite for anaphase onset and provide strong evidence that MI spindle stabilization and anaphase onset requires stable bipolar attachment of a critical mass - but, importantly, not all - chromosomes. We postulate that subtle differences in SAC-mediated control make the human oocyte inherently error-prone and provide a biological explanation for the high rate of aneuploidy in humans.
Results and Discussion
Meiotic errors in the human female represent the leading cause of pregnancy loss and congenital defects [11]. Most errors occur during the first meiotic division (MI), and their incidence is strongly influenced by maternal age [12]. Direct studies of human oocytes have revealed a striking age-related increase in chromosome alignment defects in human oocytes [13, 14]. Although it has been argued that such cells would be unable to initiate anaphase due to the actions of the SAC [15, 16], experimental studies in the mouse indicate otherwise. Specifically, alignment defects can be induced in a variety of ways, including mutations in meiotic genes (e.g., [4, 17]) or by changes that affect the endocrine environment in the ovary (e.g., [15, 18–21]) but meiosis proceeds, albeit with an increase in the occurrence of aneuploid eggs and embryos [19, 20].
Studies of the behavior of univalent chromosomes in the oocyte originally led to the suggestion that SAC-mediated control differs in the mammalian oocyte [7] and, in the intervening 13 years, debate about the oocyte SAC has continued. Although the presence of one or several univalents is tolerated in the oocyte [7, 22, 23], an excess number results in meiotic arrest. Specifically, on the C57BL/6J (“B6”) background, oocytes from Mlh1 mutant females exhibit defects in spindle assembly and fail to complete MI [17]. Meiotic recombination is virtually abolished in the absence of MLH1 protein, hence we postulated that MI spindle formation is disturbed by the presence of multiple univalent chromosomes that are unable to form bipolar attachments. Because the single X chromosome in XO females is better able to form a bipolar attachment and segregate equationally at MI in C3H/HeJ (“C3H”) than B6 females [24], we transferred the Mlh1 mutation to the C3H background to test the effect of genetic background on the meiotic behavior of multiple univalent chromosomes. Although the severe reduction in homologous recombination previously reported in the Mlh1 mutant [17] was also evident on the C3H background (Figure S1), the MI arrest phenotype was rescued and oocytes exhibited wildtype levels of polar body (PB) extrusion (Figure 1A).
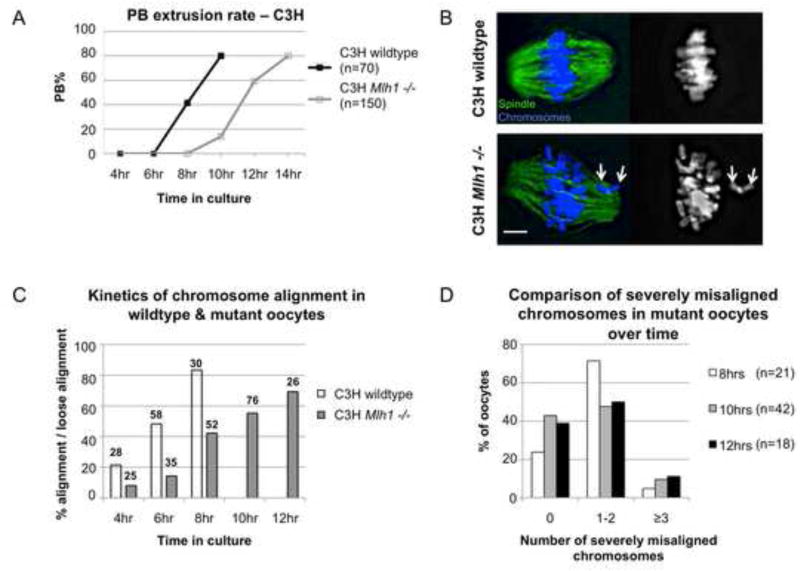
Figure 1. Oocytes from Mlh1 mutant females complete MI although a normal metaphase is not achieved.
(A) In contrast to the meiotic arrest phenotype reported for Mlh1 mutant females on the B6 background ([17] and see Figure S4), prophase arrested oocytes meiotically matured in vitro from C3H mutant females extruded a polar body (PB) at wildtype frequency. However, by comparison with wildtype siblings, polar body extrusion was delayed by 3–4 hours in mutants. (B–D) To assess chromosome alignment, groups of oocytes were fixed at successive stages of meiotic maturation (i.e., after 4, 6, 8, 10, and 12 hours in culture), immunostained with an antibody to α-tubulin (green) to detect the spindle, counterstained with DAPI (blue) to visualize the chromosomes, imaged and scored for chromosome alignment. Because a normal metaphase configuration was not observed in mutant oocytes, cells were scored as “aligned” if more than 80% of chromosomes were loosely aligned at the spindle equator. (B) Representative images of cells scored as “aligned” and “loosely aligned.” Left images show spindle and chromosomes, right images show chromosomes alone. Top panels: Wildtype oocyte showing tight metaphase alignment of all chromosomes. Bottom panels: Mutant oocyte showing loose alignment of most chromosomes, but severe misalignment of two univalents (arrows). Scale bar = 5μm. (C) An analysis of the percentage of cells exhibiting aligned (wildtype) or loosely aligned (mutant) chromosomes after 4, 6, 8, 10, and 12 hours in culture demonstrates a delay in chromosome alignment in mutant oocytes. Numbers above each bar represent sample sizes. Note that mutants on the B6 background exhibited no improvement in chromosome alignment over time (Figure S4B). (D) Although, on average, 3–5 chromosomes were misaligned in cells scored as loosely aligned, an analysis of the number of severely misaligned chromosomes (those at or behind the poles) revealed no significant difference in cells collected after 8, 10, or 12 hours in culture (χ2 = 3.74, p=0.44).
Metaphase alignment of all chromosomes is not a prerequisite for anaphase onset
To determine if rescue of the meiotic arrest phenotype was due to the improved ability of univalent chromosomes to form bipolar attachments, oocytes from C3H females were analyzed at successive stages of MI. In controls, normal metaphase alignment was achieved in over 80% of oocytes by 8 hours (Figure 1B,C). In mutants, normal metaphase alignment was almost never observed (2/214 oocytes) but, with increasing time, most cells achieved a “loose alignment” (ie., > 80% of chromosomes present at the spindle equator; Figure 1B,C).
The improved alignment of univalents suggests that, as in the XO female [24], functional separation of sister kinetochores is enhanced on the C3H genetic background. The delay in chromosome alignment by comparison with controls (Figure 1C) suggests that the ability to make bipolar attachments is acquired by univalents during early prometaphase. However, the persistence of misaligned chromosomes even after 12 hours suggests that, even on this “permissive” genetic background, not all univalents establish bipolar connections. To test this, we analyzed cells scored as loosely aligned in groups of oocytes collected after 8, 10, and 12 hours to determine if the number of severely misaligned chromosomes (i.e., those at or very near the spindle poles) diminished as cells approach anaphase (Figure 1D). The finding that misaligned chromosomes persist is consistent with timelapse studies of living cells, where clearly misaligned chromosomes were evident in the image immediately preceding anaphase in over half of the oocytes from mutant females (Figure S2). Taken together, these data indicate that anaphase onset does not require stable bipolar attachment of all chromosomes. Two lines of evidence suggest that this is not a defect unique to the C3H background but a feature of MI in the oocyte: First, oocytes from both mutant and wildtype females exposed to nocodazole during meiotic maturation displayed appropriate inhibition of anaphase onset (Figure S3), indicating that the SAC functions normally in C3H females and responds to spindle damage. Second, the ability to complete MI in the presence of multiple univalents is not unique to the C3H background. Although meiotic analyses were not conducted, oocytes from an induced mutation in a ubiquitin ligase that similarly reduces recombination levels can complete meiosis but arrest at the 2-cell stage [4].
Formation of a stable MI spindle is delayed by aberrant chromosome behavior
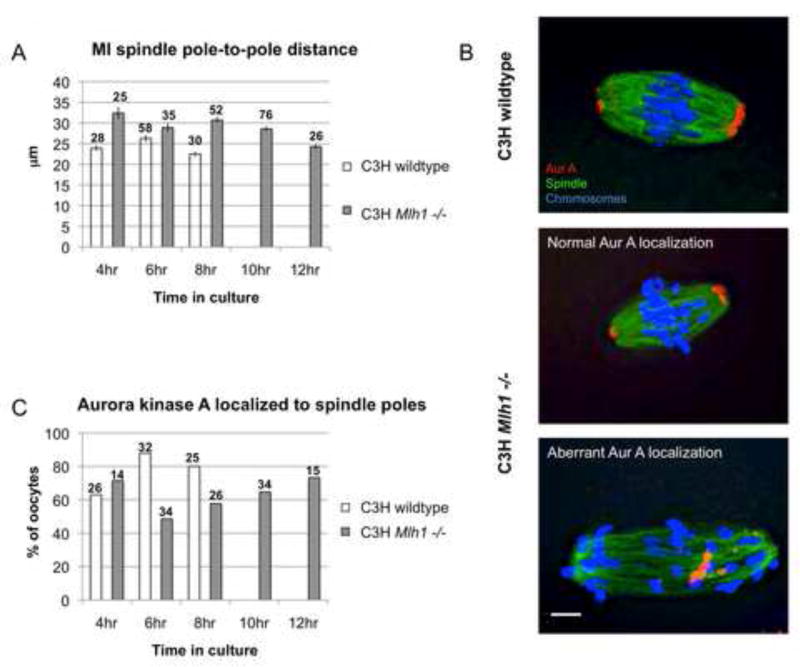
A striking phenotype of Mlh1 mutant oocytes on the B6 background is the continual elongation of the MI spindle over time in culture [17]. To assess MI spindle formation and stabilization in mutants on the C3H background, we first measured pole-to-pole spindle length in oocytes at successive stages of MI (Figure 2A). In controls, spindle length shortened as cells approached anaphase. Although the average spindle length was increased in mutants, a similar shortening occurred, and by the time of anaphase onset nearly wildtype spindle length was attained. Because Aurora kinase A localizes to the spindle poles during early prometaphase, we analyzed the temporal and spatial pattern of localization as an additional means of assessing spindle formation. By comparison with controls, the frequency of cells exhibiting aberrant Aurora A localization was increased in the mutant although, like spindle length, nearly wildtype levels of frequency of cells with normal Aurora A localization were attained by 12 hours (Figure 2B lower panels and 2C). Together these results suggest that the 2–3 hour delay in polar body extrusion in mutants (Figure 1A) reflects a delay in the formation of a stable MI spindle. Importantly, this suggests that chromosome alignment and spindle stabilization in the oocyte are interrelated, and that achievement of bipolar attachments by a critical mass of chromosomes is necessary for MI spindle stabilization.
Figure 2. MI spindle stabilization is delayed by aberrant chromosome behavior.
(A) A comparison of MI spindle length demonstrates significant changes over time in both controls (F=7.06; p<0.001) and mutants (F=8.65; p<0.001), with the shortest spindle lengths occurring around the time of anaphase onset (8 hrs in controls; 12 hrs in mutants). Although spindle length was consistently greater in mutants, at the time of anaphase onset, lengths were not significantly different between controls and mutants (t=1.98; p>0.05). Error bars denote s.e.m. and numbers above each bar represent sample sizes. (B) Examples of Aurora kinase A localization during metaphase in a wildtype oocyte (top panel) and in Mlh1 mutant oocytes with normal Aurora kinase A localization (middle panel) and aberrant localization (bottom panel). Oocytes were immunostained with antibodies to α-tubulin (green) and Aurora kinase A (red) and counterstained with DAPI (blue). Scale bar = 5μm. (C) The kinetics of Aurora kinase A localization to the spindle poles in mutants and controls. Numbers above each bar represent sample sizes. Note that in mutants on the B6 background, aberrant Aurora kinase A localization persisted. (Figure S4C, D)
Kinetochore morphology and chromosome segregation support the hypothesis that bipolar attachment of all chromosomes is not required for anaphase onset
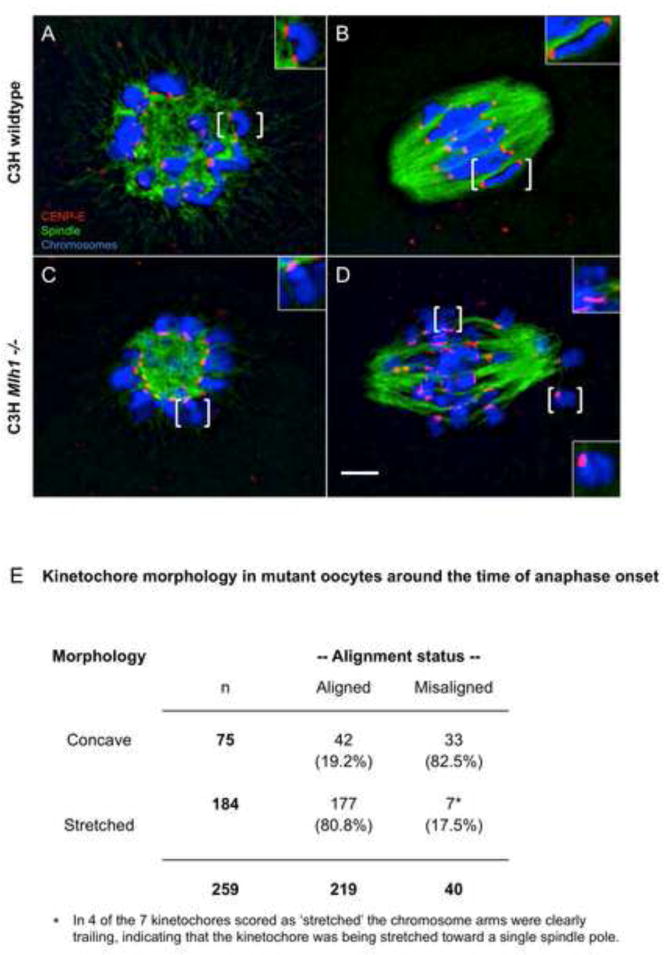
Because our results indicated that not all univalents formed stable bipolar attachments, we analyzed kinetochore morphology during MI. Oocytes were stained with an antibody to CENP-E, a kinesin-like motor protein that binds outer kinetochores and is necessary for chromosome alignment [25]. In controls, sister kinetochores exhibited a single concave-shaped CENP-E domain from early prometaphase I to metaphase I (Figure 3A,B). In mutants, the signal domain on univalents was indistinguishable from wildtype during early prometaphase (Figure 3C). However, in cells approaching anaphase onset, an additional pattern of CENP-E staining that correlated with alignment status became evident. Specifically a stretched CENP-E signal was evident on over 80% of univalents aligned at the spindle equator, whereas the concave morphology persisted on most misaligned chromosomes (Figure 3D,E).
Figure 3. Kinetochore morphology is correlated with univalent behavior.
Oocytes fixed at early prometaphase (A,C) and around the time of anaphase onset (8hrs in controls; 12 hrs in mutants) (B,D) from control and mutant females were immunostained with antibodies to α-tubulin (green) and CENP-E (red) to detect the spindle and kinetochores, respectively, and counterstained with DAPI (blue) to visualize the chromosomes. Brackets denote chromosomes shown in enlarged inset images. (A,C) In oocytes fixed during early prometaphase (i.e., after 4 hours in culture), the kinetochores of bivalents in wildtype oocytes (A) and of univalents in mutant oocytes (C) exhibited a single concave-shaped domain of CENP-E signal. (B) This morphology persisted in wildtype oocytes when kinetochores come under tension around the time of anaphase onset. (D) In mutant oocytes fixed around the time of anaphase onset, univalents aligned at the spindle equator typically exhibited a thin, stretched CENP-E signal. Scale bar = 5μm. Note that this morphology was not observed in mutant oocytes on the B6 background (Figure S4). (E) An analysis of 259 kinetochores on univalent chromosomes from 9 mutant oocytes collected around the time of anaphase onset demonstrates a highly significant correlation between alignment status and CENP-E morphology (χ2=65.9, p<0.001).
The stretching of CENP-E signals observed around the time of anaphase onset is consistent with the fact that kinetochores in the oocyte are not placed under tension until just prior to anaphase onset [26] and indicates that, although sister kinetochores have functionally differentiated (i.e., can form bipolar attachments) they remain physically connected. In maize, sister kinetochores are constrained at MI by a fused microtubule binding interface and, like the stretched CENP-E signal we observed, a stretched signal domain has been reported on sister kinetochores making bipolar attachments [27]. Thus, two lines of evidence suggest that the fused kinetochore domain on MI chromosomes does not have to separate into physically distinct kinetochore domains to support bipolar attachments.
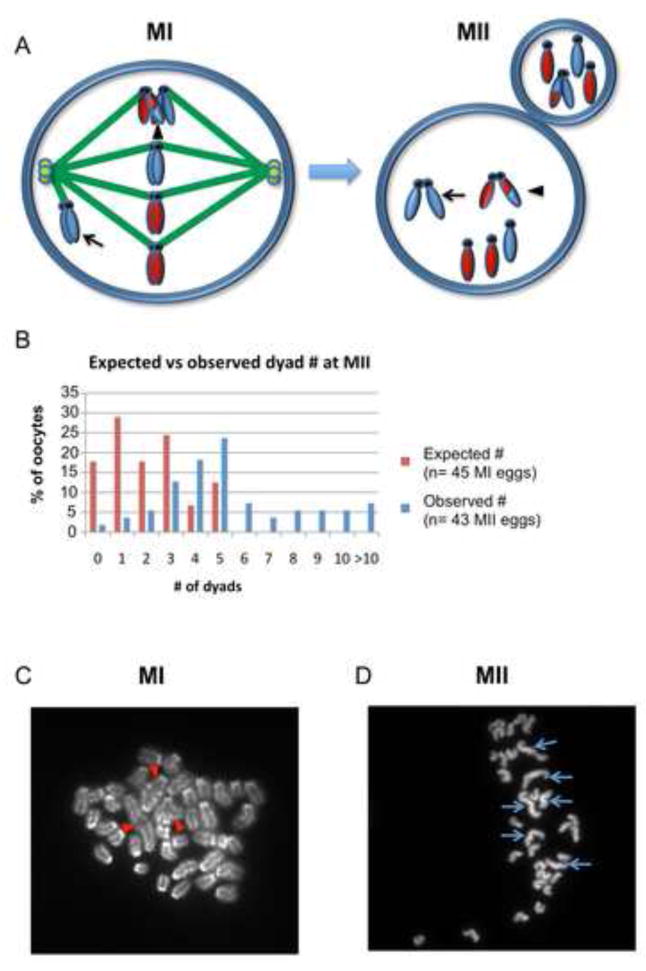
As shown schematically in Figure 4A, univalent chromosomes forming bipolar attachments and segregating equationally at MI should be present in MII arrested eggs as single chromatids (monads). In contrast, univalents forming monopolar attachments and segregating intact, as well as the few bivalents (on average, 1.9/oocyte; Figure S1) observed in mutants that presumably result from the actions of an alternate double stand break repair pathway [28] should be present as normal MII chromosomes (dyads). Assuming that all univalents form bipolar attachments and segregate equationally, mutant MII oocytes should contain, on average, 1.9 dyads (Figure 4B), but an analysis of 43 MII eggs from mutants revealed a three-fold excess, with an average of 5.9 dyads per oocyte (Figure 4B–D). This indicates that, on average, 4 univalents segregated intact. It is reasonable that some univalents forming bipolar attachments were unable to segregate their chromatids. However, failure to segregate should result in anaphase lagging and, although lagging chromosomes were a feature of mutant oocytes, their frequency in our live-cell studies was insufficient to explain the cytogenetic findings (Figure S2). Further, the correlation between our analysis of misaligned chromosomes (Figure 1D) and our cytogenetic studies is remarkable, suggesting that, on average, 4–5 univalents failed to establish a stable bipolar attachment and segregated intact at anaphase I.
Figure 4. MII chromosome analysis provides evidence of intact segregation of univalents.
(A) Schematic showing the segregation patterns that give rise to dyads and monads at MII. Homologs with an exchange (arrowhead, MI cell) will segregate reductionally, resulting in a dyad with genetically distinct chromatids (arrowhead, MII cell). In addition, a univalent that forms a monopolar attachment (arrow, MI cell) will segregate intact, resulting in a dyad with genetically identical sister chromatids (arrow, MII cell). Univalents that form bipolar attachments and segregate equationally will result in single chromatids (monads) at MII. (B) Comparison of the expected number of dyads with the number observed; expected values were based on data from the cytogenetic analysis of 45 MI oocytes (Figure S1) and assuming that dyads derive only from MI bivalents. (C) Representative image of an MI chromosome preparation showing 3 bivalents (red arrowheads) and 34 univalents. (D) Representative image of an MII chromosome preparation with 6 dyads (blue arrows) and 27 monads.
Conclusions
Taken together, our analyses of chromosome alignment, spindle formation and stabilization, kinetochore morphology and chromosome segregation suggest that, in the mammalian oocyte, anaphase can be initiated before all chromosomes establish stable bipolar attachments. Thus, in contrast to mitotic cells where the SAC prevents anaphase onset until all chromosomes form stable bipolar attachments [1, 29], the conditions necessary for the satisfaction of the checkpoint appear to differ in mammalian oocytes, with a requirement for stable attachments of a critical mass, but not all chromosomes.
Although stable end-on kinetochore attachments have been considered essential for chromosome alignment, recent studies suggest that, even in mitotic cells, lateral attachments are sufficient for chromosome congression [30]. In C.elegans, lateral attachments not only represent the major mechanism of chromosome alignment in oocytes, but conversion to end-on attachments does not appear to be required for anaphase onset [31, 32]. This novel perspective on chromosome congression in the oocyte is consistent with our findings and suggests that differences in kinetochore attachment may underlie the differences in SAC control that allow the oocyte to initiate anaphase even if all chromosomes have not formed stable bipolar attachments.
Because the production of genetically normal offspring requires accurate chromosome segregation, lack of sensitivity to detect aberrant chromosome behaviors that predispose to segregation errors seems counterintuitive. However, differences in SAC-mediated control provide a plausible explanation for the inherently high meiotic error rate in the human female and the influence of maternal age. The combined data from humans and mice indicate that a variety of factors conspire to create the human maternal age effect (reviewed in [12]). Data from studies in mice suggest that the age-related increase in meiotic errors may be driven, at least in part, by a reduction in sister chromatid cohesion during the period of protracted meiotic arrest [23, 33–35]. Other studies suggest that age-related changes in oocyte growth [20] or exposures to endocrine disrupting chemicals [15, 18, 19, 21] can affect chromosome alignment in the mammalian oocyte. Regardless of the reason for the disturbance, our results indicate that, if a threshold number of chromosomes make stable bipolar attachments, anaphase onset can occur despite the persistence of misaligned chromosomes - with aneuploidy as the unavoidable consequence. Thus, we postulate that the inability of the oocyte SAC to ensure stable bipolar attachment of all chromosomes prior to anaphase onset is a major determinant of human age-related aneuploidy.
Experimental Procedures
Mouse strains and production of Mlh1 mutants
Targeted disruption of the Mlh1 gene and analysis of mutant animals on the B6 background has been described previously [17, 36]. To assess the effect of genetic background on the mutant phenotype, the mutation was transferred to the C3H background by mating B6 carrier animals to C3H strain animals and successively backcrossing carrier offspring to C3H animals for over ten generations. The offspring from each backcross generation were genotyped by PCR using primers as described previously [36]. All animal experiments were approved by the WSU Institutional Animal Care and Use Committee. WSU is fully accredited by the American Association for Accreditation of Laboratory Animal Care, and all investigations were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [37].
Isolation and culture of oocytes from Mlh1 mutants and control siblings
For all studies, germinal vesicle (GV) stage oocytes were collected from 4–5 wk-old females. Ovaries were removed and placed in Waymouth’s MB752/1 medium (GIBCO, CA) supplemented with 10% fetal calf serum and 0.23mM sodium pyruvate as described previously [17]. After 2 hours in culture, oocytes were scored for GV breakdown, indicating resumption of MI, and any oocytes remaining at the GV stage were excluded from the experiment. Polar body extrusion was recorded every 2 hours after GV breakdown for a period 14 hours. For the analysis of checkpoint response, nocodazole (Sigma, MO) was added to the culture medium 4 hours after GV breakdown and oocytes were scored for polar body extrusion every 2 hours for 8 hours. Nocodazole was dissolved in DMSO and added to the culture medium to achieve a final concentration of 1μM; DMSO in Waymouth’s medium was used as a control.
Fixation, imaging, and analysis
For analysis of chromosome alignment and meiotic spindle formation during MI, oocytes were cultured for 4, 6, 8, 10, 12, and 14 hours before fixation. Oocytes were fixed in a microtubule stabilizing buffer (100mM PIPES, pH 7.2, 5mM MgCl2•6H2O, and 2.5mM EGTA) containing 2% formaldehyde, 0.1% Triton X-100, 0.01% aprotinin, 1mM dithilthreitol and 50% deuterium oxide [38]. Primary antibodies used in this study included mouse monoclonal (35C1) Aurora kinase A antibody (1:100, Sigma, MO), polyclonal CENP-E antibody (1:200, a gift from Don Cleveland, UC San Diego and Tim Yen, Fox Chase Cancer Center), and FITC-labeled mouse monoclonal (DM1A) α-tubulin antibody (1:150, Sigma, MO). Secondary antibodies used in this study included Rhodamine goat anti-mouse (1:200 Jackson ImmunoResearch, PA) and Rhodamine goat anti-rabbit (1:200, Jackson ImmunoResearch, PA). After staining, DAPI and antifade reagent (Invitrogen, CA) were applied to the slide to stain the chromosomes and diminish signal fading. To assess chromosome alignment in mutant oocytes, images were scored by two observers who were blinded with regard to experimental conditions. In mutants, oocytes in which most chromosomes were aligned at the spindle equator and 8 or fewer chromosomes were misaligned were scored as “loosely aligned.” Individual chromosomes in cells scored as loosely aligned were categorized as ‘severely misaligned’ if they were at, behind, or very close to the spindle pole. For CENP-E kinetochore staining, the morphology of the CENP-E signal at the kinetochores was recorded as “square/concave” or “stretched.” For pole-to-pole spindle measurements, oocytes with a collapsed spindle were excluded. All MI spindle images were deconvolved using Metamorph (Molecular Devices Inc. PA). For MII chromosome analysis, air-dried chromosome preparations were made of oocytes that had extruded a polar body as described previously [17].
Timelapse imaging
GV stage oocytes were collected as described above, incubated in Waymouth’s medium containing 1μg of Hoechst 34580 for 15 min to stain the chromosomes, and returned to medium without Hoechst for imaging. Oocytes were incubated at 37°C with 5% CO2 in air on a Nikon Biostation IM equipped with a DS-Qi1 monochrome camera (Nikon Inc., NY). Fluorescent and phase contrast images were captured every 20 – 30 min with a 20x/0.8NA dry objective lens and fluorescent excitation light was attenuated to 3% by neutral density filters.
Supplementary Material
Acknowledgments
We thank Gary Gorbsky, Terry Hassold and members of Hunt laboratory for their helpful suggestions on the manuscript and Don Cleveland and Tim Yen for generously providing antibodies. This work was supported by funds from NIH grants HD37502 (P.A.H) and T32GM08386 (S.I.N) and from the Hall Family Foundation and ESHE Fund (D.F.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 2.Eaker S, Cobb J, Pyle A, Handel MA. Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: insights into regulation of spermatogenic progress. Dev Biol. 2002;249:85–95. doi: 10.1006/dbio.2002.0708. [DOI] [PubMed] [Google Scholar]
- 3.Lipkin SM, Moens PB, Wang V, Lenzi M, Shanmugarajah D, Gilgeous A, Thomas J, Cheng J, Touchman JW, Green ED, et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet. 2002;31:385–390. doi: 10.1038/ng931. [DOI] [PubMed] [Google Scholar]
- 4.Ward JO, Reinholdt LG, Motley WW, Niswander LM, Deacon DC, Griffin LB, Langlais KK, Backus VL, Schimenti KJ, O’Brien MJ, et al. Mutation in mouse hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS Genet. 2007;3:e139. doi: 10.1371/journal.pgen.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10:207–216. doi: 10.1038/nrg2505. [DOI] [PubMed] [Google Scholar]
- 6.Kouznetsova A, Lister L, Nordenskjold M, Herbert M, Hoog C. Bi-orientation of achiasmatic chromosomes in meiosis I oocytes contributes to aneuploidy in mice. Nat Genet. 2007;39:966–968. doi: 10.1038/ng2065. [DOI] [PubMed] [Google Scholar]
- 7.LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol. 1997;139:1611–1619. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet S, Pahlavan G, Taylor S, Maro B. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction. 2003;126:443–450. doi: 10.1530/rep.0.1260443. [DOI] [PubMed] [Google Scholar]
- 9.Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202–207. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niault T, Hached K, Sotillo R, Sorger PK, Maro B, Benezra R, Wassmann K. Changing Mad2 levels affects chromosome segregation and spindle assembly checkpoint control in female mouse meiosis I. PLoS ONE. 2007;2:e1165. doi: 10.1371/journal.pone.0001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 14.Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–160. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat Res. 2008;651:82–92. doi: 10.1016/j.mrgentox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651:14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Woods LM, Hodges CA, Baart E, Baker SM, Liskay M, Hunt PA. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol. 1999;145:1395–1406. doi: 10.1083/jcb.145.7.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod. 2005;11:389–396. doi: 10.1093/molehr/gah179. [DOI] [PubMed] [Google Scholar]
- 19.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 20.Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod. 2002;17:1171–1180. doi: 10.1093/humrep/17.5.1171. [DOI] [PubMed] [Google Scholar]
- 21.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Yuan L, Liu JG, Hoja MR, Wilbertz J, Nordqvist K, Hoog C. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science. 2002;296:1115–1118. doi: 10.1126/science.1070594. [DOI] [PubMed] [Google Scholar]
- 23.Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- 24.LeMaire-Adkins R, Hunt PA. Nonrandom segregation of the mouse univalent X chromosome: evidence of spindle-mediated meiotic drive. Genetics. 2000;156:775–783. doi: 10.1093/genetics/156.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 26.Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol. 1999;146:1–12. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Dawe RK. Fused sister kinetochores initiate the reductional division in meiosis I. Nat Cell Biol. 2009;11:1103–1108. doi: 10.1038/ncb1923. [DOI] [PubMed] [Google Scholar]
- 28.Holloway JK, Booth J, Edelmann W, McGowan CH, Cohen PE. MUS81 generates a subset of MLH1-MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 2008;4:e1000186. doi: 10.1371/journal.pgen.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai S, O’Connell CB, Khodjakov A, Walczak CE. Chromosome congression in the absence of kinetochore fibres. Nat Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumont J, Oegema K, Desai A. A kinetochore-independent mechanism drives anaphase chromosome separation during acentrosomal meiosis. Nat Cell Biol. 2010;12:894–901. doi: 10.1038/ncb2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wignall SM, Villeneuve AM. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nat Cell Biol. 2009;11:839–844. doi: 10.1038/ncb1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, et al. Age-related meiotic segregation errors in Mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol. 2010;20:1529–1533. doi: 10.1016/j.cub.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Laboratory Animal Resources, C.o.L.S., National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 38.Messinger SM, Albertini DF. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100(Pt 2):289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.