Abstract
Sick preterm and term newborns are highly vulnerable to neural injury, and thus there has been a major search for new, safe and efficacious neuroprotective interventions in recent decades. Preclinical studies are essential to select candidate drugs for clinical trials in humans. This article focuses on ‘negative’ preclinical studies, i.e. studies where significant differences cannot be detected. Such findings are critical to inform both clinical and preclinical investigators, but historically they have been difficult to publish. A significant amount of time and resources is lost when negative results or nonpromising therapeutics are replicated in separate laboratories because these negative results were not shared with the research community in an open and accessible format. In this article, we discuss approaches to strengthen conclusions from negative preclinical studies and, conversely, to reduce false-negative preclinical evaluations of potential therapeutic compounds. Without being exhaustive, we address three major issues in conducting and interpreting preclinical experiments, including: (a) the choice of animal models, (b) the experimental design, and (c) issues concerning statistical analyses of the experiments. This general introduction is followed by synopses of negative data obtained from studies of three potential therapeutics for perinatal brain injury: (1) the somatostatin analog octreotide, (2) an AMPA/kainate receptor antagonist, topiramate, and (3) a pyruvate derivative, ethyl pyruvate.
Key Words: Hypoxia-ischemia, Stroke, Cerebral palsy, Topiramate, Ethyl pyruvate, Octreotide, Somatostatin, Excitotoxicity
Introduction
Protecting the perinatal brain is a healthcare priority, and the search for new, safe and efficacious neuroprotectants has been a major quest during the last decade [1]. As in other fields of neuroprotection, preclinical studies have been extensively used to select candidate drugs for clinical trials in humans. Due to the high vulnerability of sick preterm and term newborns to neural injury [2, 3], this selection process is of utmost importance. Previous articles have discussed potential reasons why interventions that were neuroprotective in preclinical studies were not protective in human controlled trials [4, 5]. This article focuses, instead, on negative preclinical studies, i.e. studies where no apparent significant differences were found. Such findings are critical to inform future research directions for both clinical and preclinical investigators, but historically they have been difficult to publish. A significant amount of time and resources is lost when negative results or nonpromising therapeutics are reproduced in separate laboratories because these negative results were not shared with the research community in an open and accessible format.
On the one hand, relative failure to publish negative studies biases the literature towards positive studies. Conversely, false-negative studies could lead to premature rejection of potentially promising neuroprotectants, reducing the number of successful treatments that reach the bedside and misinforming future research directions. This problem is largely due to the difficulty in publishing negative results. It is our view that well-designed and sufficiently powered negative studies, when replicated in different animal models and if possible in different species, should be published. Standards for such negative studies should be defined and specific tracks established to help these data to become available to the whole scientific community.
As an example of a robust negative outcome, oxidative stress is a key player in perinatal brain damage [6], making NADPH oxidase a good candidate target. However, pharmacological and genetic blockade of NADPH oxidase in two separate models of perinatal brain damage in mice failed to show any neuroprotective effects [7]. While these studies documenting failure to obtain neuroprotection when NADPH oxidase was targeted were published, this paper represents an exception.
In this article, we discuss approaches to strengthen conclusions from those preclinical studies that are negative and to reduce false-negative preclinical evaluations of potential therapeutic compounds. Without being exhaustive, we address three major issues in the conduct and interpretation of preclinical experiments, including: (a) the choice of animal models, (b) the experimental design, and (c) issues concerning statistical analyses of the experiments. This general introduction is followed by three reports of negative findings from studies that were carefully designed and for which there was a satisfactory rationale. To our knowledge, this report represents the first coordinated step towards this goal in the field of perinatal neurology.
Choice of Animal Models
Although difficult to obtain, sufficient knowledge should be available to support the validity of the selected animal model for human neonates. There are several excellent reviews of the selection of animal models for perinatal brain injury research [8, 9, 10]. As there is no ‘perfect’ animal model that completely mimics the human situation, we propose that investigators should aim to confirm the positive effects of a given drug in multiple animal models and, if possible, in different species including both rodents and larger animals. Given the issues concerning choosing a model, confirmation in multiple models is equally important to confirm negative studies.
Experimental Design: Power and Sample Size
A priori power calculations are a key step in preclinical experimental design. Failure to consider which statistical tests will be used and to appropriately power the experimental design may lead to false-negative results and premature rejection of potentially promising neuroprotectants. Unless new experiments are almost identical to others in the investigator's laboratory, using an effect size from previous experiments can lead to an over- or underestimate of the sample size required to achieve the desired power or may even lead to other design flaws. For each new experiment, a design and sample size need to be developed in the context of the study question. In publications of negative results, presenting each aspect of the sample size calculation (statistical approach to testing, expected difference between groups, hypothesized variances, and power) will assure the reviewers that the study was not underpowered to address the question of interest.
Testing neuroprotectants in new models requires reconsideration of sample size parameters. A key point in model selection is the fact that human neonates are often sick, whereas the majority of laboratory animals used for induced brain damage are healthy. In particular, systemic inflammation is present in many neonates [11]. Systemic inflammation is known to either sensitize the brain to, or protect it from, secondary insults, as well as modulating pathophysiological pathways that are activated [12, 13]. Testing neuroprotective strategies in a systemic inflammatory context is highly relevant to the clinic but requires careful consideration in preclinical experimental design. This type of design is likely to decrease the difference between groups, increase variability and increase the required sample size.
As another example, hypothermia is emerging as a standard of care, but its clinical efficacy is partial [3], and thus hypothermia would benefit from add-on therapies to further improve outcomes. However, differences between the preclinical model and the clinical situation create a conundrum. For understandable reasons, in animal models, optimized hypothermia leads to a very high level of neuroprotection [14]. How then does one proceed to design an experiment to test add-on therapies? Two very different strategies are plausible. In one scenario, optimized hypothermia is applied, which has the advantage of mimicking the protocol that is used in humans [15]. By doing so, often significantly superior neuroprotection is obtained compared to what is observed in humans, and there may be a limited potential for the add-on compound to induce significant additive neuroprotection, potentially leading to a false-negative study unless the sample size is increased sufficiently to compensate.
Alternatively, a suboptimal hypothermia protocol may be used that would produce partial neuroprotection, comparable to that observed in babies. This protocol would be different from the one used in humans, with milder or shorter periods of cooling, but it might address the real question whether the protective agent remains protective when the brain is cooled [16]. A potential underlying and, at present, undefined risk is that if the mechanisms of hypothermia and the potential cotreatment overlap and, thus, might not warrant combinatorial use, this outcome might not be clearly shown if the preclinical study used a suboptimal cooling regime. The optimal balance between these conflicting limitations is not yet clear, but a better understanding of the mechanisms of neuroprotection with hypothermia may help resolve this conundrum [17].
Other Design Issues
Many pharmacological compounds have U-shaped curve effects. An incomplete dose-response curve (too low or too high doses) might miss the therapeutic window. Furthermore, as we do not have a complete understanding of the pathophysiology of brain damage in our animal models (e.g. activated microglia seem to be deleterious at early stages of lesion formation, while they could be beneficial at later stages) [18], the optimal schedule of administration of a given drug is difficult to determine a priori. Therefore, different schedules (single vs. repeated administration, early vs. delayed administration) should be tested before discarding a promising drug. A rigorous design also includes randomization using rigorous statistical procedures. Many analytic software packages now include a randomization procedure. Blinding of the observer collecting the data is another consideration. If the experimenter cannot be blinded, this should be indicated in the report on the study.
Analysis
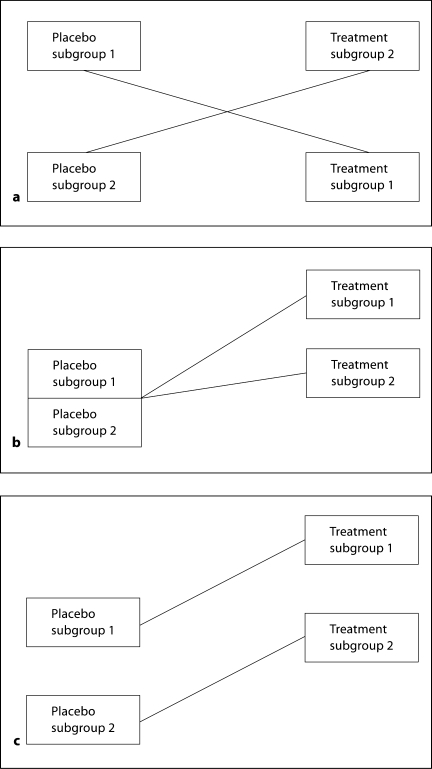
Interactions. In analysis, the potential for there to be subsets of responders and nonresponders is rarely taken into account in preclinical studies. Despite the relative genetic homogeneity of the rodents we use, it is clear that, for some drugs, careful analysis can strongly suggest two population subgroups. Defining appropriate tests of interaction to unravel this responder/nonresponder issue could be of major importance in developing future patient-based therapies. For an interaction to be present and affect the interpretation of the analysis, the variable defining the subgroup must have a relationship to both the treatment and the outcome. Ignoring an interaction in the analysis could lead to a false-negative conclusion (fig. 1a, b). For example, some drugs have protective effects on one cell type but are toxic to another cell population (fig. 1a). This can easily lead to an apparent lack of effect if data are combined across cell types. However, proper subgroup analysis could lead to the ‘rescue’ of the initial target combined with the design of a cell-specific strategy. If subgroups respond differently, the first step would be to test for an interaction effect by adding an interaction to the model being used to test for a treatment effect. The model would also include both a term for treatment and a term for subgroup. If an interaction is detected (usually using p < 0.10), the two subgroups would be analyzed separately, and additional animals would possibly be needed to have sufficient power for the subgroup analyses.
Fig. 1.
Examples of types of interaction between treatment and subgroup effects. a Interaction. Placebo subgroup 1 has a higher value than placebo subgroup 2, where higher values are better. Treatment subgroup 1 is worsening compared to placebo subgroup 1; placebo subgroup 2 has lower values, but treatment subgroup 2 is improving. If the interaction is statistically significant, the two subgroups should be analyzed separately. b Interaction. There appears to be a benefit in treatment subgroup 1, and a lesser benefit in treatment subgroup 2. If the interaction is not taken into account, there could be a modest benefit shown for both groups combined. If the interaction is statistically significant, the two subgroups should be analyzed separately. c No interaction. There is a consistent difference between placebo and treatment subgroups. The difference between placebos could reflect different baselines or different responses to factors external to the experiment. There is no need to analyze groups separately, but the confounder should be adjusted for in analysis.
Confounding Factors. False-negative results may also be obtained when there is excessive noise or great experimental variation or confounding by factors related to the outcome but not to the treatment group. If the subtypes have different baselines (e.g. sex of the pups [19]) and thus different levels of responses, but the magnitude of response as compared to placebo is similar (fig. 1c), the subtype would be a confounder. Adjusting for confounding by gender could increase the power to detect an effect by decreasing variability in the treatment estimate, decreasing the probability of a false-negative result. External factors also induce confounding. In the perinatal brain injury field, maternal stress (i.e. pregnant mothers are very sensitive to different kinds of stressors including temperature, humidity, noise or changes in habits) [20], body temperature [21] as well as the time of the day when the procedure is performed (the expression of several trophic factors such as brain-derived neurotrophic factor vary over the day) [[22], Gressens, unpubl.] may all exert confounding effects upon the final outcome. Confounding variables should be either eliminated or, if not possible, recorded and then factored into the final analysis. If a confounder is suspected, a test for interaction would be conducted. If no interaction is detected, then adjusting for confounding can be accomplished by including the potential confounder as a covariate using analysis of covariance, logistic regression or Cox proportional hazards regression, depending on the type of outcome variable. Including subgroup or other confounders as covariates could improve the precision of the treatment estimate and potentially increase the power to detect a difference.
Other Analytic Considerations
A common analytic issue is the failure to adjust for replicate measures on the same animal (unless replicates are combined into a single measure) or litter effect, as dramatically demonstrated in a simulation study using amyotrophic lateral sclerosis preclinical studies [23]. Clustering is addressed more frequently in the context of randomized trials [24]. Clustering impacts the sample size, requiring an increase over a traditional design. If clustering is not taken into account in the design but is adjusted for in the analysis as would be necessary, there is the potential for an underpowered false-negative study. Because the estimate of type I error is too low when positive within-animal or within-litter correlation (clustering) is not taken into account, there can be false-positive results – but this is not the topic of this review. Other analytic issues include failure (1) to follow intent-to-treat principles (‘as randomized, so analyzed’ [25]) in analysis, (2) to account for all animals included in the experiment, (3) to adjust for multiple comparisons where appropriate, and (4) to predefine criteria for excluding animals from analysis after randomization. While many of these errors are generally more likely to contribute to false-positive studies, a poorly designed study can also lead to false-negative results. Additionally, in convincing reviewers to publish a negative preclinical study, obvious design flaws make the study fare worse.
In summary, false-negative studies can be avoided by proper design and analyses, such as adjusting for interactions and confounding. Standards for reporting negative studies may improve the likelihood of publication. All publications of negative studies should include a clear discussion of the design (choice of model and whether the study was replicated in multiple animal models, choice of dosing schedule, methods of randomization, blinding and sample size considerations demonstrating sufficient power). Reporting and analysis should include an accounting for all animals randomized, a discussion of approaches to the analysis of interactions and confounders if some are expected and, if applicable, a report on how replicates or litters were taken into account in the analysis. Below, we give three examples of negative studies.
Study 1: Somatostatin Analog Octreotide Fails to Protect Rat Perinatal Brain against Excitotoxicity
Introduction
Brain lesions induced in newborn mice by the glutamatergic agonists ibotenate (acting on N-methyl-D-aspartic acid and metabotropic receptors) and S-willardiine (acting on AMPA-kainate receptors) mimic some aspects of white matter cysts and transcortical necrosis observed in human perinatal brain damage [26, 27]. This model has been used to successfully evaluate different classes of neuroprotective drugs including trophic factors, such as brain-derived neurotrophic factor, or neuropeptides, such as vasoactive intestinal peptide [28, 29]. Somatostatin (SST), which modulates neurotransmission [30], has been shown to limit N-methyl-D-aspartic acid-mediated neuronal death in vitro, and ischemic brain damage following permanent cerebral artery occlusion in the adult rat in vivo [31, 32]. Among the five SST receptor subtypes, SST2 receptor mRNA, binding sites and protein [33] are predominant in the developing rat brain. The goal of the present study was to test the hypothesis that octreotide, a stable SST2 receptor agonist, is neuroprotective against neonatal excitotoxic brain injury, focusing on cortical gray matter.
Methods
Sprague-Dawley rats of both sexes were used for this study. We induced excitotoxic brain lesions by intracerebrally injecting (into the neopallial parenchyma) ibotenate (10 μg; Tocris, Bristol, UK), S-willardiine (15 μg; Tocris) or ibotenate + S-willardiine into developing rat brains on postnatal day 5 (P5), as previously described [34]. Octreotide (SMS 201-995; Sigma, St. Louis, Mo., USA) was coinjected with excitotoxins. Pups were sacrificed 5 days after the excitotoxic challenge. Following paraffin embedding, we cut 16-μm-thick coronal brain sections. Every third section was stained with cresyl violet. This permitted an accurate determination of the sagittal frontooccipital diameter used as an index of the lesion volume. For each experiment, animals from different litters were randomly attributed to the different experimental groups, and for each experimental group, experiments were run in triplicate. The number of animals included in each experimental group was determined by a power calculation based on standard deviation from previous studies using the same animal model (α = 0.05 and β = 0.20; power: 80%). We needed at least 9 animals per group to detect a 20% difference between groups, assuming a control mean lesion size of 960 μm and standard deviation of 160 μm. One-way ANOVA was performed and was considered significant if p < 0.05.
Results
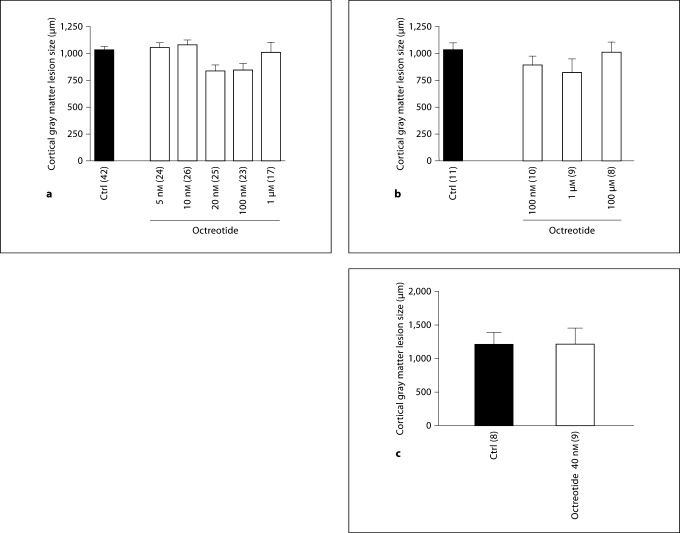
Within a large range of concentrations (from 5 nM to 1 μM), octreotide had no significant effect on the size of the cortical gray matter lesion induced by S-willardiine, ibotenate or both together, when compared to controls (fig. 2).
Fig. 2.
Lack of neuroprotection from ibotenate- or S-willardiine-induced brain injury in the neonatal rat with octreotide. Octreotide had no significant effect on the size of cortical gray matter lesions induced by S-willardiine (a), ibotenate (b) or S-willardiine + ibotenate (c). Rat pups were injected on P5 and sacrificed on P10. Numbers of animals in each group are shown in parentheses on the x-axis of each graph. Bars: mean length of brain lesions at P10. Whiskers: SEM.
Conclusion
Despite the neuroprotective effects of octreotide on in vitro excitotoxicity or adult stroke, octreotide was not neuroprotective in a model of neonatal excitotoxic brain damage. To fully exclude a potential role for SST receptor agonists, studies on other models of perinatal brain injury, including hypoxia-ischemia (HI), should be performed.
Study 2: Lack of Neuroprotection with Delayed Topiramate Treatment after Cerebral Ischemia in Near-Term Fetal Sheep
Background
Severe perinatal ischemic injury is associated with delayed onset of seizures and massive accumulation of excitotoxins and cytotoxic edema [35]. However, it remains unclear to what extent and by what mechanisms these potentially damaging events contribute to delayed cell death. For example, selective N-methyl-D-aspartate blockade, started 6 h after reperfusion (before the onset of seizures), profoundly suppressed seizure activity, but was associated with no improvement in the parasagittal cortex or basal ganglia and a modest reduction in damage to the hippocampus and temporal lobe [36]. Activation of the AMPA subtype of glutamate receptor can also be damaging [37], and in sheep, the AMPA receptor appears to remain calcium permeable throughout the second half of gestation, denoting continuing susceptibility to excessive AMPA receptor activation [38]. Therefore, we examined whether treatment with the clinically available anticonvulsant – and AMPA/kainate antagonist – topiramate would reduce neural injury after cerebral ischemia in near-term fetal sheep.
Methods
In utero, unanesthetized near-term fetal sheep were exposed to 30 min of cerebral ischemia induced by bilateral carotid artery occlusion [39]. Five hours after reperfusion, fetuses received an intravenous infusion of either normal saline (n = 7), or topiramate (Janssen-Cilag Pty. Ltd., Australia) in saline (n = 4), 25 mg over 30 min (approx. 5–7 mg/kg) followed by 8.33 mg/h for a further 24 h. The fetuses were killed after 4 days for histological assessment. EEG data were missing for 1 topiramate fetus. Data were analyzed by ANOVA; for physiological data, changes over time were treated as repeated measures to allow for repeated sampling.
Results
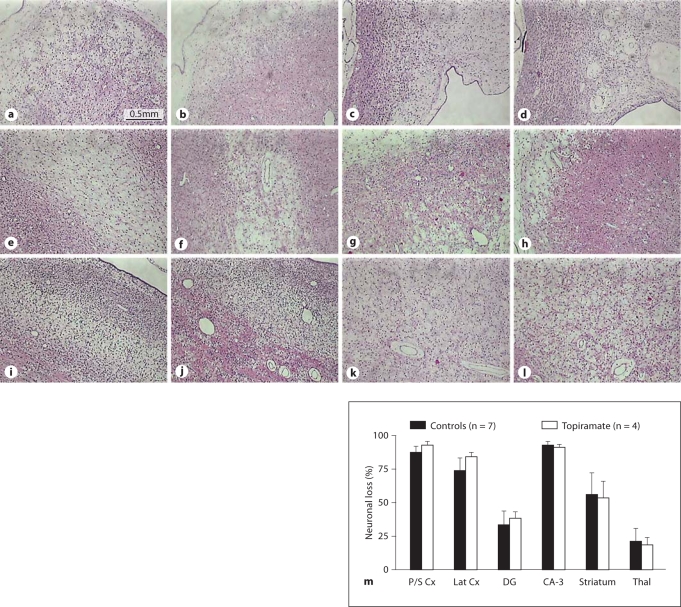
Cerebral ischemia was associated with severe parasagittal cortical infarction in all fetuses. There was extensive neuronal loss in the lateral (temporal) lobe, the hippocampus and thalami, and infarction in key white matter areas including the intragyral and periventricular white matter and the corpus callosum. There was no significant effect of topiramate on damage in any of these regions, and in particular, no reduction in cortical damage (fig. 3). Topiramate was associated with transient suppression of EEG activity, but seizures recurred in all cases within 1–2 h despite the maintenance infusion. The secondary rise in impedance (a measure of cytotoxic edema) was not reduced after topiramate infusion (mean: 24–48 h; controls 128 ± 15% baseline vs. topiramate 135 ± 5.6%, mean ± SD). Residual EEG power (–12.5 ± 5.3 dB vs. −19 ± 0.9 dB) and 90% spectral edge frequency (6.4 ± 1.1 vs. 5.7 ± 0.9 Hz) were markedly suppressed in the final 24 h of recovery in both groups.
Fig. 3.
a–l Representative photomicrographs of coronal sections (8 μm; acid fuchsin/thionine staining for neuronal loss, pink acidophilic neurons) illustrating histopathological changes 96 h after 30 min of cerebral ischemia in near-term fetal sheep treated with saline (a, c, e, g, i, k) or topiramate (b, d, f, h, j, l). There was extensive parasagittal cortical necrosis in both hemispheres of the brain in both groups. ×25. Scale bar = 0.5 mm. a, b Parasagittal cortex. c, d Corpus callosum. e, f Parasagittal intragyral white matter. i, j Lateral (temporal lobe) cortex. k, l Periventricular white matter. m Percent neuronal loss 96 h after 30 min of cerebral ischemia in near-term fetal sheep treated with saline or topiramate, in the parasagittal cortex (P/S Cx), the lateral or temporal cortex (Lat Cx), the dentate gyrus (DG) and cornu ammonis region 3 (CA-3) of the hippocampus, and the striatum and thalamic nuclei (Thal). Data are means ± SEM.
Discussion
Disappointingly, this preliminary study suggests that delayed treatment with topiramate, using a strategy broadly consistent with clinical anticonvulsant use, does not have a strong neuroprotective or white matter-protective effect. It is possible that protection requires higher doses or much earlier treatment. Nevertheless, a dose of 5 mg/kg, even as a single bolus, is known to rapidly achieve therapeutic cerebrospinal fluid levels for at least 24 h in the piglet [40], and plasma levels in newborns [41]. Schubert et al. [42] reported protection with a 50 mg/kg loading dose, but not with 20 mg/kg, after HI in the piglet. However, they initiated the infusion very early (1 h) after reperfusion and, of concern, high-dose treatment was associated with increased white matter apoptosis. Given that in this paradigm, seizures and the rise in excitotoxins do not typically start until 5.5–9 h after ischemia [35, 43], and that moderate hypothermia alone started after a similar delay was significantly protective [44], the present data provide further evidence that these events are primarily epiphenomena of cell death.
Although the present study was relatively modest in size, it had 80% power to detect a reduction in cortical neuronal loss of at least 20%, based on a vehicle mean of 88%, standard deviation 10, and two-tailed α of 0.05. We cannot exclude the possibility that small or sex-specific beneficial effects of topiramate might have been observed in a larger cohort of animals powered sufficiently to enable evaluation of potential confounding factors. Future studies should focus on early postresuscitation treatment, and on cotreatment with hypothermia [16].
Study 3: Contradictory Outcome of Studies of Ethyl Pyruvate Neuroprotection after HI in Neonatal Rats
Introduction
Epidemiologic and experimental data implicate HI in the etiology of perinatal brain injury, which may lead to cerebral palsy. Apart from a partial protective effect of hypothermia, there are currently no effective therapeutic strategies for limiting perinatal brain injury. Ethyl pyruvate (EP) is a pyruvate derivative that has been shown to possess anti-inflammatory properties [45], and has been reported to inhibit cerebral injury in adult rats after middle cerebral artery occlusion with a wide therapeutic window [46, 47]. Recently, it was shown that EP provided impressive protection (by 50%) in neonatal Sprague-Dawley rats subjected to HI as well [48]. Encouraged by these results, our specific aim was to investigate whether this beneficial effect of EP was related to improved substrate delivery to mitochondria. However, as a first step, we intended to reproduce the protective response to settle the optimal dose and timing of treatment in our laboratory, using a similar neonatal rat model of HI [49].
Methods
Sixty P8 Wistar rats received unilateral ligature of the left common carotid artery followed by 50 min of hypoxia at 36°C with 7.8% O2. Just after hypoxia, pups were intraperitoneally injected with a single dose of either 10 (EP10 group) or 40 mg/kg (EP40 group) of EP dissolved in Ringer's solution [50], while control pups received an injection of Ringer's solution as vehicle at the same time. Three days after the insult, animals were killed by intracardiac perfusion of paraformaldehyde. Outcome was assessed by scoring macroscopic brain injury. In order to decide the number of animals required, we performed a power calculation based on standard deviation from previous studies using the same animal model (α = 0.05 and β = 0.15; power: 85%). We needed at least 11 animals per group to detect a 33% difference between groups, assuming a vehicle mean score of 3.0 and a standard deviation of 0.75. The actual power of the study – recomputed under the same assumptions used for sample size calculations, but with the sample sizes that were achieved – was 98%. Data were analyzed using GraphPad Prism 4.0 by the Mann-Whitney test.
Results
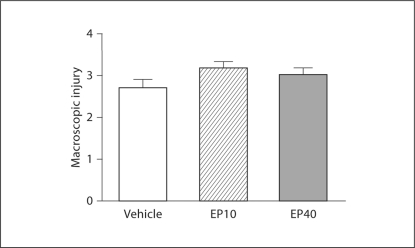
All the pups seemed to tolerate EP administration well. Nevertheless, EP administration after HI did not reduce the severity of infarction at either 10 or 40 mg/kg compared to the vehicle group (vehicle vs. EP10: p = 0.2; vehicle vs. EP40: p = 0.6) (fig. 4).
Fig. 4.
Lack of neuroprotection using EP after HI in neonatal rats. No difference in HI brain injury was detected between rat pups treated with EP (EP10: n = 13; EP40: n = 17) and those who received vehicle (n = 30). Data represent means of macroscopic injury scores ± SEM, 3 days after exposure to HI.
Discussion
In contrast to previous studies showing that EP has neuroprotective effects in adult and neonatal rats after HI, we were unable to detect an effect on brain injury. The results disagree with those of a recent paper showing that diverse doses of EP administered to P7 Sprague-Dawley rats before or within 30 min after HI provided up to 50% protection associated with calpain inhibition and anti-inflammatory actions [48]. In that study, no significant effect was associated with a dose of 10 mg/kg, but higher doses were effective, and the optimal dose was 50 mg/kg administered 10 min after HI. The therapeutic window was limited to 30 min, which is shorter than previously reported for adult rats after middle cerebral artery occlusion [46].
The settings of our two studies investigating the effect of EP on the immature brain after HI were quite similar: the doses (10 and 40 vs. 10, 30, 50, 100, 250 and 500 mg/kg), age (P8 and P7), gender (mixed gender in both studies), timing of treatment (10 min after vs. 30 min prior, or 10 or 30 min after HI), animal model (the Vannucci model [49], with 7.8% oxygen in both studies, but 50 vs. 150 min of exposure time), anesthesia (isoflurane) and extent and severity of the injury observed in the vehicle controls were comparable. The two main differences were the rat strain and the solution used to dissolve EP. We used Wistar rats, whereas Shen et al. [48] used Sprague-Dawley rats. However, both rat strains were outbred, and both strains are often utilized in the Vannucci model without any major differences reported in the literature.
The control group was larger than the treatment groups in the present study as both treatment groups were compared with the same control group in order to increase the study's power. This can be criticized as an increased number of animals in only one of the groups marginally increases power, and a better strategy would be to increase numbers in all groups. Nevertheless, in spite of uneven numbers of animals per group, this study had robust power to detect a 30% or greater difference between groups, a much smaller improvement than the 50% difference reported by Shen et al. [48]. Finally, Shen et al. [48] used saline as vehicle for EP, whereas we used Ringer's solution. However, EP solubilized in Ringer's solution has been shown to be an efficient neuroprotectant in adult injury models [46, 50], suggesting that protection with EP is unlikely to depend on the choice of vehicle.
Conclusion
Taken together, these data show that quite similar experimental settings can produce very different results in different laboratories, which are not easily explained by differences in experimental setup.
Acknowledgements
This article and the research studies reported herein were supported by grants from Inserm, Université Paris Diderot, APHP (Contrat d'Interface to Dr. Pierre Gressens and to Dr. Pascal Dournaud), PremUP, Fondation Roger de Spoelberch, Fondation Grace de Monaco and Fondation LeDucq, Health Research Council of New Zealand, Swedish Government grant to researchers in Public Health Service at the Sahlgrenska University Hospital (ALFGBG-142881), Swedish Research Council (2009-2630), Neurobid, European Union grant FP7 (241778), Åhléns Foundation, Frimurare barnhusfonden, Medical Research Council strategic award (MRC; United Kingdom, P19381), Medical Research Council (VR; Sweden, 2010-3396), ALF-LUA (Sweden, ALFGBG2863), and Wellcome Trust (Programme Grant WT094823MA), NINDS U01NS043127 and NICHD 052064.
References
- 1.Degos V, Loron G, Mantz J, Gressens P. Neuroprotective strategies for the neonatal brain. Anesth Analg. 2008;106:1670–1680. doi: 10.1213/ane.0b013e3181733f6f. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS, editors. Board on Health Sciences Policy. Washington: Institute of Medicine of the National Academies; 2007. Preterm Birth Causes, Consequences, and Prevention. Committee on Understanding Premature Birth and Assuring Healthy Outcomes. [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBow SB, Clark DL, MacLellan CL, Colbourne F. Incomplete assessment of experimental cytoprotectants in rodent ischemia studies. Can J Neurol Sci. 2003;30:368–374. doi: 10.1017/s0317167100003097. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB. Neuroprotection in acute ischaemic stroke: a tale of for whom the bell tolls? Lancet. 2000;355:1925–1926. doi: 10.1016/S0140-6736(00)02318-7. [DOI] [PubMed] [Google Scholar]
- 6.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 7.Doverhag C, Keller M, Karlsson A, Hedtjarn M, Nilsson U, Kapeller E, Sarkozy G, Klimaschewski L, Humpel C, Hagberg H, Simbruner G, Gressens P, Savman K. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol Dis. 2008;31:133–144. doi: 10.1016/j.nbd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;422:85–88. doi: 10.1111/j.1651-2227.1997.tb18353.x. [DOI] [PubMed] [Google Scholar]
- 9.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 10.Hagberg H, Ichord R, Palmer C, Yager JY, Vannucci SJ. Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev Neurosci. 2002;24:364–366. doi: 10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- 11.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 12.Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 13.Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- 14.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–169. doi: 10.1016/j.nurx.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George SA, Bennet L, Weaver-Mikaere L, Fraser M, Bouwmans J, Mathai S, Skinner SJM, Gunn AJ. White matter protection with insulin-like growth factor 1 and hypothermia is not additive after severe reversible cerebral ischemia in term fetal sheep. Dev Neurosci. 2011 doi: 10.1159/000329923. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–1465. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 17.Drury P, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Semin Fetal Neonatal Med. 2010;15:287–292. doi: 10.1016/j.siny.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 20.Rangon CM, Fortes S, Lelièvre V, Leroux P, Plaisant F, Joubert C, Lanfumey L, Cohen-Salmon C, Gressens P. Chronic mild stress during gestation worsens neonatal brain lesions in mice. J Neurosci. 2007;27:7532–7540. doi: 10.1523/JNEUROSCI.5330-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoresen M. Supportive care during neuroprotective hypothermia in the term newborn: adverse effects and their prevention. Clin Perinatol. 2008;35:749–763, vii. doi: 10.1016/j.clp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Bova R, Micheli MR, Qualadrucci P, Zucconi GG. BDNF and trkB mRNAs oscillate in rat brain during the light-dark cycle. Brain Res Mol Brain Res. 1998;57:321–324. doi: 10.1016/s0169-328x(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 23.Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG, Ramasubbu J, Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- 24.Campbell MK, Grimshaw JM. Cluster randomised trials: time for improvement. The implications of adopting a cluster design are still largely being ignored. BMJ. 1998;317:1171–1172. doi: 10.1136/bmj.317.7167.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen J, Christensen K, Murray J, Ekbom A. An Introduction to Epidemiology for Health Professionals, ed 1. New York: Springer Science and Business Media; 2010. [Google Scholar]
- 26.Marret S, Mukendi R, Gadisseux JF, Gressens P, Evrard P. Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol. 1995;54:358–370. doi: 10.1097/00005072-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Plaisant F, Clippe A, Vander Stricht D, Knoops B, Gressens P. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic Biol Med. 2003;34:862–872. doi: 10.1016/s0891-5849(02)01440-5. [DOI] [PubMed] [Google Scholar]
- 28.Husson I, Rangon CM, Lelièvre V, Bemelmans AP, Sachs P, Mallet J, Kosofsky BE, Gressens P. BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb Cortex. 2005;15:250–261. doi: 10.1093/cercor/bhh127. [DOI] [PubMed] [Google Scholar]
- 29.Gressens P, Marret S, Hill JM, Brenneman DE, Gozes I, Fridkin M, Evrard P. Vasoactive intestinal peptide prevents excitotoxic cell death in the murine developing brain. J Clin Invest. 1997;100:390–397. doi: 10.1172/JCI119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Rauca C, Schäfer K, Höllt V. Effects of somatostatin, octreotide and cortistatin on ischaemic neuronal damage following permanent middle cerebral artery occlusion in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:633–638. doi: 10.1007/s002109900136. [DOI] [PubMed] [Google Scholar]
- 32.Forloni G, Lucca E, Angeretti N, Chiesa R, Vezzani A. Neuroprotective effect of somatostatin on nonapoptotic NMDA-induced neuronal death: role of cyclic GMP. J Neurochem. 1997;68:319–327. doi: 10.1046/j.1471-4159.1997.68010319.x. [DOI] [PubMed] [Google Scholar]
- 33.le Verche V, Kaindl AM, Verney C, Csaba Z, Peineau S, Olivier P, Adle-Biassette H, Leterrier C, Vitalis T, Renaud J, Dargent B, Gressens P, Dournaud P. The somatostatin 2A receptor is enriched in migrating neurons during rat and human brain development and stimulates migration and axonal outgrowth. PLoS ONE. 2009;4:e5509. doi: 10.1371/journal.pone.0005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sfaello I, Baud O, Arzimanoglou A, Gressens P. Topiramate prevents excitotoxic damage in the newborn rodent brain. Neurobiol Dis. 2005;20:837–848. doi: 10.1016/j.nbd.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckman PD. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Tan WK, Williams CE, Gunn AJ, Mallard CE, Gluckman PD. Suppression of postischemic epileptiform activity with MK-801 improves neural outcome in fetal sheep. Ann Neurol. 1992;32:677–682. doi: 10.1002/ana.410320511. [DOI] [PubMed] [Google Scholar]
- 37.Gressens P, Spedding M, Gigler G, Kertesz S, Villa P, Medja F, Williamson T, Kapus G, Levay G, Szenasi G, Barkoczy J, Harsing LG., Jr The effects of AMPA receptor antagonists in models of stroke and neurodegeneration. Eur J Pharmacol. 2005;519:58–67. doi: 10.1016/j.ejphar.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Dean JM, Fraser M, Shelling AN, Bennet L, George S, Shaikh S, Scheepens A, Gunn AJ. Ontogeny of AMPA and NMDA receptor gene expression in the developing sheep white matter and cerebral cortex. Brain Res Mol Brain Res. 2005;139:242–250. doi: 10.1016/j.molbrainres.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galinkin JL, Kurth CD, Shi H, Priestley MA, Loepke AW, Adamson PC. The plasma pharmacokinetics and cerebral spinal fluid penetration of intravenous topiramate in newborn pigs. Biopharm Drug Dispos. 2004;25:265–271. doi: 10.1002/bdd.408. [DOI] [PubMed] [Google Scholar]
- 41.Filippi L, la Marca G, Fiorini P, Poggi C, Cavallaro G, Malvagia S, Pellegrini-Giampietro DE, Guerrini R. Topiramate concentrations in neonates treated with prolonged whole body hypothermia for hypoxic ischemic encephalopathy. Epilepsia. 2009;50:2355–2361. doi: 10.1111/j.1528-1167.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 42.Schubert S, Brandl U, Brodhun M, Ulrich C, Spaltmann J, Fiedler N, Bauer R. Neuroprotective effects of topiramate after hypoxia-ischemia in newborn piglets. Brain Res. 2005;1058:129–136. doi: 10.1016/j.brainres.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 43.Williams CE, Gunn AJ, Synek B, Gluckman PD. Delayed seizures occurring with hypoxic-ischemic encephalopathy in the fetal sheep. Pediatr Res. 1990;27:561–565. doi: 10.1203/00006450-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 45.Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. Crit Care Med. 2003;31:S51–S56. doi: 10.1097/00003246-200301001-00008. [DOI] [PubMed] [Google Scholar]
- 46.Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- 47.Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the postischemic brain. Brain Res. 2005;1060:188–192. doi: 10.1016/j.brainres.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Shen H, Hu X, Liu C, Wang S, Zhang W, Gao H, Stetler RA, Gao Y, Chen J. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 50.Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]