Abstract
Background
Selenium is an element necessary for normal cellular function, but it can have toxic effects at high doses. We investigated an outbreak of acute selenium poisoning.
Methods
A case was defined as the onset of symptoms of selenium toxicity in a person within 2 weeks after ingesting a dietary supplement manufactured by “Company A,” purchased after January 1, 2008. We conducted case finding, administered initial and 90-day follow-up questionnaires to affected persons, and obtained laboratory data where available.
Results
The source of the outbreak was identified as a liquid dietary supplement that contained 200 times the labeled concentration of selenium. Of 201 cases identified in 10 states, 1 person was hospitalized. The median estimated dose of selenium consumed was 41 749 μg/d (recommended dietary allowance is 55 μg/d). Frequently reported symptoms included diarrhea (78%), fatigue (75%), hair loss (72%), joint pain (70%), nail discoloration or brittleness (61%), and nausea (58%). Symptoms persisting 90 days or longer included fingernail discoloration and loss (52%), fatigue (35%), and hair loss (29%). The mean initial serum selenium concentration of 8 patients was 751 μg/L (reference range, ≤125 μg/L). The mean initial urine selenium concentration of 7 patients was 166 μg/24 h (reference range, ≤55 μg/24 h).
Conclusions
Toxic concentrations of selenium in a liquid dietary supplement resulted in a widespread outbreak. Had the manufacturers been held to standards used in the pharmaceutical industry, it may have been prevented.
Selenium is a naturally occurring mineral required for good health. It is obtained from food, and the recommended dietary allowance is 55 μg/d for persons 14 years or older, with a tolerable upper intake limit of 400 μg/d.1,2 The amount of selenium available in a diverse diet with meat, grains, vegetables, and nuts is typically sufficient to negate the necessity for supplementation.3 Selenium toxicity can occur with acute or chronic ingestion of excess selenium. Symptoms of selenium toxicity include nausea; vomiting; nail discoloration, brittleness, and loss; hair loss; fatigue; irritability; and foul breath odor (often described as “garlic breath”).1,2,4–6
Selenium is found in the environment in soil. Soils of certain areas in the Great Plains and western United States4 as well as other parts of the world have high concentrations of selenium, which are taken up by plants. For example, chronic selenium toxicity was endemic in parts of China until recently.6 Outbreaks of acute selenium poisoning are rare, but have been reported.7,8
In March 2008, a chiropractor in Florida noted common symptoms of gastrointestinal illness and hair loss among several of his patients. In response to their symptoms, these patients had doubled the dose of a dietary supplement they had purchased at the chiropractic office. The patients subsequently noted worsening symptoms, including nail discoloration. Two couples contacted the local health department, where investigators identified the common exposure and initiated an investigation. Active case finding was initiated by the local health department, including contacting the chiropractor, who provided a list of patients to whom the dietary supplement had been sold. These patients were interviewed. Simultaneously, the US Food and Drug Administration (FDA) initiated an independent investigation as a result of similar complaints, at which time other agencies, including the poison control center and state health department, were notified. The dietary supplement was identified as the common exposure among all affected persons.
METHODS
A case of selenium poisoning was defined as hair loss, nail discoloration or brittleness, or 2 or more of the following symptoms: muscle or joint pains, headache, foul breath, fatigue/weakness, gastrointestinal symptoms, or cutaneous eruption. Symptom onset must have occurred within 2 weeks after the person ingested a liquid dietary supplement manufactured by “Company A,” purchased after January 1, 2008.
Case finding was conducted on a national level through multiple mechanisms. Company A’s list of all retail locations and persons who had placed an Internet or telephone order for the implicated product was obtained. State health departments contacted retail outlets within their respective states and obtained lists of customers known to have purchased the implicated product. A voluntary product recall and FDA and state press releases stimulated spontaneous self-reports of consumption and illness to state health departments, poison control centers, and the FDA’s Safety Information and Adverse Event Reporting System (MedWatch). Attempts were made to contact any person who reported consumption of the supplement, as well as those listed as product recipients with either the company or a retail distributor.
A questionnaire was administered to 227 affected persons identified in 9 states: Florida, Georgia, Kentucky, Michigan, North Carolina, Pennsylvania, Tennessee, Texas, and Virginia. In addition, 5 states (Florida, Georgia, Michigan, North Carolina, and Tennessee) administered follow-up questionnaires approximately 90 days after the initial interviews.
Questions addressed demographics, amount of product consumed, symptoms, and dates of onset and resolution of symptoms. The selenium dose was calculated as grams of selenium consumed per kilogram of body weight, after the date on which the first bottle of contaminated product had been opened.
Seven affected patients in Tennessee provided 24-hour urine specimens for testing of selenium concentration at the time of initial interview and at 1 week and 1 month thereafter. Standard laboratory methods were used to determine urine selenium concentrations, compared with blank controls.9 Testing was performed at the Vanderbilt University Medical Center, Nashville, Tennessee. Eight patients provided results of serum selenium testing ordered by their physicians from commercial laboratories.
Information about the product was obtained from Company A. After the outbreak was recognized, the FDA conducted inspections of the manufacturing and supply plants and tested the product from the implicated lots to measure selenium concentation.
Data were analyzed by using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina). This investigation was determined by the Centers for Disease Control and Prevention’s human subjects review to be a public health outbreak response.
RESULTS
The implicated product was marketed as a dietary supplement, as stated on the label and the Internet, suitable for the “entire family” to provide a balance of nutrients to “maintain energy and sustain health.” The product contained a blend of 16 vitamins, 12 elements with labeled concentrations and 58 “trace elements,” 18 amino acids, 3 essential fatty acids, Spirulina, coenzyme Q10, and anti-oxidants, suspended in an ionic colloidal liquid. The label instructions directed adults to take 1 capful (1 fl oz [30 mL]) of the supplement daily, or one-half teaspoon (2.5 mL) for each 20 lb (9.07 kg) of body weight daily for children 5 years or older. On the basis of the reported symptom complex and the laboratory test results of the implicated product obtained by the distributor, selenium was suspected to be the source of illness. The product was labeled as containing 200 μg of selenium per fluid ounce (30 mL) in the form of sodium selenite, an inorganic form of selenium. The FDA subsequently tested the product and determined the selenium concentration to be 40 800 μg/1 oz, approximately 200 times the labeled concentration. Chromium was also identified as being elevated at 17 times the labeled concentration of 200 μg/1 oz. This was less than the amount known to cause adverse health effects among either humans or animals.10,11 None of the affected patients reported symptoms typical of chromium toxicity (eg, gastrointestinal hemorrhage, liver dysfunction, renal failure). All other tested ingredients were within the labeled concentrations.
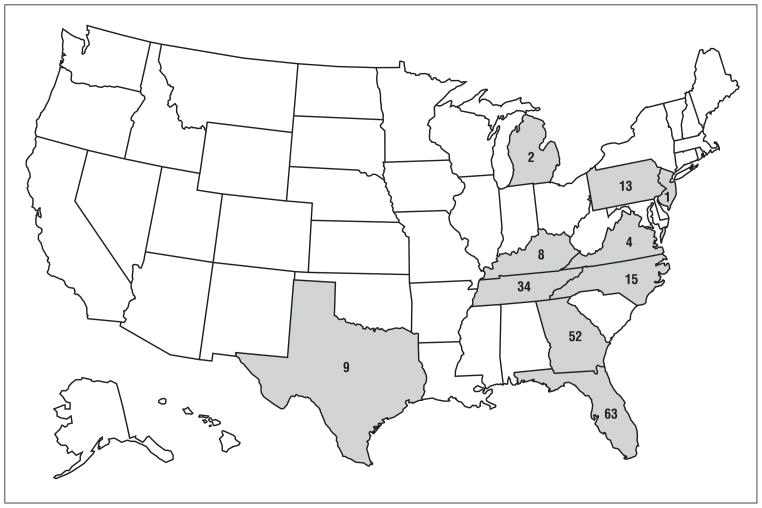
The incriminated lots of 1200 bottles of the product were manufactured in September 2007, and distribution began in January 2008. Product distribution was primarily in the eastern United States, with the highest concentration in the Southeast. This was reflected in the geographic distribution of cases (Figure 1). The product was recalled in late March 2008.
Figure 1.
Distribution of cases of acute selenium poisoning after ingestion of a misformulated dietary supplement
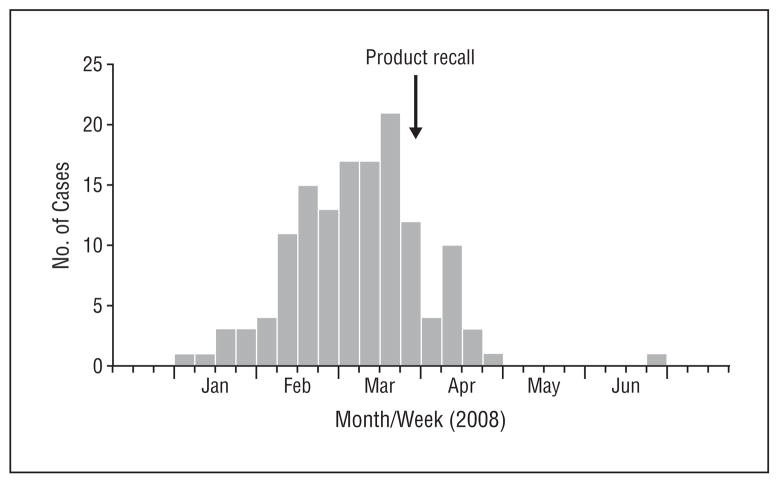
Structured telephone interviews were administered to all persons identified as having consumed the product. Of 227 consumers, 201 (89%) met the case definition for selenium poisoning. The remaining 26 consumers who did not meet the case definition reported no or mild symptoms. Illness onset extended from January 5 through June 11, 2008 (Figure 2). The median patient age was 54 years (range, 4–92 years) and 121 (60%) were female. One-hundred forty-nine (74%) were white, and 15 (7%) were black. At the time of the initial interview, 118 (59%) of the patients had sought care at private physicians’ offices, clinics, or emergency departments; 83 (41%) had not brought their symptoms to medical attention. One patient was hospitalized; none died.
Figure 2.
Epidemic curve by date of onset of illness after consumption of a misformulated dietary supplement. The arrow indicates date of voluntary product recall.
The median period over which patients had consumed the misformulated product was 29 days (range, 1–109 days). Among 156 patients with data available, the median estimated amount of selenium ingested was 989 mg (range, 41–5875 mg), for a median of 41 585 μg/d (range, 3400–244 800 μg/d; recommended dietary allowance, 55 μg/d). Among 98 patients with weight and dose available, the median dose ingested was 12.8 mg/kg (range, 0.5–115.4 mg/kg).
The most frequently reported symptoms included diarrhea (78%), fatigue (72%), hair loss (70%), joint pain (67%), nail discoloration or brittleness (61%), nausea (57%), and headache (45%) (Table 1). The proportion of scalp hair lost ranged from 10% to 100%, with a median of 50%. Foul breath (37%) and cutaneous eruption (26%) were also commonly reported.
Table 1.
Reported Symptoms of Selenium Toxicity After Consuming a Misformulated Dietary Supplement
| Symptoms | Patients, No. (%) (n=201) |
|---|---|
| Diarrhea | 156 (78) |
| Fatigue | 144 (72) |
| Hair loss | 140 (70) |
| Joint pain | 135 (67) |
| Nail discoloration/brittleness | 122 (61) |
| Nausea | 115 (57) |
| Headache | 90 (45) |
| Tingling | 78 (39) |
| Vomiting | 52 (26) |
| Fever | 43 (21) |
| Ataxia | 27 (13) |
A follow-up questionnaire was administered to 104 of 150 patients (69%) in Florida, Georgia, and Tennessee, at a median of 106 days (range, 43–206 days) after symptom onset. Among patients originally reporting specific symptoms and who were interviewed 90 days or more after symptom onset, symptoms persisting 90 days or more included fingernail discoloration and loss (52%), fatigue (35%), hair loss (29%), joint pain (26%), memory loss (22%), and muscle aches (22%) (Table 2).
Table 2.
Persistence of Symptoms 90 Days or More After Cessation of a Misformulated Dietary Supplement
| Symptoms | No. of Cases With Data Available | No. (%) With Symptom Lasting ≥ 90 Days |
|---|---|---|
| Fingernail discoloration, brittleness, or loss | 62 | 32 (52) |
| Fatigue | 63 | 22 (35) |
| Toenail discoloration | 49 | 16 (33) |
| Hair growth | 62 | 18 (29) |
| Hair loss | 56 | 16 (29) |
| Joint pain | 65 | 17 (26) |
| Memory loss | 83 | 18 (22) |
| Muscle pain/aches | 77 | 17 (22) |
| Weakness | 80 | 16 (20) |
| Mood changes | 81 | 14 (17) |
| Tingling | 76 | 9 (12) |
| Foul breath | 83 | 9 (11) |
| Nausea | 83 | 9 (11) |
Of the 78 patients reporting hair loss, 14 (18%) reported complete loss of scalp hair. One patient (1%) reported total body hair loss. Of the 74 patients who reported fingernail discoloration or brittleness, 28 (38%) reported nail loss.
Initial serum selenium concentrations were obtained from 8 patients at a median of 1 day after cessation of product consumption (range, 0–33 days). Initial serum selenium concentrations ranged from 321 to 1500 μg/L, with a mean of 761 μg/L and a median of 664 μg/L; 3 were higher than 1000 μg/L (reference mean, 125 μg/L).12 Initial urine specimens from 7 patients were obtained at a median of 9 days after cessation of product consumption (range, 6–37 days). Initial urine selenium concentrations ranged from 55 to 227 μg/d, with a mean of 166 μg/d and a median of 179 μg/d (reference mean for Tennessee residents, 55 μg/d).13 Serum and urine selenium concentrations decreased gradually with time, with values returning to normal by weeks 1 to 2 for urine and starting at week 6 for serum.
The supplement was distributed by Company A, a small privately owned company in Atlanta, Georgia. The product had been on the market for 12 years without any reported problems. Company A received the finished product from a manufacturer in Arkansas, which in turn received ingredients from suppliers in Louisiana.
The FDA conducted inspections of the manufacturing and supply plants after the outbreak was identified. Company A had recently changed the manufacturer of the product, and the misformulated lots were the first lots produced after the change. Employee error at one of the ingredient suppliers was determined to be the cause of the increased selenium in the product.
COMMENT
A misformulated liquid dietary supplement resulted in 201 cases of selenium poisoning, but the actual number of affected persons was likely greater. Outbreaks of selenium poisoning are rare; the last occurred in 1983 and involved 12 persons who had consumed inappropriately potent selenium tablets as a dietary supplement.7,8 A single case of selenium poisoning in 1996 was attributed to vitamins with elevated amounts of selenium.14 Occasional cases of acute selenium poisoning are caused by unintentional or suicidal ingestion.5 Most recently, 21 polo ponies died after receiving a supplemental injection containing toxic amounts of selenium.15 Collaboration among epidemiologists, clinicians, and toxicologists during this outbreak investigation provided an opportunity to identify the circumstances that led to the event, describe the clinical manifestations and their duration, and observe the kinetics of serum and urine selenium concentrations among affected persons.
Case reports and epidemiologic studies from previous poisonings, foreign and domestic, reveal a wide range of symptoms similar to those described herein.1,6–8,14,16 Notably, approximately one-fourth of the patients (23%) in this outbreak reported fever, a symptom not previously associated with selenium toxicity. Although only 3 patients had recorded their temperatures, they were substantially elevated at 38.4°C to 39.5°C. Whether fever can be attributed directly to selenium toxicity, represents a general sense of malaise, or is the result of concurrent infection could not be determined.
Persistence of symptoms was also notable; patients often continued to experience symptoms 90 days after the exposure to selenium had ended. This was true not only for the hair and nail changes, which are expected to require substantial time to return to normal, but also for constitutional symptoms, including memory loss, mood swings, fatigue, musculoskeletal complaints, and garlic breath.
Progression of symptoms with time often mirrors the distribution of selenium from its entry into the gastrointestinal tract to the well-perfused internal organs and to other less-perfused tissues.1,5,14,17 Excessive amounts of selenium commonly cause gastrointestinal effects (eg, diarrhea and vomiting). Subsequent distribution of excessive amounts of selenium into musculoskeletal tissues has been reported to cause muscle pain and cramps as well as joint pain. There are no proven antidotes or curative treatments for selenosis. Treatment involves stopping the exposure and providing supportive care for symptoms.1
The serum selenium concentrations in this outbreak were all substantially elevated and were comparable to or higher than values previously reported in association with selenium toxicity from dietary supplements,5,14,18 although they were lower than values that have been reported after attempted suicides.5 The serum selenium concentrations reported during this outbreak are high for subjects ingesting inorganic forms of selenium. Ingestion of organic selenium in the form of selenomethionine is associated with much higher serum selenium concentrations than ingestion of inorganic forms.19 Biological samples (eg, urine and serum) correlate poorly with the degree of toxicity among humans. Selenium undergoes a unique concentration-dependent triphasic elimination. Depending on the actual dose ingested, different and unpredictable serum levels of selenium can result. As evidenced during this outbreak, selenium is sequestered in different organ tissues, and its slow metabolism accounts for the persistence of symptoms.1,5,17,20,21
This episode of selenium toxicity caused by a misformulated commercially distributed dietary supplement presented unique clinical and public health challenges. Given the rarity of selenium toxicity, along with the array of nonspecific symptoms, recognizing the diagnosis can be difficult. Furthermore, a substantial proportion of patients had not yet sought medical attention at the time they were contacted by public health investigators. During interviews, patients often stated they had not suspected that a health product made them ill, and thus never mentioned to their health care providers that they were taking the implicated dietary supplement, despite experiencing weeks of unexplained symptoms. Patients even reported increasing their dose in an attempt to ameliorate symptoms. This highlights the importance of patients informing their health care providers about all dietary supplements, herbal remedies, and over-the-counter medications, in addition to prescription medications.
After recognition of the initial cluster, additional case finding was conducted by tracing sales of the product, press releases to the general public, referrals from patients calling poison control centers, FDA field offices, and FDA’s MedWatch. Because of nonspecific symptoms and limited health care–seeking behavior among affected persons, the outbreak was probably even larger than recognized.
This investigation was limited by potential recall bias among patients and a limited number of clinical specimens available for laboratory testing. While the observation of fever in some patients is interesting, we were unable to determine conclusively that selenium toxicity was the cause. There were also substantial barriers to sharing proprietary or personally identifiable information among investigating agencies. Policy differences regarding the sharing of identifiable information among federal, state, regulatory, and nonregulatory agencies are complex and can impede the efficient flow of sensitive data, even during an acute outbreak investigation. In this case, for example, identifiable information from persons calling MedWatch was not shared directly with the health departments. Rather, FDA staff had to ask the callers to contact the health department themselves.
Unfortunately, examples abound in which dietary supplements have been associated with toxicity.22–25 Human error during manufacturing resulted in toxic concentrations of selenium in this dietary supplement. Dietary supplements such as vitamins, minerals, and herbal products are not subject to premarket review or approval for safety, efficacy, or Good Manufacturing Practices (GMPs). Under the Federal Food, Drug, and Cosmetic Act,25 the manufacturer of a finished dietary supplement product is responsible for ensuring the safety and the quality of the final product, which includes all ingredients received from suppliers. That responsibility includes testing of received ingredients, including pre-mixed ingredients and the final product, for compliance with predetermined specifications for identity and composition. In September 2007, the FDA issued guidelines for the gradual implementation of GMPs on the basis of the number of employees of a manufacturer of dietary supplements. However, the GMP regulations, which govern the manufacturing process but not necessarily the outcome, were not required of all suppliers and final manufacturers at the time of processing the product implicated during this outbreak.25 Had the manufacturers been held to standards used in the pharmaceutical industry, this outbreak may have been prevented. Gaps in existing regulations present a significant public health risk, and attention should be directed at correcting them to prevent recurring outbreaks such as this.
Acknowledgments
Funding/Support: Dr Burk is supported in part by National Institutes of Health grant DK058763.
Footnotes
Financial Disclosure: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author Contributions: Ms MacFarquhar and Dr Jones had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MacFarquhar, Wolkin, Burk, Dunn, Schaffner, and Jones. Acquisition of data: MacFarquhar, Broussard, Melstrom, Hutchinson, Wolkin, Burk, Dunn, and Hammond. Analysis and interpretation of data: MacFarquhar, Broussard, Melstrom, Hutchinson, Wolkin, Martin, Burk, Green, Hammond, Schaffner, and Jones. Drafting of the manuscript: MacFarquhar, Melstrom, Hutchinson, Wolkin, Dunn, Hammond, Schaffner, and Jones. Critical revision of the manuscript for important intellectual content: MacFarquhar, Broussard, Melstrom, Hutchinson, Martin, Burk, Dunn, Green, Hammond, and Jones. Statistical analysis: MacFarquhar, Hutchinson, Martin, and Green. Administrative, technical, and material support: Broussard, Melstrom, Hutchinson, Wolkin, Martin, Dunn, Hammond, Schaffner, and Jones. Study supervision: MacFarquhar, Hutchinson, Wolkin, Dunn, Schaffner, and Jones.
Additional Contributions: Joshua G. Schier, MD, Center for Environmental Health, Centers for Disease Control and Prevention, provided technical assistance. Nupur A. Sashti, MPH, Communicable and Environmental Disease Services, Tennessee Department of Health, Samir Hanna, MD, MSPH, Communicable and Environmental Disease Services, Tennessee Department of Health, and Ashley N. Webb, MS, PharmD, Carolinas Poison Center, provided data acquisition support. Carina Blackmore, DVM, PhD, Florida Department of Health, provided supervision over initial acquisition of data. None received compensation for their contributions.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicologic Profile for Selenium. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2003. [Google Scholar]
- 2.Institute of Medicine (IOM) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. Selenium; pp. 284–324. [Google Scholar]
- 3.Levander OA. Scientific rationale for the 1989 recommended dietary allowance for selenium. J Am Diet Assoc. 1991;91(12):1572–1576. [PubMed] [Google Scholar]
- 4.Fan AM, Kizer KW. Selenium-nutritional, toxicologic, and clinical aspects. West J Med. 1990;153(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- 5.Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006;36(4):409–420. [PubMed] [Google Scholar]
- 6.Yang GQ, Wang SZ, Zhou RH, Sun SZ. Endemic selenium intoxication of humans in China. Am J Clin Nutr. 1983;37(5):872–881. doi: 10.1093/ajcn/37.5.872. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control (CDC) Selenium intoxication—New York. MMWR Morb Mortal Wkly Rep. 1984;33(12):157–158. [PubMed] [Google Scholar]
- 8.Galliot-Guilley M. Toxicity with superpotent selenium. FDA Drug Bull. 1984;14(2):19. [PubMed] [Google Scholar]
- 9.Sheehan TMT, Gao M. Simplified fluorometric assay of total selenium in plasma and urine. Clin Chem. 1990;36(12):2124–2126. [PubMed] [Google Scholar]
- 10.Anderson RA, Bryden NA, Polansky MM. Lack of toxicity of chromium chloride and chromium picolinate in rats. J Am Coll Nutr. 1997;16(3):273–279. doi: 10.1080/07315724.1997.10718685. [DOI] [PubMed] [Google Scholar]
- 11.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicologic Profile for Chromium. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- 12.Niskar AS, Paschal DC, Kieszak SM, et al. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res. 2003;91(1):1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 13.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15(4):804–810. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 14.Clark RF, Strukle E, Williams SR, Manoguerra AS. Selenium poisoning from a nutritional supplement. JAMA. 1996;275(14):1087–1088. doi: 10.1001/jama.1996.03530380029025. [DOI] [PubMed] [Google Scholar]
- 15.Florida Department of Agriculture and Consumer Services. [Accessed September 15, 2009];Polo horses likely died from selenium overdose. http://www.doacs.state.fl.us/press/2009/04282009.html.
- 16.Yang G, Yin S, Zhou R, et al. Studies of safe maximal daily dietary Se-intake in a seleniferous area in China, part II: relation between Se-intake and the manifestation of clinical signs and certain biochemical alterations in blood and urine. J Trace Elem Electrolytes Health Dis. 1989;3(3):123–130. [PubMed] [Google Scholar]
- 17.Suzuki KT, Ogra Y. Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam. 2002;19(10):974–983. doi: 10.1080/02652030210153578. [DOI] [PubMed] [Google Scholar]
- 18.Sutter ME, Thomas JD, Brown J, Morgan B. Selenium toxicity: a case of selenosis caused by a nutritional supplement. Ann Intern Med. 2008;148(12):970–971. doi: 10.7326/0003-4819-148-12-200806170-00015. [DOI] [PubMed] [Google Scholar]
- 19.Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors. 2001;14(1–4):107–114. doi: 10.1002/biof.5520140115. [DOI] [PubMed] [Google Scholar]
- 20.Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr. 2003;133(5 suppl 1):1517S–1520S. doi: 10.1093/jn/133.5.1517S. [DOI] [PubMed] [Google Scholar]
- 21.Lockitch G. Selenium: clinical significance and analytical concepts. Crit Rev Clin Lab Sci. 1989;27(6):483–541. doi: 10.3109/10408368909114596. [DOI] [PubMed] [Google Scholar]
- 22.Dara L, Hewett J, Lim JK. Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World J Gastroenterol. 2008;14(45):6999–7004. doi: 10.3748/wjg.14.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller CA, Meier KH, Olson KR. Seizures reported in association with use of dietary supplements. Clin Toxicol (Phila) 2005;43(1):23–30. doi: 10.1081/clt-44771. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Engel A, Fattinger K, et al. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J Hepatol. 2007;47(4):521–526. doi: 10.1016/j.jhep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Center for Food Safety and Applied Nutrition, US Food and Drug Administration. [Accessed February 11, 2009];Overview of dietary supplements. http://www.fda.gov/Food/DietarySupplements/ConsumerInformation/ucm110417.htm.