Abstract
CD40L and BAFF, members of TNF superfamily, play critical roles in B cell survival and activation, and in the regulation of humoral immunity. We previously reported that the adaptor molecule Act1 functions as a negative regulator of CD40- and BAFF-mediated B cell survival. Here we demonstrated that mice deficient in Act1 developed systemic autoimmune disease with histological and serological features of human Sjogren’s syndrome, in association with SLE-like nephritis. Analyses of Act1−/−CD40−/− and Act1−/−BAFF−/− mice revealed that Act1 regulates different stages of the disease development through its impact on both CD40-and BAFF-mediated pathways. We found that Act1 modulates the survival of auto-reactive B cells mainly through its negative regulatory role in BAFF-mediated cell survival, while the effect of Act1 on autoantibody production is probably through its modulation on CD40-mediated T-cell dependent antibody response. The impact of Act1 on both BAFF and CD40 pathways establishes Act1−/− mice as a unique model to study distinct steps of autoimmunity and regulation of self-tolerance.
Keywords: B cells, Autoimmunity, Systemic Lupus Erythematosus, Rheumatoid Arthritis, Autoantibodies
Introduction
Through B-cell development, B cell repertoire is generated to recognize a vast diversity of exogenous antigens and defend against pathogens {Hardy, 2001 543/id;Martin, 2002 544/id}. The B cell repertoire is tightly regulated to avoid damaging immune responses and to maintain self-tolerance. Disruption of the mechanisms regulating B-cell survival, maturation and activation leads to development of autoimmunity.
Sjogren’s syndrome (SS) is a systemic autoimmune disorder characterized by profound lymphocytic infiltration of the lachrimal and salivary glands {Manoussakis, 2001 720/id;Manoussakis, 2004 721/id;MacSween, 1967 722/id}. This chronic inflammatory process leads to diminished function of the glands resulting in symptoms of xerophthalmia (dry eyes) or xerostomia (dry mouth). B cell hyper-activation is a predominant feature of Sjogren’s syndrome, which in human patients is characterized by hypergammaglobulinemia and production of autoantibodies {Slobbe, 1991 725/id;Haneji, 1997 723/id;Manoussakis, 2001 720/id;Manoussakis, 2004 721/id;MacSween, 1967 722/id}. The occurrence of anti-SS-A/Ro and anti-SS-B/La, the two most commonly detected autoantibodies in Sjogren’s syndrome patients has been directly associated with recurrent lymphocyte infiltration of the exocrine glands, tissue damage and secretory dysfunction. Sjogren’s syndrome is classified as primary or secondary depending on the absence or presence of conjunction with other systemic autoimmune diseases such as systemic lupus erythematosus (SLE), which is characterized by production of anti-nuclear autoantibodies and immune complex deposition in the kidney. Kidney frailer, as a result of lupus nephritis is the leading cause of fatality in human patients with SLE {Manoussakis, 2004 721/id}.
CD40L and BAFF, two members of TNF superfamily, play critical roles in the regulation of B cell homeostasis. BAFF (also known as BlyS, TALL-1, zTNF4, THANK, and TNFSF 13B) has emerged as a critical regulator of B cell survival and maturation. Mice deficient in BAFF display severe loss of mature B cells and attenuated antibody responses {Schonbeck U and Libby P, 2002 428/id;van Kooten, 1997 402/id;Bishop, 2001 427/id;Lei, 1998 416/id;Thompson, 2001 490/id;Mackay, 2002 541/id;Mackay, 2003 542/id}. The interaction between CD40 and its ligand CD40L is required for T-cell dependent B cell activation and antibody production {Grammer, 2000 476/id;Bishop, 2001 427/id}. Mice deficient in CD40L or CD40 are unable to mount primary or secondary antibody response to a T cell-dependent antigen, do not form GCs and display blockage of the immunoglobulin isotype switching {Kawabe, 1994 545/id;Xu, 1994 546/id}. Excessive BAFF expression and CD40-CD40L signaling have been linked to initiation and progression of many autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and Sjogren’s syndrome (SS) {Brink, 2006 637/id;Mackay, 2007 727/id;Biancone, 1999 406/id}. BAFF transgenic mice display mature B cell hyperplasia and symptoms of SLE and SS. Consistent with this, serum levels of BAFF are elevated in autoimmune prone NZB mice and human patients with SLE and SS {Groom, 2002 678/id}. Similarly, CD40L transgenic mice have increased peripheral B cell number and display SLE-like symptoms, including lupus nephritis with immunoglobulin deposition in the kidney {Lutgens, 2002 508/id}.
Previously we found that CD40- and BAFF-mediated survival is significantly increased in Act1-deficient B cells, implicating a negative or attenuating regulatory role of Act1 in B cell survival {Qian, 2004 609/id}. Consistent with this finding, Act1-deficient mice revealed a general increase in peripheral B cells, culminating in lymphadenopathy, splenomegaly, hypergammaglobulinemia, inflammation in multiple tissues, and the formation of autoantibodies. These results indicate that Act1 plays an important role in the regulation of humoral immunity and autoimmunity.
In this manuscript, we report that Act1-deficient mice developed disease that closely resembles Sjogren’s syndrome in association with SLE. Self-reactive B cells in Sjogren’s syndrome patients produce high levels of anti-SS-A/Ro and anti-SS-B/La autoantibodies. Importantly, although these autoantibodies have not been detected in BAFF transgenic mice, Act1-deficient mice did produce high titers of anti-SS-A/Ro and anti-SS-B/La, indicating the advantage of the Act1-deficient mice as an animal model for human Sjogren’s syndrome. Furthermore, whereas production of anti-Ro and anti-La autoantibody was no longer detected in CD40−/−Act1−/− double knockout mice, the production of anti-Ro and anti-La was still detectable in the BAFF−/−Act1−/− mice, indicating that the role of BAFF is dispensable for the production of these autoantibodies. These results suggest that the loss of negative regulation on CD40 pathway is likely the cause for the development of anti-Ro and anti-La autoantibody in Act1-deficient mice. On the other hand, it is also critical to note that significant degree of Sjogren’s syndrome was retained in Act1−/−CD40−/− double deficient mice. All of the B cell subpopulations except marginal zone B cells were still significantly higher in the Act1−/−CD40−/− double knockout mice as compared to the CD40−/− mice, suggesting that the regulatory role of Act1 on these B cell subpopulations is CD40-independent. Importantly, BAFF stimulation resulted in much enhanced B cell survival in the Act1−/−CD40−/− double knockout B cells as compared to CD40−/− B cells and the increased B cell populations observed in Act1-deficcient mice were abolished in Act1−/−BAFF−/− double deficient mice. Our studies demonstrate the Act1-deficient mice are a new animal model for human Sjogren’s syndrome with intrinsic B cell defects in BAFF and CD40-mediated pathways.
Results
Act1-deficent mice develop Sjogren’s syndrome
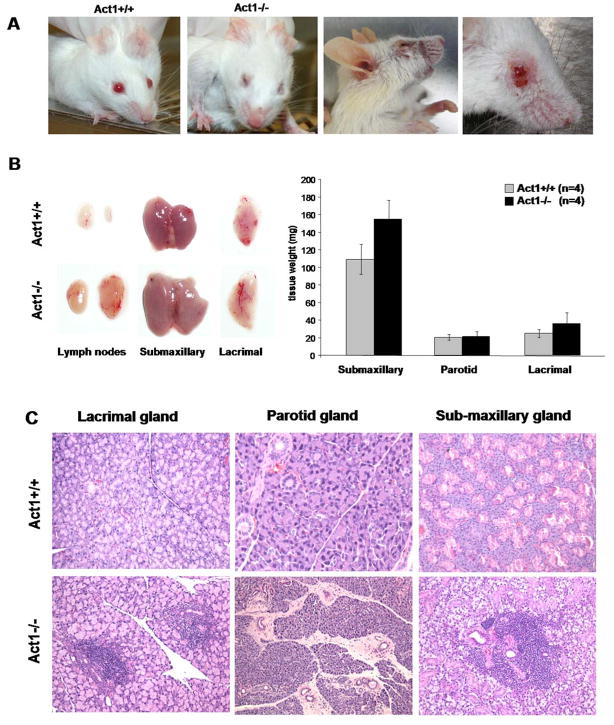
During breeding of Act1-deficient mice, we observed that almost all of both males and females in the mixed Balb/c/129sv background had difficulties in maintaining fully opened eyelids beginning around three weeks of age. This phenomenon was confirmed in mice after back-crossing onto pure Balb/c background for 10 generations. Act1-deficent mice developed symptoms of blepharitis and mouth inflammation. Due to excessive scratching of the affected eyes, some of these mice developed skin lesions around the eyes (Figure 1A). This phenotype of Act1-deficient mice closely resembles the symptoms of dry eye and dry mouth in human patients with Sjogren’s syndrome, resulting from a progressive damage of the exocrine glands. Inflammation of salivary glands (sialadenitis) accompanied with prominent loss of their secretory function is a major manifestation of Sjogren’s syndrome in human patients. Upon dissection of Act1-deficient mice, we observed that most of these mice had enlarged submaxillary glands (Fig. 1B). In addition, the lymph nodes, localized proximal of these glands were greatly enlarged (Fig. 1B). Histological analyses revealed profound lymphocyte infiltration in lacrimal, parotid and submaxillary glands in Act1-deficient mice but not in wild-type mice (Figure 1C). Most of the infiltrates were detected in the peri-vascular or peri-ductal areas of the glands. Furthermore, lymphocyte aggregates and fibrosis formation were also detected in the gland tissue.
Figure 1. Symptoms of Sjogren’s Syndrome in Act1-deficient mice.
(A) Dry eyes and skin lesions around the eyes of Act1−/− but not in Act1+/+ (littermate control) mice. Mice shown are 4 and 6 month old. (B). 4 Act1−/−and 4 Act1+/+ littermate control mice (4–6 month old) were sacrificed and the glands were dissected and weighted. The left panel shows the visibly enlarged and inflamed submaxillary and lacrimal glands and cervical lymph nodes from a representative Act1−/− mouse as compared to wild type control mouse. The data in the right panel represents the average (mean ± SD) of the combined weight of the right and left glands of the mice in each group. (C) H&E staining of lacrimal, parotid and submaxillary glands. Paraffin sections show massive lymphocyte infiltration in the glands of Act1−/−, but not Act1+/+ littermate control mice. All images are shown at a magnification x100.
Exocrine glands from Act1-deficient mice are infiltrated by a large number of B cells
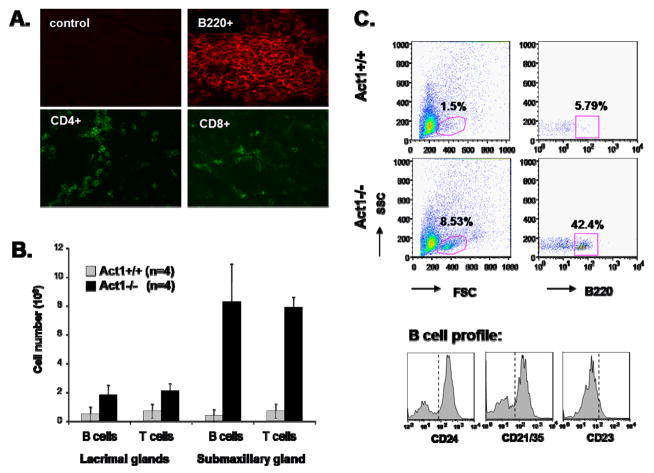
Immunofluorescent staining of the lacrimal gland (Figure 2A) and FACS analysis of the cell content of the lacrimal and submaxillary glands (Figure 2B) showed that the infiltrated cells were mostly B220+ B cells, but a significant number of CD4+ T cells and CD8+ T were also detected. The infiltrated B-cells were further identified as marginal zone (MZ)-like B cells (CD21hiCD23loCD24hi) and CD5+ B1 cells (data not shown), which were also found in the salivary glands of BAFF transgenic mice with Sjogren’s syndrome {Groom, 2002 678/id}.
Figure 2. B cell infiltration in lacrimal and salivary glands of Act1-deficient mice.
(A) Representative immunostaining of lacrimal glands from 6 month old Act1−/− mice with anti-B220, anti-CD4 and anti-CD8 fluorescence-labeled antibodies. Control represents staining with fluorescence-labeled isotype control antibody. (B) Glands from 5 (4–6 month old) Act1−/− or Act1+/+ littermate control mice were dissected and subjected to collagenase digestion. The total number of leukocytes per gland was counted using a hemocytometer. Cells were stained with anti-B220 (B-cells) and anti-CD3 (T-cells). The percentage of each cell subpopulation was analyzed using BD FACSCaliber and the absolute cell number was calculated according to the total number of infiltrated cells in each gland. The data presented are the average (mean ± SD) T or B cell number in lachrymal and submaxillary gland of wild type and Act1-deficient mice. (C) Upper plots are representative staining patterns, indicating the percentage of infiltrating B-cell (B220+) in glands from wild type and Act1-defcient mice. Lower panel shows the phenotype of the gated B220+ cells from Act1−/− mice after staining with anti-CD24, anti-CD21/35 and anti CD23 antibodies.
Act1-deficient mice develop anti-SSA/Ro and anti-SSB/La antoantibodies
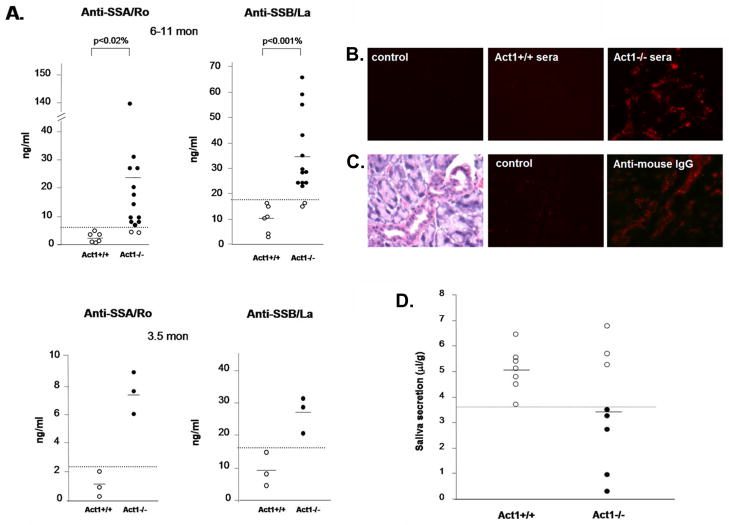
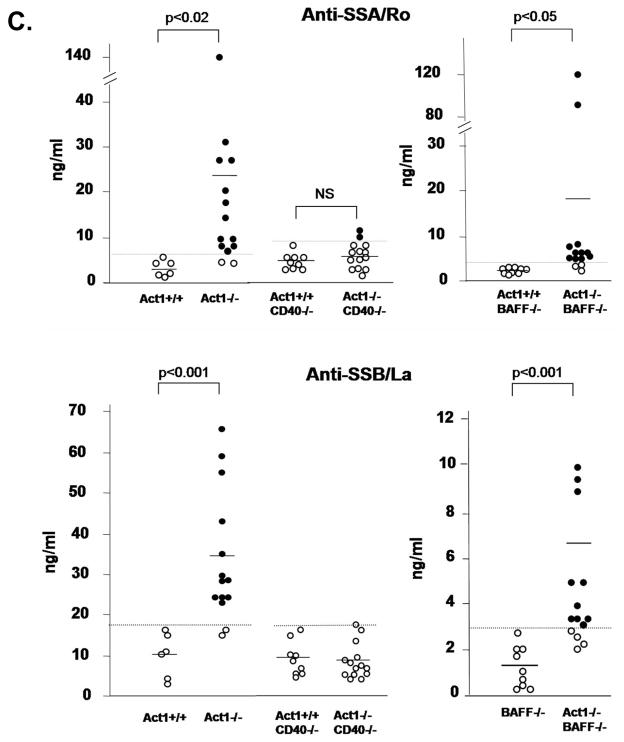
Since anti-SSA/Ro and anti-SSB/La autoantibodies are often associated with human Sojgren’s syndrome, we tested the presence of these autoantibodies in sera of wild-type and Act1-deficient mice, in a specific ELISA assay using recombinant mouse SSA and SSB antigens {Kinoshita, 1999 728/id}. High titers of anti-SSA/Ro and anti-SSB/La were detected in the sera of both young (3.5 months) and about 85% of the aged (6–11 months) Act1-deficient mice, but not in wild-type control mice (Figure 3A). The rheumatoid factor (RF), commonly found in autoimmune diseases such as SLE and Sjogren’s syndrome {Manoussakis, 2004 721/id;MacSween, 1967 722/id;Mackay, 1999 555/id;Kotzin, 1984 566/id}, was also detected in Act1-deficient mice but not in their littermates (Figure 3B). To investigate if the autoantibodies produced in Act1-deficient mice can recognize autoantigens in normal mouse glands, sera from wild-type and Act1-deficient mice were used to stain the normal lacrimal gland tissue sections. Whereas the sera from Act1-deficient mice showed strong auto-reactivity to lacrimal gland tissues (Figure 3C), there was no detectable auto-reactivity in the sera from wild-type littermates. Furthermore, immunofluorescent staining showed antibody deposition in the ductal tubes and some acinar cells of the exocrine glands from the Act1-deficient mice (Figure 3D). Act1-deficient mice showed reduced flow rate of the saliva production as compared to their wild type controls (Fig. 4), indicating that lymphocyte infiltration and antibody deposition lead to destruction of secretory function of the salivary glands. Taken together, these data suggest that Act1 deficiency leads to development of Sjogren’s syndrome (including auto-antibody production and immune complex deposition), which results in dysfunction of the exocrine glands.
Figure 3. Sjogren’s Syndrome-associated autoantibodies in Act1-deficient mice.
(A) Titers of anti-SSA/Ro and anti-SSB/La autoantibodies detected by ELISA. 14 Act1−/− mice and 6 Act1+/+ mice age between 6 and 11 months (upper panel) and 3 pairs of 3.5 months old Act1−/− and Act1+/+ mice (bottom panel) were used. The ELISA results were detected at A450 and the concentration of the antibodies in the serum of each individual mouse were calculated according to a standard curve. Short horizontal bars indicate the mean. A grey line is drawn across the graph to show the lowest value in Act1+/+ mice. The values below the grey line are considered positive (black filled dots). (B) Autoantigen staining of normal glands. Normal lacrimal glands from 3 month-old mice were incubated with or without sera from 8 month-old Act1+/+ or Act1−/− mice and stained with fluorescence-labeled rat anti-mouse IgG. The images are shown at a magnification x400. (C) Immunoglobulin deposition in the lacrimal gland. The lacrimal glands from Act1−/− or Act1+/+ mice were unstained (control) or stained with rat anti-mouse IgG. H&E staining of lacrimal gland (left panel) is included to show the localization of the complex deposition in the gland ductal. The images are shown at x400 magnification. (D) Reduced saliva secretion in the Act1-deficient mice. The secretion of saliva was determined from 8 Act1−/− or 7 Act1+/+ mice (aged 8–10 month) after pilocarpine stimulation. Values are normalized by body weight. Short horizontal bars indicate the mean. A grey line is drawn across the graph to show the lowest value in Act1+/+ mice. The values below the grey line are considered positive for reduced secretion.
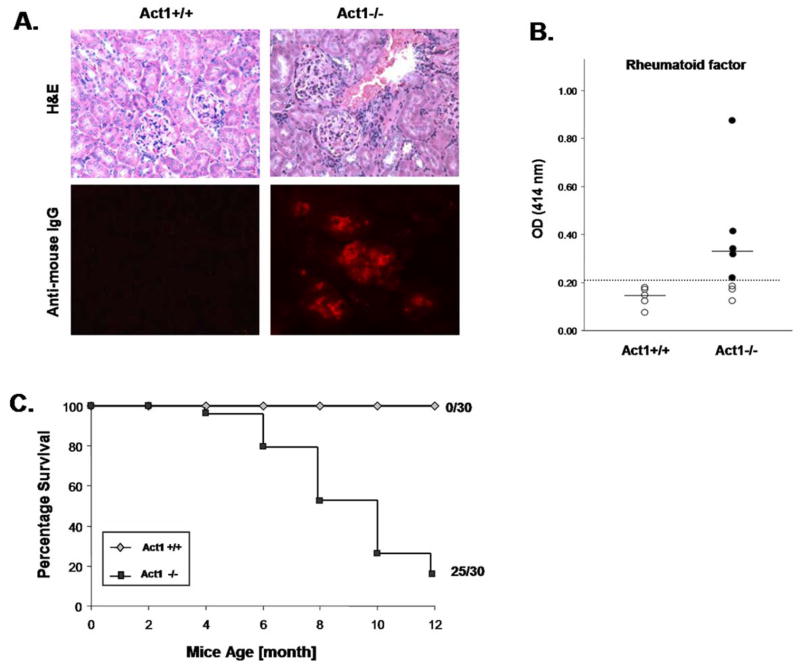
Figure 4. Act1-deficnet mice develop SLE-like nephritis, increased RF and show decreased survival rate.
(A) Representative H&E staining of kidneys sections from Act1−/− mice or control littermates showing the kidney glomeruli (x100 magnification). The lower panel is a representative immunohistochemical staining of kidney glomeruli of 8 month old Act1+/+ or Act1−/− mice (n=4) using rat anti-mouse IgG. (B) Levels of rheumatoid factor (RF) in 4 to 10 months old Act1−/− (n=9) or Act1+/+ (n=5) mice measured by ELISA. Short horizontal bars represent the mean. A grey line is drawn across the graph to show the lowest value in Act1+/+ mice. The values below the grey line are considered positive (black filled dots). (C) Survival rates of Act1−/− mice. 30 pairs of Act1−/− mice and Act1+/+ mice were observed for mortality during a period of 12 months.
Act1-deficient mice develop SLE-like glomerulonephritis
In human patients, Sjogren’s syndrome is frequently accompanied with other systemic autoimmune disorders, including SLE. Previously we found that Act1-deficient mice produce high levels of anti-histone, anti-ssDNA and anti-dsDNA autoantibodies {Qian, 2004 609/id}. Histological analysis of kidney sections from Act1-deficient mice (8–12 months) showed significant lymphocyte infiltration in the kidneys (including glomeruli) (Figure 5A). Analysis of Ig deposition in the kidneys revealed the presence of anti-DNA autoantibodies and IgG complex in the glomeruli of Act1-deficient mice, but not in the control mice (Fig. 5B), suggesting that Act1 deficiency also leads to development of SLE-like glomerulonephritis. Act1-deficient mice display severe symptoms of Sjogren’s-like syndrome in combination with SLE-like disease, which probably contribute to the significant increase of the morbidity and mortality of the Act1-deficnet mice (Figure 1A and 5C).
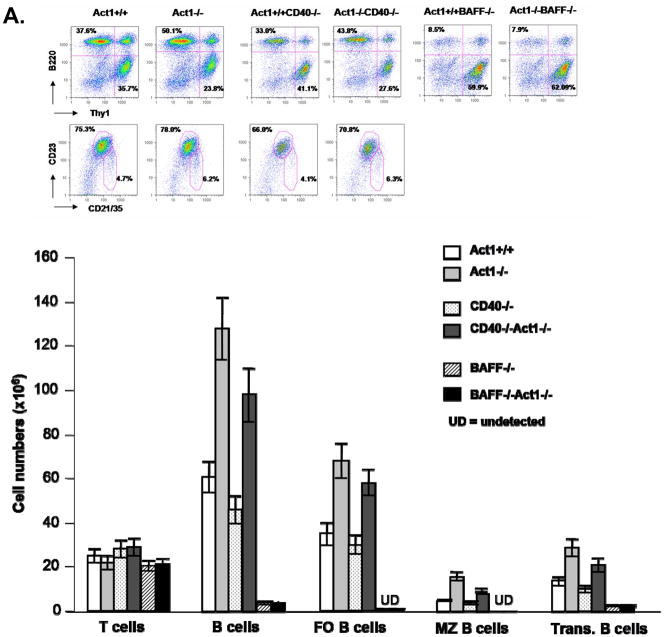
Figure 5. Differential role of CD40- and BAFF-pathways in the development of Sjogren’s phenotype in the Act1-deficient mice.
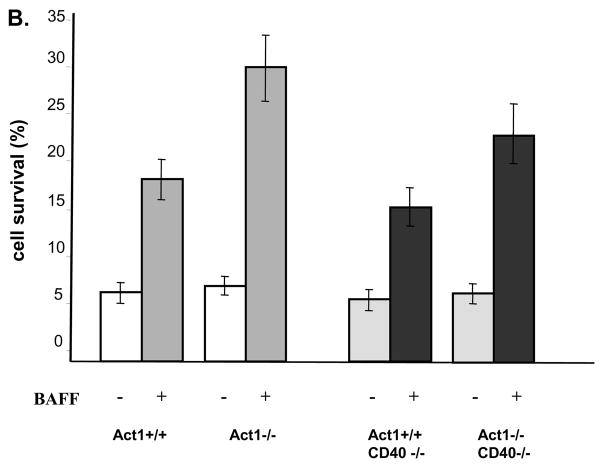
(A) FACS analysis of splenocytes from three pairs of 8 week old Act1+/+, Act1−/−, CD40−/−, Act1−/−CD40−/−or BAFF−/− and BAFF−/−Act1−/− mice. Upper panel shows a representative staining pattern and the percentage of T cells (Thy.1+), B cells (B220+), follicular (B220+CD21intCD23hi) and marginal zone (B220+CD21hiCD23lo) B-cells. Transitional B cells were defined as (B220+IgMhiIgDlo). Lower panel shows the absolute number of cells for each cell subset. The standard deviation indicates the differences between the individual mice in each group. (B) Enhanced ex vivo BAFF-mediated survival of Act1−/−CD40−/− B cell. Purified splenic B cells from 8-week-old Act1+/+, Act1−/−, CD40−/− and Act1−/−CD40−/− mice were untreated or treated with recombinant BAFF for 4 days. Cell survival was analyzed by FACS as described in the Materials and methods. The data presented are the average of percentage of cell survival for each mice group (n=4). (C) CD40, but not BAFF is required for production of SS-associated autoantibodies. The titer of Anti-SSA/Ro (upper panel) and ant-SSB/La (lower panel) in the serum from 8–10 months old Act1+/+ (n=6), Act1−/− (n=13), Act1+/+CD40−/−(n=9) and Act1−/− CD40−/− (n=14) were measured by ELISA. Short horizontal bars indicate the mean. The significant P values are shown. A grey line is drawn across the graph to show the lowest value in Act1+/+ mice. The values below the grey line are considered positive (black filled dots).
The production of anti-SSA/Ro and anti-SSB/La antoantibodies in Act1-deficient mice is CD40-dependent
B cell hyper activation is a dominant feature of Sjogren’s syndrome. Although BAFF- and CD40-mediated signaling act synergistically in promoting B cell survival, they regulate different aspects of B cell function. While BAFF-BAFFR interaction is critical for survival and maturation of the B cells in the periphery, CD40-CD40L interaction is required for T cell-dependant B cell activation, germinal center formation and antibody production. The enhanced survival of B cells is the main mechanism for pathogenesis of Sjogren-like disease in BAFF transgenic mice. We previously found that all of the peripheral B cell subpopulations [transitional B cells (B220+IgMhiIgDlo), follicular B cells (B220+CD21intCD23hi) and marginal zone B cells (B220+CD21hiCD23lo)] were significantly increased in Act1-deficient mice as compared to wild-type littermate control mice, indicating that increased B cell survival is likely to be one of mechanisms for the autoimmune pathogenesis in Act1-deficient mice {Qian, 2004 609/id}. Interestingly, the same B cell subpopulations except marginal zone B cells were still significantly higher in the Act1−/−CD40−/− double knockout mice as compared to the CD40−/− mice, suggesting that the regulatory role of Act1 on these B cell subpopulations is CD40-independent (Figure 6A). Importantly, BAFF stimulation resulted in significantly enhanced B cell survival in the Act1−/−CD40−/− double knockout B cells as compared to CD40−/− B cells (Figure 6C). Taken together, these results suggest that Act1 probably has a more important impact on BAFF-mediated than CD40-mediated B cell survival in vivo. To test this hypothesis, we compared peripheral B cell population between BAFF−/− and Act1−/−BAFF−/− double knockout mice. In the BAFF−/− spleen, the total splenic B cells, transitional B cells and follicular B cells are dramatically reduced. Importantly, the deletion of Act1 gene in the BAFF−/− background (Act1−/−BAFF−/−) did not lead to increase in the peripheral B cell subpopulations (including transitional B cells, follicular B cells and marginal B cells) (Figure 6B), indicating that Act1 exerts its regulatory role on B cell survival mainly through its impact on BAFF-mediated pathway. In addition to B cell hyperplasia, autoantibody production is another important feature in autoimmune diseases. To determine the contribution of BAFF and CD40 pathways in the production of anti-SS-A/Ro and anti-SS-B/La antibodies in Act1-deficient mice, we examined whether these autoantibodies are present in BAFF−/−Act1−/− and CD40−/−Act1−/− double deficient mice. Sera from 14 CD40−/−Act1−/− double deficient mice and 9 CD40-deficient littermate control mice were analyzed by ELISA for anti-Ro and anti-La. Our results showed that production of anti-Ro and anti-La autoantibody was no longer detectable in the double knockout mice CD40−/−Act1−/− (Figure 7A), indicating that CD40 signaling is required for the autoantibody production. Although the mature B cells were greatly reduced in Act1−/−BAFF−/− deficient mice, significant levels of autoantibodies were still produced in these double deficient mice (ten out of fourteen mice) (Figure 7B), indicating that BAFF signaling is not required for the development of autoantibodies. These data clearly demonstrate Act1 regulates autoimmunity through CD40 and BAFF pathways by differential mechanisms (Figure 8).
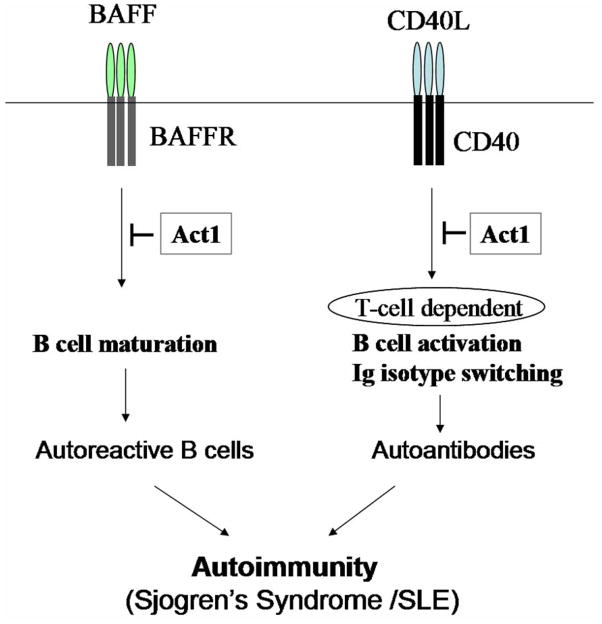
Figure 6. Schematic representation of the regulatory role of Act1 in BAFF-and CD40-mediated autoimmunity.
The BAFF-BAFFR interaction is required for B cell maturation while the CD40-CD40L interaction leads to T cell-dependent B cell activation and immunoglobulin isotype switching. Act1 regulates autoimmunity through its dual impact on BAFF-mediated B cell survival and CD40-mediated autoantibody production. While Act1 inhibits BAFF-mediated B cell maturation and survival, Act1 deficiency results in cell hyperplasia leading to autoimmunity. On the other hand, Act1 inhibits CD40-mediated T cell-dependent response and antibody production. Act1 deficiency leads to autoantibody production in a CD40-dependent, BAFF-independent manner, contributing to pathogenesis of autoimmunity.
Discussion
We previously showed that genetic loss of Act1 leads to lymphadenopathy and splenomegaly, B-cell hyperplasia and hyper-immune responses. Here we reported that Act1-deficient mice spontaneously developed Sjogren’s syndrome, associated with lymphocyte infiltration of exocrine glands, autoantibody production and complex deposition in the exocrine glands, resulting in symptoms of dry eyes and dry mouth. Act1-deficient mice also developed SLE-like phenotype, including lymphocyte infiltration of the kidney, and immunoglobulin complex deposition in kidney glomeruls, suggesting that Act1−/− mice developed secondary Sjogren’s syndrome in association with SLE-nephritis.
BAFF transgenic mice have been extensively investigated as an animal model for human Sjogren’s syndrome {Mackay, 1999 555/id;Mackay, 2007 727/id;Groom, 2002 678/id}. While the BAFF transgenic mice share several important features with human Sjogren’s syndrome, there are significant differences between BAFF transgenic mice and human Sjogren’s syndrome. First of all, self-reactive B cells in Sjogren’s syndrome patients produce high levels of anti-SS-A/Ro and anti-SS-B/La autoantibodies, whereas these autoantibodies have not been detected in BAFF transgenic mice. Importantly, Act1-deficient mice did produce high titers of anti-SS-A/Ro and anti-SS-B/La, indicating the advantage of the Act1-deficient mice as an animal model for human Sjogren’s syndrome. It is important to point out that whereas production of anti-Ro and anti-La autoantibody was no longer detected in CD40−/−Act1−/− double knockout mice, anti-Ro and anti-La were still produced in the BAFF−/−Act1−/− mice, indicating that BAFF signaling is dispensable for the production of these autoantibodies. Taken together, these results suggest that the loss of negative regulation on CD40 pathway is likely the cause for the development of anti-Ro and anti-La autoantibody in Act1-deficient mice. These findings are consistent with the fact that BAFF transgenic mice do not develop anti-Ro and anti-La autoantibody. Another major difference between BAFF transgenic model of Sjogren’s syndrome and human Sjogren’s syndrome is the role of T cells. Cells infiltrating the glands of BAFF transgenic mice are essentially marginal zone-like B cells, whereas in humans larger numbers of T cells infiltrate the gland tissues, although B cells are also present. Furthermore, BAFF transgenic mice on TCR−/− background retained all of the autoimmune phenotype indistinguishable from those of BAFF transgenic mice. The fact that the production of anti-Ro and anti-La autoantibody in Act1-deificient mice is CD40-dependent implicates the critical role of T cells in the development of Sjogren’s syndrome in Act1-deficient mice. In support of this, significant number of infiltrating T-cells was detected in the exocrine glands of Act1-deficient mice.
It is critical to note that significant degree of Sjogren’s syndrome was observed in Act1−/−CD40−/− double deficient mice. Importantly, all of the B cell subpopulations except marginal zone B cells were still significantly higher in the Act1−/−CD40−/− double knockout mice as compared to the CD40−/− mice, suggesting that the regulatory role of Act1 on these B cell subpopulations is CD40-independent. In support of this, BAFF stimulation resulted in much enhanced B cell survival in the Act1−/−CD40−/− double knockout B cells as compared to CD40−/− B cells. The increased B cell subpopulations in Act1-deficient mice were indeed abolished when they were crossed onto BAFF-deficient background. Taken together, these results suggest that Act1 may have a more important impact on BAFF-mediated than CD40-mediated B cell survival in vivo.
Coordinated regulation of T and B cell-mediated immune responses plays a critical role in the control and modulation of autoimmune diseases. Whereas Act1 molecule negatively regulates B cell-mediated humoral immune responses through its inhibitory function in CD40L and BAFF signaling, our recent studies have shown that Act1 is also a key positive signaling component for IL-17 signaling pathway, critical for TH17-mediated autoimmune and inflammatory responses. The dual functions of Act1 are evident in Act1-deficient mice that displayed B cell-mediated autoimmune phenotypes, but are resistant to TH17-dependent EAE and colitis. Such seemingly opposite functions of Act1 in CD40-BAFFR and IL-17R signaling are orchestrated by different domains of Act1 molecule. Whereas Act1 interacts with the IL-17R through the C-terminal SEFIR domain, Act1 is recruited to CD40 and BAFFR indirectly, through its interaction with the adaptor TRAF3 (Giltiay and Li, unpublished information). Such delicate regulatory mechanisms mediated by Act1 may provide a common vehicle to promote the balance between host defense to pathogens and tolerance to self.
In conclusion, we found that Act1-deficient mice developed Sjogren’s –like disease in association with lupus nephritis. Through investigation of the double deficient mice of CD40−/−Act1−/− and BAFF−/−Act1−/−, we demonstrated that Act1 modulates autoimmunity through CD40 and BAFF by different mechanisms. Importantly, Act1−/−CD40−/− and Act1−/−BAFF−/− mice remained significant levels of autoimmune phenotype, indicating that both lymphoproliferation and production of autoantibodies can independently contribute to the development of Sjogren’s – like disease. The Act1-deficient mice represent a novel model for investigation of humoral immunity and autoimmune diseases with intrinsic defects in B cell functions. Future studies will investigate the potential use of Act1 in modulating autoimmunity and prevention of autoimmune diseases.
Methods
Mice
Act1−/−, Act1−/−CD40−/− and Act1−/−BAFF−/− mice were generated as described previously {Qian, 2004 609/id}. Act1−/− mice from Balb/c/129sv background were back crossed on to wild-type Balb/c mice for 10 generations for the experiments. Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Cleveland Clinic.
Detection of autoantibodies
Anti-SSA/Ro and Anti-SSB/La autoantibodies were detected using commercial ELISA assay (Alpha Diagnostic, Int., San Antonio, TX) according to the manufacture’s instructions. Sera from Act1−/−mice or wild-type littermate controls, as well as from CD40−/−, CD40−/−Act1−/−, BAFF−/−, BAFF−/−Act1−/− mice were used at 1:100 dilution. Both positive and negative controls were provided by the manufacture.
RF in the serum of wild-type and Act-deficient mice was determined by ELISA. Briefly, polystyrene plates were coated with 20 μg/ml NIP (4-hydroxy-3-iodo-5-nitrophenylacetyl) conjugated BSA (Biosearch Technologies), followed by incubation with 10 μg/ml monoclonal mouse anti-NIP antibody and sera from wild-type and Act1-deficient mice at 1:50 dilution. The assay was then developed using HRP-labeled anti-mouse kappa antibody. Results are presented as OD value at 414 nm.
Measurement of saliva production
Act1-deficient and wild-type littermate controls, aged 8–10 months, were anesthetized using nembutol and injected intra-peritoneally with 0.5 mg/g (bw) muscarinic agonist pilocarpine (Sigma, St. Louis, MO) to stimulate saliva production. Saliva was collected using micro-capillary pipette for 14 minutes starting immediately after the injection. The saliva secretory volumes were normalized to the body weight of each mouse.
Histopathology and Immunostaining
Lachrymal, parotid and submaxilary glands or kidneys were dissected from the mice and fixed in 10% formalin in PBS and embedded in paraffin. Sections were then cut and stained with hematoxylin and eosin (H&E) and examined by light microscopy.
For immune fluorescent staining of the lymphocyte infiltration, the lacrimal glands from Act1-deficient mice were collected and embedded in Tissue-Tek OCT compound (Sakura Finetek USA Inc., Torrance, CA) and slowly frozen over liquid nitrogen. Cryostat sections (6–8 μm) were dried and fixed in ice-cold acetone for 10 min. The slides were incubated in 0.05M PH 6.0 citrate buffer and heated until boiling. After washing with PBS the slides were blocked for 1 hr with 1% BSA in PBS, followed by incubation for 1 hr at RT with rat anti-mouse B220, rat anti-mouse CD4 and anti-mouse CD8 (BD Bioscience). The slides were washed with PBS and then incubated with Alexa-goat anti-rat IgG secondary antibody (Molecular Probes) for 1 hr. The slides were washed and covered with mounting medium before fluorescent microscopy.
Autoantigen staining and immunoglobulin deposition in lacrimal glands and kidneys was detected using fluorescein M.O.M. kit (Vector Laboratory), according to the instructions. Briefly the tissues were frozen sectioned, fixed in acetone and then air dried. The sections were washed with PBS and blocked with M.O.M. mouse Ig blocking reagent. For autoantigen staining the sections were incubated without or with sera from Act1-deficient mice or littermate control mice for 30 min. Sections were then stained with M.O.M. biotinylated rat anti-mouse IgG reagent for 10 min, washed with PBS and stained with Fluorescein Avidin DCS for 5 min. The sections were washed with PBS and covered with mounting medium for fluorescent microscopy.
B cell isolation and B cell survival assay
Mouse spleens were collected under sterile conditions and splenocytes were gently smashed through a nylon cell strainer into RPMI medium supplied with 10% FCS to create a single – cell suspension. Cells were centrifuged at 1,000 rpm for 5 min. and the pellet was re-suspended in 1 ml per spleen RBC lysis buffer for 5 min at room temperature. The remaining white blood cells were collected and panned on 150 cm plates pre-coated with anti-mouse B220 antibody for two rounds to get total B cells. For the cell survival assay, splenic B cells from naive mice were incubated at concentration of 3×106 cells/ml in RPMI medium plus 10% FCS, untreated or treated with anti-CD40 antibody or BAFF ligand for 4 days. Annexin V-FITC apoptosis detection kit was used for detecting apoptotic cells and propidium iodide used for detecting dead cells in the flow analysis. The percentage of remaining live cells was determined by flow analysis using BD FACSCalibur instrument.
Flow cytometry
Single-cell suspensions from the mice spleens were stained with conjugated mAbs, including PE anti-B220, anti-IgM, anti-CD21, PerCP anti-B220 and FITC anti-CD5, anti-IgD, anti-CD21, anti-Thy1, anti-CD24, APC anti-CD23 and biotinylated anti-CD23 and anti-B220, followed by allophycocyanin-streptoavidin (all from BD Biosciences, San Diego, CA).
Cell associated fluorescence was detected on BD FACScan instrument and analyzed with Cell Quest software (BD Biosciences). The cell content of the glands was analyzed after collagenase digestion. Single-cell suspensions were stained using PE or PerCP-conjugated anti-B220 antibody and FITC-conjugated anti-CD3 antibody. Flow cytometry was performed on BD FACSCaliber and data was analyzed using FlowJo software (Tree Star)
Supplementary Material
Acknowledgments
This study is supported by the National Institutes of Health (AI 065470 to X.L.) and the Leukemia and Lymphoma Society (Y.Q.).
Nonstandard abbreviations used
- BAFF
B lymphocyte-activating factor belonging to the TNF superfamily
- BAFFR
BAFF receptor
- H&E
hematoxylin and eosin
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- CD40L
CD40 ligand
- Ig
immunoglobulin
- SS
Sjogren’s Syndrome
- SLE
systemic lupus erythematosus
- TD
T cell-dependent
- TI
T cell-independent
Footnotes
This study is supported by the National Institutes of Health (AI 065470 to X.L.) and the Leukemia and Lymphoma Society (Y.Q.).
References
- 1.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 3.Manoussakis MN, Moutsopoulos HM. Sjogren’s syndrome: Current concepts. Adv Intern Med. 2001;47:191–217. [PubMed] [Google Scholar]
- 4.Manoussakis MN, Georgopoulou C, Zintzaras E, Spyropoulou M, Stavropoulou A, Skopouli FN, Moutsopoulos HM. Sjogren’s syndrome associated with systemic lupus erythematosus: Clinical and laboratory profiles and comparison with primary Sjogren’s syndrome. Arthritis Rheum. 2004;50:882–891. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 5.MacSween RN, Goudie RB, Anderson JR, Armstrong E, Murray MA, Mason DK, Jasani MK, et al. Occurrence of antibodyto salivary duct epithelium in Sjogren’s disease, rheumatoid arthritis, and other arthritides. A clinical and laboratory study. Ann Rheum Dis. 1967;26:402–411. doi: 10.1136/ard.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slobbe RL, Pruijn GJ, Damen WG, van der Kemp JW, van Venrooij WJ. Detection and occurrence of the 60-and 52-kD Ro (SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol. 1991;86:99–105. doi: 10.1111/j.1365-2249.1991.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276:604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 8.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2002;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Kooten C, Banchereau J. Functional role of CD40 and its ligand. Int Arch Allergy Immunol. 1997;113:393–399. doi: 10.1159/000237614. [DOI] [PubMed] [Google Scholar]
- 10.Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 11.Lei XF, Ohkawara Y, Stampfli MR, Mastruzzo C, Marr RA, Snider D, Xing Z, Jordana M. Disruption of antigen-induced inflammatory responses in CD40 ligand knockout mice. J Clin Invest. 1998;101:1342–1353. doi: 10.1172/JCI1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 13.Mackay F, Browning JL. BAFF: A fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 14.Mackay F, Schneider P, Rennert P, Browning J. BAFFANDAPRIL: A tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 15.Grammer AC, Lipsky PE. CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signalingmechanisms. Adv Immunol. 2000;76:61–178. doi: 10.1016/s0065-2776(01)76019-1. [DOI] [PubMed] [Google Scholar]
- 16.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, et al. The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 18.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: Fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Biancone L, Cantaluppi V, Camussi G. CD40-CD154 interaction in experimental and human disease (review) Int J Mol Med. 1999;3:343–353. doi: 10.3892/ijmm.3.4.343. [DOI] [PubMed] [Google Scholar]
- 21.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutgens E, Daemen MJ. CD40-CD40L interactions in atherosclerosis. Trends Cardiovasc Med. 2002;12:27–32. doi: 10.1016/s1050-1738(01)00142-6. [DOI] [PubMed] [Google Scholar]
- 23.Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, et al. Act1, a negative regulator in. Immunity. 2004;21:575–587. doi: 10.1016/j.immuni.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita G, Purcell AW, Keech CL, Farris AD, McCluskey J, Gordon TP. Molecular chaperones are targets of autoimmunity in Ro(SSA) immune mice. Clin Exp Immunol. 1999;115:268–274. doi: 10.1046/j.1365-2249.1999.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotzin BL, Lafferty JA, Portanova JP, Rubin RL, Tan EM. Monoclonal anti-histone autoantibodies derived from murine models of lupus. J Immunol. 1984;133:2554–2559. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.