Abstract
Objective
Previous investigations suggest that agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) may mediate a hypertensive response through dysregulation of the endothelin-1 system. AT1-AA induced hypertension is attenuated by AT1 receptor and/or Endothelin-1 type A receptor antagonists. This study was undertaken to determine if AT1-AA induced hypertension is associated with renal endothelial dysfunction.
Study Design
We compared the vascular reactivity of renal interlobar arteries from normal pregnant control rats and AT1-AA chronically infused pregnant rats in the presence and absence of endothelin type A (ETA) receptor antagonism. Renal endothelial function was tested using isolated renal interlobar arteries in a pressure myograph that were exposed to acetylcholine or sodium ntiroprusside.
Results
Vasodilatory responses to the endothelial dependent agonist acetylcholine were impaired in AT1-AA rats (74±10%) compared to NP controls (95±5%, p<0.05). In the presence of endothelin type A (ETA) receptor antagonism, no differences were observed between controls or the AT1-AA treated group in regard to endothelial dependent (acetylcholine) relaxation.
Conclusion
AT1-AA induced hypertension during pregnancy is associated with disparate renal endothelial responses to acetylcholine. The difference in renal vascular responses between AT1-AA and NP rats is abolished by ETA receptor blockade.
Keywords: Hypertension, angiotensin, pregnancy, endothelial dysfunction
Introduction
The initiating event in early onset preeclampsia is postulated to involve reduced placental perfusion that leads to hypertension during pregnancy by mechanisms not yet elucidated.1,2 Recent studies have suggested that the production of agonistic autoantibodies to the angiotensin II (AngII) type I receptor (AT1-AA) may be an important link between placental ischemia and hypertension in preeclamptic women.3–8 The AT1-AA induces signaling in vascular cells including activating protein-1, calcineurin and nuclear factor kappa B activation which are blocked by an AT1 receptor antagonist.3,4,7 Recent studies by Zhou et al. demonstrate that immunoglobulin isolated from preeclamptic women increased systolic blood pressure and causes renal pathology four days after retro-orbital injection into pregnant mice.7,8 This hypertensive response was attenuated by administration of an AT1 receptor antagonist. While these findings suggest that AT1-AAs from preeclamptic women increases blood pressure in pregnant mice, possibly by activation of the AT1 receptor, it remains unclear by what mechanism purified AT1-AA mediates hypertension, renal or vascular function during pregnancy.
We recently reported that the hypertension in response to reductions in uterine perfusion pressure in pregnant rats (RUPP) is associated with circulating levels of the AT1-AA.9 Moreover, we found that the increased blood pressure response in RUPP pregnant rats decreased markedly by antagonism of the AT1 receptor. In addition, we previously reported that the hypertension in response to chronic AT1-AA infusion is associated with significant increases in placental and renal expression of preproendothelin and this blood pressure response is attenuated by administration of a selective endothelin type A (ETA) receptor antagonist.10 In contrast, ETA receptor blockade had no significant effect on blood pressure in the normal pregnant animal suggesting a role for ET-1 in mediating AT1-AA induced hypertension11,12. However, there are still a number of unanswered questions that remain in this field of investigation. While AT1-AA causes significant hypertension and renal pathology, the role of the AT1-AA to impaired renal hemodynamics, proteinuria, or renal vascular function during pregnancy is unclear. Therefore, this current investigation was performed in order to evaluate the potential role of the AT1-AA to mediate renal endothelial vascular function during pregnancy.
Materials and Methods
All studies were performed in timed pregnant Sprague Dawley rats purchased from Harlan Inc. (Indianapolis IN). Animals were housed in a temperature controlled room (23°C) with a 12:12 light: dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Rat AT1-AA was isolated and purified from a pregnant female hAogen × male hRen (MDC, Berlin) rat cross that develops hypertension associated with production of the AT1-AA beginning on day 13 of gestation.13 The complete details of rat AT1-AA isolation and purification have been previously published.3,13 Measurement of arterial pressures in chronically instrumented conscious rats was determined in all groups of pregnant rats at day 19 of gestation as previously described.9,10
Chronic administration of rat AT1-AA
To determine if AT1-AA contributes to renal endothelial dysfunction, purified rat AT1-AA was infused from day 12 to 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific Corporation, Palo Alto, CA) into normal pregnant rats (n=5) and normal pregnant rats orally treated (drinking water) with the ETA receptor antagonist (ABT-627, 5mg/kg/day) for 5 days(n=3). Normal pregnant rats (n=5) and Normal pregnant rats treated with ETA receptor antagonist (n=3) alone served as controls. Blood pressure and vascular function was compared among the groups on day 19 of gestation.
Vascular studies
On day 19 of gestation, animals were sacrificed, kidneys were removed, and the renal interlobar arteries were isolated from and prepared for vessel reactivity studies in a pressure myograph as previously described.14 The interlobar artery was equilibrated at a baseline intraluminal pressure of 75 mmHg. Vasodilation was assessed in vessels that were preconstricted with phenylephrine (10−5 M). This dose of phenylephrine has previously been shown to induce a sustained vasoconstriction in renal vessels and there was no difference in phenylephrine induced constriction at this concentration.14,15 After preconstriction, concentration responses to acetylcholine and sodium nitroprusside (10−9 to 10−4 M) were performed to assess endothelial dependent and smooth muscle dependent vasodilation, respectively. The degree of vessel response to acetylcholine and sodium nitroprusside was determined by measuring the change in the lumen diameter via an electronic caliper with each subsequent exposure to increasing concentrations of these vasodilatory agents. Data are presented as the percent dilation relative to the preconstricted inner diameter of the vessel.
Statistics
All data are expressed as mean+/− standard error. Differences between control and experimental groups were analyzed using the student t-test. Differences between multiple groups were analyzed via one way ANOVA with a Student Newman-Keuls Post Hoc test. Associations were considered significant if the p-value was < 0.05.
Results
AT1-AA induced hypertension
As we have previously reported, chronic administration of rat AT1-AA into normal pregnant rats significantly increases blood pressure.12 In addition, administration of a selective ETA receptor antagonist for 7 days to normal pregnant control rats and pregnant rats chronically treated with AT1-AA abolished AT1-AA induced hypertension. This study was performed to determine if the AT1-AA induced hypertension was associated with altered renal vascular function and whether differences in renal vascular function are abolished after ETA blockade.
AT1-AA induced renal vascular dysfunction
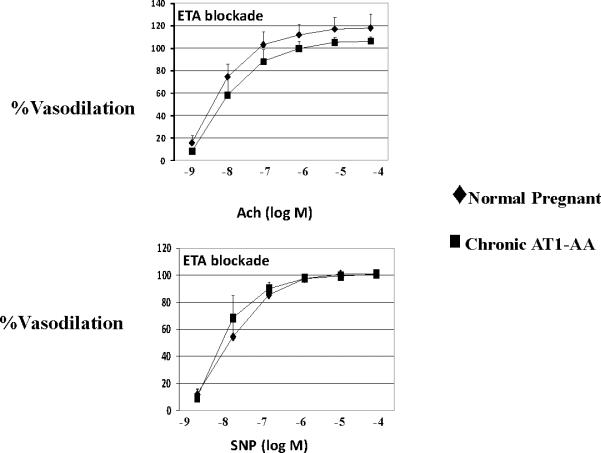
Renal endothelial function was tested using isolated renal interlobar arteries in a pressure myograph. Vasodilatory responses to the endothelial dependent agonist acetylcholine (10−8 M) were lower in AT1-AA rats (74±10%) compared to NP controls (95±5%, p<0.05). The response to the smooth muscle dependent agonist sodium nitroprusside was not different (Figure 1). However, in the presence of the ETA receptor antagonist, vasodilatory responses to the endothelial dependent agonist acetylcholine were not significantly different between the controls and those rats exposed to chronic excess levels of the AT1-AA (Figure 2).
Figure 1.
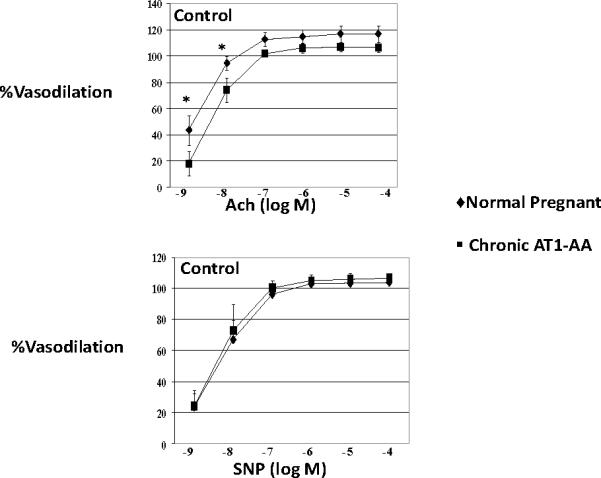
Vasodilatory responses to the endothelial dependent agonist acetylcholine (ACh) (10-8 M) were impaired in AT1-AA rats (74±10%) compared to NP controls (95±5%, p<0.05). The response to sodium nitroprusside (SNP) was not different.
Figure 2.
In the presence of endothelin type A (ETA) receptor antagonism, no differences were observed between controls or the experimental group in regard to endothelial dependent (ACh=acetylcholine) or smooth muscle dependent (SNP=sodium nitroprusside) relaxation.
Comment
There is growing evidence to suggest that dysregulation of the tissue-based and circulating reninangiotensin system (RAS) may be involved in the pathophysiology of preeclampsia.16–19 One mechanism by which this system may exert its effects is through the excess production of an agonistic autoantibody to the angiotensin II type 1 receptor (AT1-AA).17,21 This autoantibody is thought to be a central mediator of several pathways in preeclampsia. However, both the specific mechanisms that lead to excess production and the mechanisms whereby AT1-AA increases blood pressure during pregnancy remain unclear. One proposed mechanism of AT1-AA induced hypertension includes stimulation of the endothelin-1 system, one hallmark of endothelial dysfunction, and a characteristic of preeclampsia. We recently demonstrated that increasing levels of AT1-AA to that observed in preeclamptic women and in placental ischemic rats, led to increased mean arterial pressure (MAP) in pregnant rats via activation of the endothelin system.12 In the current investigation, we evaluated renal endothelial function during chronic AT1-AA infusion. Vasodilatory responses to the endothelial dependent agonist acetylcholine were reduced in AT1-AA induced hypertensive pregnant rats compared to normal pregnant controls. The response to sodium nitroprusside was not different suggesting normal smooth muscle function. Furthermore, the response to acetylcholine was not different between animals treated with the Endothelin Type A receptor antagonist. The fact that renal vascular function is different between AT1-AA and vehicle treated animals, but not in animals treated with ETA receptor blockers, suggests that one mechanism whereby AT1-AA could contribute to hypertension during pregnancy is via alterations in renal vascular function that are dependent upon the ET-1 pathway.
Our laboratory has been evaluating the role of this autoantibody in a pregnant RUPP rat model. We recently demonstrated that placental ischemic-induced hypertension in pregnant rats is associated with increased circulating levels of the AT1-AA.22 In addition, we demonstrated that chronic elevation of TNF alpha in pregnant rats is associated with increased production of the AT1-AA.9 Moreover, we discovered that the hypertension in response to placental ischemia in pregnant rats and in response to chronic infusion of TNF alpha in pregnant rats was markedly attenuated by antagonism of the AT1 receptor. Collectively, these novel findings indicate that placental ischemia and TNF alpha are important stimuli of AT1-AA production during pregnancy and that activation of the AT1 receptor appears to play an important role in the hypertension produced by placental ischemia and TNF alpha in pregnant rats. However, the mechanism(s) by which the autoantibody mediates hypertension has remained undefined.
Although the present study demonstrates that infusion of the AT1-AA is associated with altered renal endothelial function, whether these local vascular changes promote renal hemodynamic impairment that leads to hypertension is not clear. In addition, the stimulus and mechanism whereby the AT1-AA is produced in response to placental ischemia or TNF alpha during pregnancy remains undefined. Experiments designed to inhibit the production of the AT1-AA in pregnant RUPP rats could contribute to our understanding of the pathophysiological role of the autoantibody during pregnancy.
Acknowledgments
Sources of Funding This work was supported by AHA SDG0835472N; NIH grants HL78147, HL51971, HL085907, and HL092284. Ralf Dechend is supported by the German Research Foundation (DFG 631/7-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no financial or other conflicts of interest to disclose.
References
- 1.Roberts JM, Lain KY. Recent Insights into the pathogenesis of preeclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Dechend R, Homuth V, Wallukat G, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 4.Dechend R, Viedt C, Muller DN, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. 1. [DOI] [PubMed] [Google Scholar]
- 5.Dechend R, Muller DN, Wallukat G, et al. Activating auto-antibodies against the AT1 receptor in preeclampsia. Autoimmun Rev. 2005;4:61–65. doi: 10.1016/j.autrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Dechend R, Homuth V, Wallukat G, et al. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce preeclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaMarca BB, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Elevated agonistic autoantibodies to the angiotensin type 1 (AT1-AA) receptor in response to placental ischemia and TNF alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaMarca BB, Speed J, Fournier L, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: Effect of TNF alpha blockade. Hypertension. 2008;52:1–5. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaMarca BB, Chandler D, Grubbs L, et al. Role of sex steroids in modulating TNF alpha induced changes in vascular function and blood pressure. Am J Hypertens. 2007;20:1216–1221. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamarca BB, Parrish MR, Ray LF, et al. Hypertension in Response to Autoantibodies to the Angiotensin II Type I Receptor (AT1-AA) in Pregnant Rats: Role of Endothelin-1. Hypertension. 2009;54:905–90. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walther T, Wallukat G, Jank A, et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MJ, Jernigan NL, Drummond HA, et al. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res. 2006;54:24–29. doi: 10.1016/j.phrs.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 289:F891–901. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 16.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herse F, Dechend R, Harsem NK, et al. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension. 2007;49:604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 19.Levesque S, Moutquin JM, Lindsay C, Roy MC, Rousseau F. Implication of an AGT haplotype in a multigene association study with pregnancy hypertension. Hypertension. 2004;43:71–78. doi: 10.1161/01.HYP.0000104525.76016.77. [DOI] [PubMed] [Google Scholar]
- 20.Mello G, Parretti E, Fatini C, et al. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension. 2005a;45:86–91. doi: 10.1161/01.HYP.0000149950.05182.a3. [DOI] [PubMed] [Google Scholar]
- 21.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dechend R, Llinas M, Caluwaerts S, et al. Agonistic autoantibodies to the AT1 receptor in rat models of preeclampsia: induced by chronic reductions in uterine perfusion pressure (RUPP) and low dose TNF alpha infusion. Hypertension. 2006;48(4):e35. [Google Scholar]