Abstract
Accurate chromosome segregation during mitosis is critical for maintaining genomic stability. The spindle checkpoint is a cellular surveillance system that ensures the fidelity of chromosome segregation. In response to sister chromatids not properly captured by spindle microtubules, the spindle checkpoint interferes with the functions of Cdc20, the mitotic activator of the anaphase-promoting complex or cyclosome (APC/C), thereby blocking APC/C-mediated degradation of securin and cyclin B to delay anaphase onset. This review summarizes the recent progress in the mechanisms by which checkpoint proteins inhibit APC/C, the conformational and enzymatic activation of checkpoint proteins, and the emerging roles of APC/C-dependent ubiquitination in checkpoint inactivation.
Keywords: Mitosis, Chromosome Segregation, Cell Cycle, Ubiquitination
1. Introduction
The cell division cycle consists of a series of temporally and spatially regulated events. Certain cell cycle processes need to be completed before others are initiated. For example, DNA replication needs to be completed before chromosome segregation occurs. The periodic rise and fall of the cyclin-dependent kinase (Cdk) activities are critical for the orderly progression of the cell cycle. An important mechanism that regulates the Cdk activities is timely degradation of cyclins and Cdk inhibitors through the ubiquitin-proteasome pathway.
The anaphase-promoting complex or cyclosome (APC/C) is a multisubunit ubiquitin ligase that regulates multiple cell cycle transitions, including the metaphase–anaphase transition and exit from mitosis [1–3]. Two APC/C activators, Cdc20 and Cdh1, directly bind to APC/C, activate its ubiquitin ligase activity, and contribute to its substrate recognition and specificity. Cdc20 is the mitotic activator of APC/C while Cdh1 mainly interacts with APC/C in telophase and G1. Securin and mitotic cyclins are the major substrates of APC/CCdc20 during mitosis. Degradation of securin and cyclin B activates separase, which cleaves the cohesin complex and triggers sister-chromatid separation. Cyclin B degradation also inactivates Cdk1 and promotes mitotic exit (Fig. 1).
Fig. 1.
The spindle checkpoint. The anaphase-promoting complex or cyclosome (APC/C) is regulated by the spindle checkpoint during mitosis. During prometaphase, improperly attached kinetochores (red dots) turn on the spindle checkpoint and produce signals to inhibit APC/CCdc20. After all pairs of sister kinetochores (green dots) are properly attached to spindle microtubules at metaphase, the spindle checkpoint is turned off, and APC/CCdc20 is activated. Active APC/CCdc20 mediates the ubiquitination and degradation of securin and cyclin B, which in turn leads to separase activation, Cdk1 inactivation, and chromosome segregation and mitotic exit.
The preservation of genome integrity is a critical task of cell division. To accomplish this task, cells duplicate their chromosomes during S phase and hold sister chromosomes together through sister-chromatid cohesion. In mitosis, the spindle checkpoint monitors the microtubule attachment to and the tension between the two opposing kinetochores of all pairs of sister chromatids [4,5] (Fig. 1). A single unattached kinetochore in a mammalian cell is sufficient to activate the spindle checkpoint, which blocks APC/CCdc20 activation and anaphase onset. Only when all pairs of sister kinetochores are properly captured by spindle microtubules and are under tension, APC/CCdc20 is activated, leading to the degradation of securin and cyclin B, activation of separase, removal of sister-chromatid cohesion, and sister-chromatid separation. In this review, we discuss recent findings that have advanced our understanding of spindle checkpoint activation and inactivation, and highlight the mutual antagonism between the spindle checkpoint and its major target APC/CCdc20.
2. Inhibition of APC/CCdc20 by the spindle checkpoint
The core components of the spindle checkpoint include the mitotic arrest deficiency (Mad) 1–3 and budding uninhibited by benomyl (Bub) 1–3 proteins. They were initially discovered through genetic studies in the budding yeast and were later shown to be conserved from yeast to man [4,5]. Upon checkpoint activation, these proteins use multiple strategies to inhibit APC/CCdc20 upon checkpoint activation, including the stoichiometric binding to and sequestration of Cdc20 by Mad2 and BubR1, and phosphorylation of Cdc20 by Bub1 [3] (Fig. 2).
Fig. 2.
Generation of APC/C-inhibitory checkpoint signals by unattached kinetochores. Mad2 and BubR1 directly bind to Cdc20, forming a larger mitotic checkpoint complex (MCC; BubR1–Bub3–Cdc20–Mad2) to inhibit APC/CCdc20. Unattached kinetochores recruit and activate Mad2 and BubR1 to promote MCC assembly. The KMN (Knl1–Mis12–Ndc80) network of kinetochore proteins provides a major platform for the recruitment of checkpoint proteins, including Bub1 and BubR1. BubR1 and Bub1 bind to Cdc20 through conserved KEN-boxes (indicated as black bars) and other motifs. The kinetochore targeting of the Mad1–Mad2 core complex also depends on Mps1 and Ndc80. Kinetochore-bound Mad1–Mad2 catalyzes O–C conformational activation of Mad2. MCC binds to and inhibits APC/CCdc20 through blocking substrate recognition. Bub1 phosphorylates Cdc20 and contributes APC/CCdc20 inhibition.
2.1. Mechanism of APC/C-dependent ubiquitination
To review how the spindle checkpoint inhibits APC/C, we briefly summarize how APC/C ubiquitinates its substrates. APC/C is a multisubunit ubiquitin ligase distantly related to the cullin-RING family of ubiquitin ligases. The subcomplex of Apc2 (a cullin-like subunit) and Apc11 (a RING-containing subunit) recruits and activates ubiquitin-conjugating enzymes (UBCs) [6]. Cdc20 or Cdh1 helps to recruit substrates to APC/C for efficient ubiquitination [1–3]. All APC/C substrates contain small peptide motifs that are critical for their recognition by APC/C, often referred to as APC/C degrons. The destruction box (D-box, with a consensus of RXXLXXXXN) and the KEN-box (with a consensus of KEN) are two well-characterized APC/C degrons. Cdc20 and Cdh1 contain a C-terminal WD40 domain, which has been shown to contact D-box directly through chemical crosslinking [7]. In addition to Cdc20 or Cdh1, Apc10 has been implicated in D-box recognition [8,9]. Recent electron microscopy (EM) studies confirm and extend these previous findings, and show that the D-box binds at the interface formed by Cdh1 and Apc10 [10–12]. How the KEN-box is recognized remains unclear. It is also unclear why many APC/C substrates contain both D- and KEN-boxes.
2.2. Inhibition of Cdc20 by Mad2 and BubR1
Mad2 and BubR1 (the vertebrate homolog of yeast Mad3) directly bind to Cdc20 and inhibit APC/CCdc20 in vitro [13,14]. Mad2 and BubR1 together inhibit APC/C with greater efficiency than either alone does [15]. Indeed, Mad2 and BubR1 are parts of a larger mitotic checkpoint complex (MCC) that contains Mad2, BubR1, Bub3, and Cdc20, and inhibit APC/CCdc20 synergistically in vivo [16,17]. On the other hand, depletion of either Mad2 or BubR1 from human cells by RNA interference (RNAi) accelerates mitotic progression and shortens the mitotic duration between nuclear envelope breakdown and anaphase onset [18]. Depletion of both has a greater effect than depletion of either alone, suggesting that Mad2 and BubR1 might have residual APC/C-inhibitory activities in the absence of each other.
2.3. Conformational activation of Mad2
Mouse genetic studies have demonstrated the physiological importance of the Mad2–Cdc20 interaction in maintaining chromosomal stability [19]. Consistently, binding of Mad2 to Cdc20 is tightly regulated by the spindle checkpoint. Efforts to understand this regulation have led to the discovery that Mad2 is an unusual two-state protein [13,20–26]. Mad2 has two native folds: a latent open conformer (O-Mad2; also known as N1-Mad2) and a closed conformer (C-Mad2; also known as N2-Mad2) that is more active in Cdc20 binding and APC/C inhibition. The current available evidence is consistent with the following model for the conformational activation of Mad2 [27,28] (Fig. 2). Mad2 binds to its upstream regulator Mad1 throughout the cell cycle, forming a tight Mad1–C-Mad2 core complex. Upon spindle checkpoint activation, the Mad1–Mad2 core complex is targeted to unattached kinetochores, and further recruits cytosolic O-Mad2 through O–C Mad2 dimerization. The asymmetric O–C Mad2 dimerization induces a conformational change of O-Mad2, which adopts a transient intermediate conformation (I-Mad2). I-Mad2 can complete the O–C conformational activation and dissociate from the Mad1–Mad2 core to become C-Mad2 without a ligand, which then binds Cdc20. Alternatively, I-Mad2 is directly captured by kinetochore-bound Cdc20, forming the Mad2–Cdc20 complex. How Mad2 binding to Cdc20 inhibits APC/C is not understood.
2.4. BubR1/Mad3 as a pseudo-substrate inhibitor of APC/CCdc20
BubR1 inhibits the activity of APC/CCdc20 more effectively than Mad2 does in vitro [14,15]. BubR1 contains an N-terminal tetratricopeptide repeat (TPR) domain required for kinetochore targeting, a conserved GLEBS motif that mediates Bub3 binding, and a C-terminal kinase domain. The kinase domain of BubR1 is not conserved in yeast and is not required for APC/C inhibition. Many studies have been devoted to dissect the functions of various domains of BubR1 and to understand the mechanism by which BubR1 inhibits APC/CCdc20 [29–35].
Yeast Mad3 contains KEN boxes, which are required for Mad3 binding to Cdc20 and for the spindle checkpoint function of Mad3 [29–31]. Furthermore, the KEN boxes of Mad3 compete with KEN-box-containing APC/C substrates for binding to Cdc20. The requirement for the KEN box of BubR1 in Cdc20 binding and in the spindle checkpoint is conserved in higher organisms, including human, mouse, and fly [32,33,35]. Interestingly, BubR1/Mad3 itself does not undergo APC/CCdc20-dependent degradation. Therefore, these results suggest that BubR1/Mad3 acts as a competitive, pseudo-substrate inhibitor of APC/CCdc20.
These findings raise two unresolved questions. First, why is BubR1 not ubiquitinated by APC/CCdc20 and degraded? Acetylation of BubR1 at K250 by the acetyltransferase PCAF has been suggested to allow BubR1 to escape APC/CCdc20-dependent ubiquitination [36]. The underlying mechanism of how acetylation prevents BubR1 ubiquitination is not established, however. Second, two major APC/CCdc20 substrates, cyclin B and securin, contain a D-box. If BubR1 uses its KEN-box to compete with substrates for binding to APC/CCdc20, how does it inhibit D-box-dependent ubiquitination? Along this vein, several studies have shown that human BubR1 contains other Cdc20-binding motifs, in addition to the KEN-boxes [14,35,37]. It will be interesting to test whether these BubR1 motifs block D-box-dependent ubiquitination by APC/CCdc20.
2.5. Inhibition of APC/C by MCC
Although BubR1 and Mad2 can independently inhibit APC/CCdc20 in vitro, they most likely collaborate with each other to inhibit APC/CCdc20 in cells by forming MCC. By using purified chromosomes from mitotic arrested HeLa extract, Kulukian et al. have investigated the role of unattached kinetochores in MCC generation and APC/CCdc20 inhibition [38]. Unattached kinetochores enhance inhibition of APC/C by purified MCC components (Mad2, BubR1, and Bub3) and binding of BubR1 to APC/CCdc20. This enhancement depends on kinetochore-bound Mad1–Mad2 core complex and Mad2 dimerization. These results suggest that unattached kinetochores produce active Mad2 (C-Mad2) and assemble an initial Mad2–Cdc20 complex to promote further interaction with BubR1–Bub3. Thus, this study provides further support a role of unattached kinetochores in the formation of MCC (Fig. 2).
How does MCC inhibit APC/CCdc20? Recent EM studies of APC/C bound to Cdh1 or bound to MCC have provided key insights into this question [10–12,39]. It appears that Cdc20 alone and Cdc20 as a component of MCC bind to APC/C in different orientations and at different sites. In other words, binding of BubR1–Bub3 and Mad2 to Cdc20 alters the interaction between Cdc20 and APC/C. In doing so, they may compromise the substrate-recognition site formed between Cdc20 and Apc10. Therefore, MCC inhibits the activity of APC/C Cdc20 by blocking productive substrate binding both competitively and non-competitively.
2.6. Inhibition of APC/C by Cdc20 phosphorylation
Cdc20 can be phosphorylated by Bub1, MAPK, and Cdk1 [40–42]. Phosphorylation of Cdc20 at S153 by Bub1 has been shown to inhibit the activity of APC/CCdc20 [40]. Similar to BubR1, Bub1 contains KEN-boxes that interact with Cdc20 [43]. KEN-box-dependent binding then recruits Cdc20 to Bub1 and enables efficient phosphorylation of Cdc20 by Bub1. Bub1 is phosphorylated in mitosis, and the chromosome-bound Bub1 is hyperphosphorylated, suggesting that the kinase activity of Bub1 might be enhanced during checkpoint activation [44]. It is unclear how phosphorylation of Cdc20 inhibits APC/CCdc20. It is also unknown whether and how Bub1-mediated phosphorylation of Cdc20 regulates MCC formation in cells.
3. Kinetochore signaling in the spindle checkpoint
The existence of a single unattached kinetochore within a cell is sufficient to activate spindle checkpoint. The spindle checkpoint proteins are targeted to unattached kinetochores in a hierarchical fashion. Although the kinetochore is a massive protein assembly with more than 90 proteins, the KMN network that consists of Knl1/blinkin, the Ndc80 complex (Ndc80–Nuf2–Spc25–Spc24), and the Mis12 complex (Dsn1–Nsl1–Nnf1–Mis12) has emerged as a major receptor for both microtubules and checkpoint proteins [45] (Fig. 2 & 3).
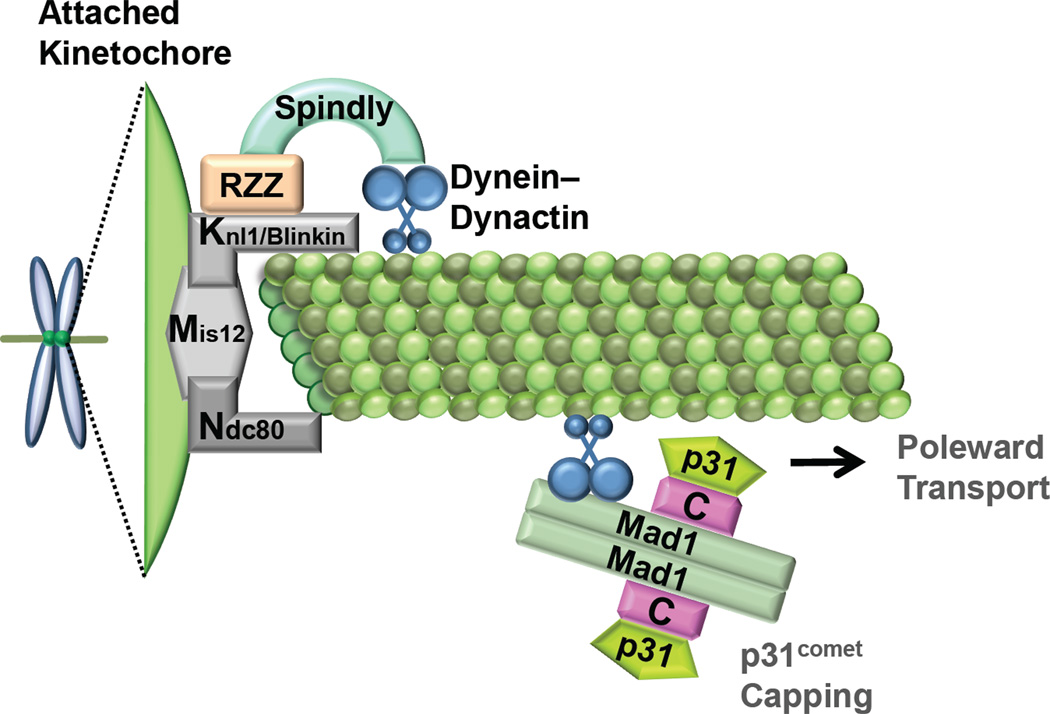
Fig. 3.
Spindle checkpoint inactivation by dynein- and microtubule-dependent removal of Mad1–Mad2 from kinetochores and by p31comet binding to C-Mad2. Dynein–dynactin is recruited to properly attached kinetochores by RZZ and spindly, and mediates the poleward transport of Mad1–Mad2. p31comet binds to C-Mad2 in the Mad1–Mad2 core complex and blocks the recruitment and activation of cytosolic O-Mad2.
3.1. Aurora B as a tension sensor
Aurora B as a part of the chromosome passenger complex (CPC; Aurora B–INCENP–survivin–borealin) lies at the top of the hierarchy for targeting checkpoint proteins to kinetochores [46]. Aurora B also promotes proper kinetochore-microtubule attachment by phosphorylating several proteins in the KMN network and thus destabilizing improper microtubule binding to kinetochores [47]. Aurora B is located at the inner centromere. The degree of phosphorylation of Aurora B substrates at the kinetochores is inversely correlated with the distance between Aurora B and its substrates [48]. It has been postulated that tension between an opposing pair of kinetochores may cause deformation of kinetochores and a physical separation between Aurora B and its substrates, thereby influencing the phosphorylation status of key kinetochore and checkpoint proteins. Aurora B can, in principle, act as a sensor for the tension (or lack thereof) across kinetochores.
3.2. Recruitment of Bub1 and BubR1
Bub1 localizes to kinetochores in mitosis and mediates the targeting other mitotic regulators to kinetochores, including BubR1 and Plk1 [49]. Ectopic targeting of Bub1 to telomeres is sufficient to recruit Bub3 and Mad3 (the yeast counterpart of BubR1) to telomeres in fission yeast [50], suggesting a direct connection between these proteins in their kinetochore targeting. Indeed, it has recently been shown that Bub1 and BubR1 are recruited to kinetochores through their interactions with blinkin/Knl1 [51]. RNAi-mediated depletion of blinkin causes defective Bub1 and BubR1 kinetochore localization while the kinetochore localization of other KMN network components, is unaffected. The N-terminal TPR domains of Bub1 and BubR1 interact directly with adjacent but distinct conserved motifs in the N-terminal region of blinkin [51,52]. Therefore, blinkin is the major receptor of Bub1 and BubR1 at the kinetochores. On the other hand, the kinetochore targeting of Bub1 and BubR1 is dependent on Bub3 [35,53]. The requirement of Bub3 in Bub1 and BubR1 kinetochore targeting is not mechanistically understood.
In addition to its functions in the spindle checkpoint, Bub1 phosphorylates the C-terminal tail of histone H2A and contributes to the recruitment of the Sgo1–PP2A complex to centromeres, where it protects the centromeric pool of cohesin from Plk1- and Wapl-dependent removal [54–62]. It is unclear, however, whether the kinetochore targeting of Bub1 is required for efficient H2A phosphorylation at the centromeres and for the centromere targeting of the Sgo1–PP2A complex.
3.3. Mps1 as a regulator of Mad1 and Mad2 kinetochore targeting
It is well established that Mps1 has a conserved function in regulating the kientochore localization of Mad1 and Mad2 [63]. Recent studies using chemical inhibitors of Mps1 have revealed additional intricacies in this regulation [64–68]. In particular, Hewitt et al. have presented data to suggest that Mps1 regulates O-Mad2 recruitment to the Mad1–Mad2 core complex in mitosis of human cells [64]. By using a chemical inhibitor AZ3146 to inhibit Mps1 during different cell cycle stages, they have shown that inhibition of the Mps1 kinase activity before mitotic entry disrupts the kinetochore localization of both Mad1 and Mad2. However, inhibition of Mps1 after mitotic entry prevents O-Mad2 recruitment to kinetochore, but does not affect the kinetochore localization of the Mad1–Mad2 core complex. Therefore, these findings suggest that the recruitment of cytosolic O-Mad2 to kinetochores and possibly Mad2 conformational activation is regulated by upstream checkpoint signaling and is thus sensitive to checkpoint status. How Mps1 regulates Mad1 and O-Mad2 recruitment to kinetochores is not understood.
How is Mps1 itself targeted to and activated at the kinetochores? The kinetochore targeting of Mps1 requires the Ndc80 complex [69]. Mps1 activation is dependent on autophosphorylation events on its activation loop [70]. Chemically induced dimerization of Mps1 is sufficient to activate Mps1 [70]. Mps1 indeed forms oligomers in mitotic human cells [64]. Therefore, the current evidence suggests that Mps1 undergoes dimerization and trans-autophosphorylation at kinetochores, leading to its activation. Interestingly, inhibition of its kinase activity leads to further accumulation of Mps1 at kinetochores, suggesting the existence of negative feedback regulation of Mps1 [71].
3.4. Kinetochore targeting of APC/C
The target of the spindle checkpoint, APC/C, is recruited to improperly attached kinetochores in several organisms, in a manner dependent on the activities of the spindle checkpoint and Cdk1 [72–74]. Interestingly, a key APC/C substrate, cyclin B, is also localized at unattached kinetochores during prometaphase and is transported to spindle poles after microtubule attachment in a process requiring dynein/dynactin [75]. The kinetochore localization of cyclin B is attenuated in Mad2-depleted cells. Although the functional importance of the kinetochore localization of APC/C and cyclin B remains to be established, these results raise the intriguing possibility of local inhibition of APC/C at unattached kinetochores by the spindle checkpoint.
4. Spindle checkpoint silencing
After all sister chromatids are properly attached to kinetochores, the spindle checkpoint needs to be inactivated to release its inhibition of APC/CCdc20, allowing anaphase onset. Several mechanisms have been implicated in spindle checkpoint silencing (Fig. 3 & 4), including dynein-dependent removal of checkpoint proteins from kinetochores, p31comet binding to and inhibition of C-Mad2, Mad2 phosphorylation, and APC/C-dependent ubiquitination and proteolysis.
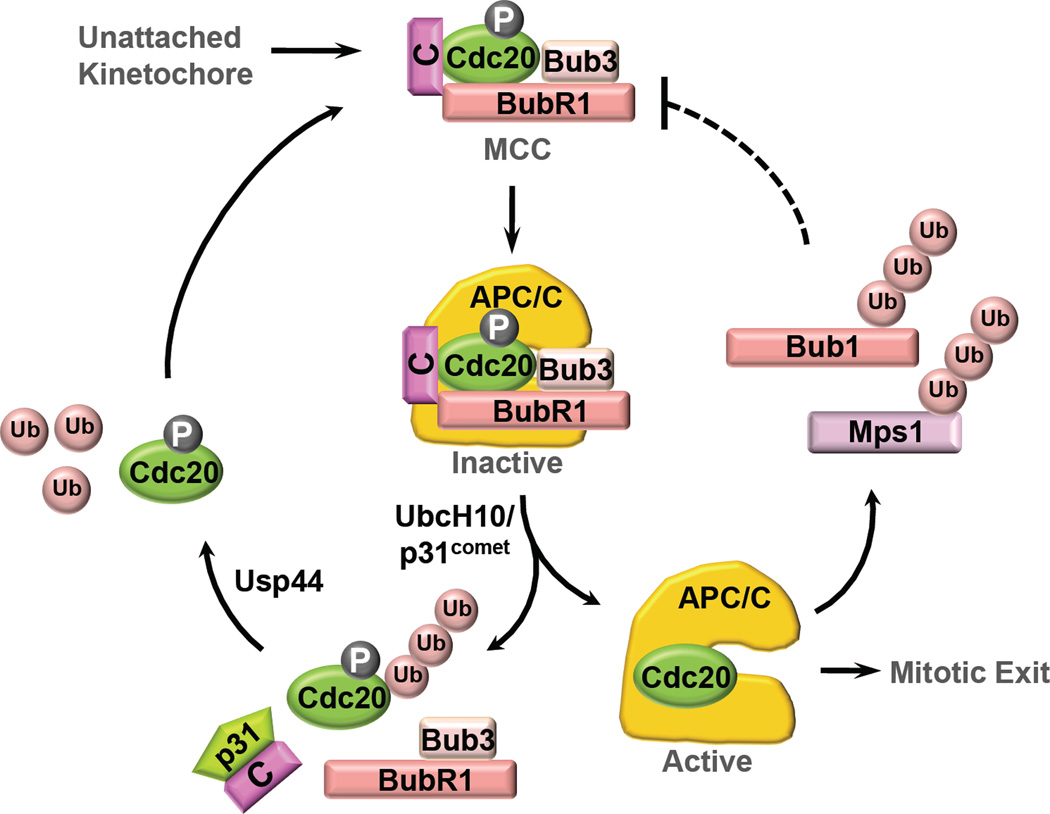
Fig. 4.
Mutual regulation between APC/CCdc20 and the spindle checkpoint. APC/C targets spindle checkpoint proteins, including Bub1 and Mps1, for ubiquitination and degradation, and indirectly regulates the kinetochore-dependent production of MCC. When bound to APC/C, Cdc20 as a subunit of MCC undergoes autoubiquitination, which is stimulated by UbcH10 and p31comet and removed by Usp44. Paradoxically, Cdc20 autoubiquitination has been proposed to regulate both checkpoint activation and inactivation.
4.1. Dynein-mediated removal of checkpoint proteins from kinetochores
The dynein/dynactin complex, a minus-end-directed microtubule motor, is required for transporting Mad1 and Mad2 from kinetochores to spindle poles following kinetochore-microtubule attachment [76]. Blocking this transport by microinjection of a dynein antibody causes prolonged metaphase arrest, indicating a crucial role of this process in checkpoint silencing. Recently, growing evidence has implicated the RZZ complex and spindly in regulating this process (Fig. 3).
RZZ is an evolutionarily conserved kinetochore complex consisting of Rod–Zwich–ZW10 [77]. It is transported from kinetochores to spindle poles during checkpoint silencing. Spindly is another conserved kinetochore protein that regulates the dynein-mediated transport of Rod and Mad2 at metaphase [78–82]. It is also transported from kinetochores to spindle poles in a dynein-dependent manner following kinetochore-microtubule attachment. The kinetochore localization of spindly depends on the RZZ complex. Depletion of Spindly reduces dynein localization at kinetochores. Moreover, a conserved motif in spindly has been shown to be critical for targeting dynein to kinetochores [81,82]. Expression of a spindly mutant with this motif mutated in cells depleted of endogenous spindly prevents the dynein-dependent removal of checkpoint proteins, resulting in defective checkpoint inactivation. Therefore, it appears that spindly bridges the interaction between RZZ and dynein, and is critical for checkpoint inactivation (Fig. 3).
4.2. p31comet binding to C-Mad2
The Mad2-binding protein, p31comet (initially called Cmt2), adopts a tertiary fold similar to that of Mad2 [83,84]. It selectively binds to the active C-Mad2 conformer and blocks its function in two ways [84–86]. First, it binds to the Mad1–Mad2 core complex and prevents the further recruitment and activation of O-Mad2 (Fig. 3). Second, it binds to Cdc20-bound C-Mad2 and neutralizes the Mad2-inhibitory effects on APC/CCdc20. Consistent with its Mad2-inhibitory activity, overexpression of p31comet causes mitotic exit in presence of the spindle poison, nocodazole. Conversely, depletion of p31comet delays mitotic exit during the recovery from nocodazole-induced mitotic arrest. Therefore, p31comet is critical for checkpoint silencing. How p31comet blocks the APC/CCdc20-inhibitory activity of Mad2 without disrupting the Mad2–Cdc20 interaction is not understood.
4.3. Phosphorylation of Mad2
Mad2 is phosphorylated on multiple sites in its C-terminal region [87]. Phosphorylation appears to inhibit Mad2 function, as Mad2 mutants with multiple phosphorylatable residues mutated to aspartates cannot bind to Mad1 or Cdc20. In a recent study, we have further characterized the effects of Mad2 phosphorylation [88]. We show that phosphorylation of Mad2 at a single site, S195, hinders its O–C conformational change. Consistently, the phospho-mimicking Mad2S195D mutant fails to bind to Cdc20, a low-affinity ligand. Interestingly, Mad2S195D still binds to high-affinity ligands, such as Mad1 and MBP1 (an artificial Mad2-binding peptide), forming ligand-bound C-Mad2. Mad2S195D overexpression causes spindle checkpoint defects in human cells. Mad2S195D appears to bind to the Mad1–Mad2 core complex with higher affinity, forming a stable Mad1–Mad2–Mad2 complex. Thus, we speculate that phosphorylation of Mad2 at S195 may prevent unscheduled activation of Mad2. Alternatively, it may contribute to checkpoint inactivation by capping the Mad1–Mad2 complex and blocking further conformational activation of Mad2, a function that is analogous to that of p31comet. The kinase responsible for phosphorylating Mad2 S195 is unknown. Given the function of Mps1 in recruiting O-Mad2 to the Mad1–Mad2 core complex at the kinetochores, it will be interesting to test whether Mps1 phosphorylates this site on Mad2.
In the same study [88], we have discovered a peculiar effect of the monomeric mutant of Mad2, Mad2R133E,Q134A. We have shown previously that overexpression of another monomeric Mad2 mutant, Mad2R133A, in HeLa cells causes mitotic arrest in the absence of spindle damage, indicating Mad2 dimerization per se is not required for APC/CCdc20 inhibition [22]. By contrast, overexpression of Mad2R133E,Q134A in human cells not only does not cause mitotic arrest, but also inhibits the functions of the endogenous Mad2 in a dominant-negative manner [88]. Because Mad2R133E,Q134A retains its ability to bind to Cdc20, this result suggests that the Mad2 dimerization helix interacts with a yet unidentified protein in cells, and this interaction is critical for APC/CCdc20 inhibition.
4.4. APC/C-dependent Cdc20 ubiquitination
APC/C is the molecular target of the spindle checkpoint. Conversely, APC/C targets several spindle checkpoint proteins for degradation, including Mps1 and Bub1 [89–91]. A recent study has implicated APC/C-dependent ubiquitination in checkpoint silencing [92]. In this study, Zeng et al. discover a small molecule called TAME, which prevents APC/C activation by Cdc20 and Cdh1. TAME causes mitotic arrest in human cells. Paradoxically, this mitotic arrest is dependent on the spindle checkpoint, as Mad2 depletion or Aurora B inhibition alleviates TAME–mediated mitotic arrest. A possible explanation for this finding is that partial inhibition of APC/C prevents checkpoint inactivation, but allows cyclin B degradation and mitotic exit in the absence of checkpoint signaling.
The targets of APC/C in checkpoint inactivation remain elusive. Artificial elevation of Mps1 levels or activity can prevent mitotic exit [89], although it remains to be demonstrated whether a non-degradable form of Mps1 delays mitotic exit. A non-degradable mutant of Bub1 with its KEN-boxes mutated does not delay anaphase onset and mitotic exit [90]. A caveat of this study is that the KEN-boxes of Bub1 are also required for efficient Cdc20 phosphorylation [43]. The non-degradable Bub1 mutant is also partially defective in its checkpoint function. Finally, Cdc20 itself undergoes autoubiquitination [93] (Fig. 4). There are conflicting results on the functions of Cdc20 ubiquitination.
In yeast, Cdc20 autoubiquitination and degradation require Mad2 and Mad3 binding, and have been proposed as an APC/CCdc20-inhibitory checkpoint mechanism [93]. As discussed above, EM studies suggest that Cdc20 in complex with checkpoint proteins binds to different sites on APC/C, as compared to Cdc20 alone. It is possible that the repositioning of Cdc20 caused by Mad2 and Mad3 binding blocks ubiquitination of securin and cyclin B, but favors Cdc20 autoubiquitination.
Cdc20 autoubiquitination is a conserved process in mammalian cells, and is stimulated by p31comet and UbcH10 [94,95]. In two related studies, Cdc20 ubiquitination is reported to cause dissociation of Mad2 from Cdc20 [94,95] (Fig. 4). Moreover, Usp44, a ubiquitin isopeptidase, has been proposed to prevent the autoubiquitination of Cdc20 and maintain the Mad2–Cdc20 interaction during checkpoint activation. Thus, these two studies implicate Cdc20 autoubiquitination as an important mechanism for checkpoint inactivation.
In a later study, Nilsson et al. have constructed a lysine-less Cdc20 mutant that cannot be ubiquitinated [96]. They show that this mutant still dissociates from Mad2 when the checkpoint is inactivated, suggesting that Cdc20 autoubiquitination is not the sole mechanism to dissociate Mad2 from Cdc20. Interestingly, ectopic expression of the lysine-less Cdc20 mutant, even to low levels, causes checkpoint defects. It then appears that Cdc20 ubiquitination might be required for the establishment of the spindle checkpoint, although the underlying mechanism is unclear. Further studies are needed to resolve these apparent discrepancies with respect to Cdc20 autoubiquitination and to uncover the molecular target(s) of APC/C during checkpoint inactivation.
5. Concluding Remarks
Impressive progress has been made towards understanding the molecular mechanisms of the spindle checkpoint. The spindle checkpoint uses an intricate intracellular signal transduction network to sense the existence of unattached or untense kinetochores in the cell, and to block the activity of APC/CCdc20. Checkpoint proteins are targeted to kinetochores in a hierarchical fashion, leading to their conformational or enzymatic activation. The activated checkpoint proteins then inhibit APC/CCdc20 through multiple mechanisms. Upon proper microtubule attachment at all kinetochores, the checkpoint is inactivated to allow APC/CCdc20 activation and anaphase onset. APC/CCdc20-mediated ubiquitination has emerged as a major mechanism for checkpoint inactivation. The mutual antagonism between APC/C and the spindle checkpoint produces negative feedback regulation in this system and allows cells to respond rapidly to the status of kinetochore-microtubule attachment.
Many significant questions in the spindle checkpoint remain unanswered, however. How does APC/C recognize substrates at the atomic level? How do Mad2 and BubR1 synergistically bind to and inhibit Cdc20? What are the kinetochore receptors for Mps1 and Mad1? What are the molecular target(s) of APC/CCdc20 in checkpoint inactivation? Future studies aimed at answering these questions will deepen our understanding of this fascinating cellular system that ensures the fidelity of chromosome segregation.
Acknowledgments
Research in the Yu laboratory is supported by the National Institutes of Health, the Welch Foundation (I-1441), and the Howard Hughes Medical Institute. S.K. is the recipient of a CPRIT predoctoral training grant. We apologize to colleagues whose primary research was not cited due to space limitation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 2.Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20:3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- 3.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 5.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 6.Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell. 2001;12:3839–3851. doi: 10.1091/mbc.12.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Carroll CW, Morgan DO. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat Cell Biol. 2002;4:880–887. doi: 10.1038/ncb871. [DOI] [PubMed] [Google Scholar]
- 9.Carroll CW, Enquist-Newman M, Morgan DO. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr Biol. 2005;15:11–18. doi: 10.1016/j.cub.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber A, Stengel F, Zhang Z, Enchev RI, Kong EH, Morris EP, et al. Structural basis for the subunit assembly of the anaphase-promoting complex. Nature. 2011;470:227–232. doi: 10.1038/nature09756. [DOI] [PubMed] [Google Scholar]
- 11.da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, et al. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buschhorn BA, Petzold G, Galova M, Dube P, Kraft C, Herzog F, et al. Substrate binding on the APC/C occurs between the coactivator Cdh1 and the processivity factor Doc1. Nat Struct Mol Biol. 2011;18:6–13. doi: 10.1038/nsmb.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Z, Bharadwaj R, Li B, Yu H. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 15.Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol Biol Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002;14:706–714. doi: 10.1016/s0955-0674(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 18.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Fang X, Wei Z, York JP, Zhang P. Loss of spindle assembly checkpoint-mediated inhibition of Cdc20 promotes tumorigenesis in mice. J Cell Biol. 2009;185:983–994. doi: 10.1083/jcb.200904020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, et al. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat Struct Biol. 2000;7:224–229. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Tang Z, Rizo J, Yu H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol Cell. 2002;9:59–71. doi: 10.1016/s1097-2765(01)00435-x. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, Tang Z, Xia G, Wassmann K, Matsumoto T, Rizo J, et al. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat Struct Mol Biol. 2004;11:338–345. doi: 10.1038/nsmb748. [DOI] [PubMed] [Google Scholar]
- 23.Sironi L, Mapelli M, Knapp S, De Antoni A, Jeang KT, Musacchio A. Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 2002;21:2496–2506. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol. 2005;15:214–225. doi: 10.1016/j.cub.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Mapelli M, Massimiliano L, Santaguida S, Musacchio A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell. 2007;131:730–743. doi: 10.1016/j.cell.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Li B, Liu CJ, Tomchick DR, Machius M, Rizo J, et al. Insights into mad2 regulation in the spindle checkpoint revealed by the crystal structure of the symmetric mad2 dimer. PLoS Biol. 2008;6:e50. doi: 10.1371/journal.pbio.0060050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mapelli M, Musacchio A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr Opin Struct Biol. 2007;17:716–725. doi: 10.1016/j.sbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Luo X, Yu H. Protein metamorphosis: the two-state behavior of Mad2. Structure. 2008;16:1616–1625. doi: 10.1016/j.str.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton JL, Solomon MJ. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 2007;21:655–667. doi: 10.1101/gad.1511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King EM, van der Sar SJ, Hardwick KG. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE. 2007;2:e342. doi: 10.1371/journal.pone.0000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sczaniecka M, Feoktistova A, May KM, Chen JS, Blyth J, Gould KL, et al. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C) J Biol Chem. 2008;283:23039–23047. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malureanu LA, Jeganathan KB, Hamada M, Wasilewski L, Davenport J, van Deursen JM. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C(Cdc20) in interphase. Dev Cell. 2009;16:118–131. doi: 10.1016/j.devcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmani Z, Gagou ME, Lefebvre C, Emre D, Karess RE. Separating the spindle, checkpoint, and timer functions of BubR1. J Cell Biol. 2009;187:597–605. doi: 10.1083/jcb.200905026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Arcy S, Davies OR, Blundell TL, Bolanos-Garcia VM. Defining the molecular basis of BubR1 kinetochore interactions and APC/C-CDC20 inhibition. J Biol Chem. 2010;285:14764–14776. doi: 10.1074/jbc.M109.082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elowe S, Dulla K, Uldschmid A, Li X, Dou Z, Nigg EA. Uncoupling of the spindle-checkpoint and chromosome-congression functions of BubR1. J Cell Sci. 2010;123:84–94. doi: 10.1242/jcs.056507. [DOI] [PubMed] [Google Scholar]
- 36.Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport J, Harris LD, Goorha R. Spindle checkpoint function requires Mad2-dependent Cdc20 binding to the Mad3 homology domain of BubR1. Exp Cell Res. 2006;312:1831–1842. doi: 10.1016/j.yexcr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16:105–117. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzog F, Primorac I, Dube P, Lenart P, Sander B, Mechtler K, et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323:1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 41.Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 42.D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang J, Yang M, Li B, Qi W, Zhang C, Shokat KM, et al. Structure and substrate recruitment of the human spindle checkpoint kinase Bub1. Mol Cell. 2008;32:394–405. doi: 10.1016/j.molcel.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen RH. Phosphorylation and activation of Bub1 on unattached chromosomes facilitate the spindle checkpoint. EMBO J. 2004;23:3113–3121. doi: 10.1038/sj.emboj.7600308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 46.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 47.Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi W, Tang Z, Yu H. Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol Biol Cell. 2006;17:3705–3716. doi: 10.1091/mbc.E06-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rischitor PE, May KM, Hardwick KG. Bub1 is a fission yeast kinetochore scaffold protein, and is sufficient to recruit other spindle checkpoint proteins to ectopic sites on chromosomes. PLoS ONE. 2007;2:e1342. doi: 10.1371/journal.pone.0001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiyomitsu T, Obuse C, Yanagida M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell. 2007;13:663–676. doi: 10.1016/j.devcel.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Bolanos-Garcia VM, Kiyomitsu T, D'Arcy S, Chirgadze DY, Grossmann JG, Matak-Vinkovic D, et al. The crystal structure of the N-terminal region of BUB1 provides insight into the mechanism of BUB1 recruitment to kinetochores. Structure. 2009;17:105–116. doi: 10.1016/j.str.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klebig C, Korinth D, Meraldi P. Bub1 regulates chromosome segregation in a kinetochore-independent manner. J Cell Biol. 2009;185:841–858. doi: 10.1083/jcb.200902128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell. 2006;10:575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci U S A. 2004;101:18012–18017. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr Biol. 2005;15:353–359. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 57.Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–517. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- 58.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 59.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 60.Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- 61.Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 62.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 63.Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 64.Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, et al. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol. 2010;190:25–34. doi: 10.1083/jcb.201002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santaguida S, Tighe A, D'Alise AM, Taylor SS, Musacchio A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol. 2010;190:73–87. doi: 10.1083/jcb.201001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tighe A, Staples O, Taylor S. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J Cell Biol. 2008;181:893–901. doi: 10.1083/jcb.200712028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwiatkowski N, Jelluma N, Filippakopoulos P, Soundararajan M, Manak MS, Kwon M, et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat Chem Biol. 2010;6:359–368. doi: 10.1038/nchembio.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maciejowski J, George KA, Terret ME, Zhang C, Shokat KM, Jallepalli PV. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190:89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 70.Kang J, Chen Y, Zhao Y, Yu H. Autophosphorylation-dependent activation of human Mps1 is required for the spindle checkpoint. Proc Natl Acad Sci U S A. 2007;104:20232–20237. doi: 10.1073/pnas.0710519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jelluma N, Dansen TB, Sliedrecht T, Kwiatkowski NP, Kops GJ. Release of Mps1 from kinetochores is crucial for timely anaphase onset. J Cell Biol. 2010;191:281–290. doi: 10.1083/jcb.201003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acquaviva C, Herzog F, Kraft C, Pines J. The anaphase promoting complex/cyclosome is recruited to centromeres by the spindle assembly checkpoint. Nat Cell Biol. 2004;6:892–898. doi: 10.1038/ncb1167. [DOI] [PubMed] [Google Scholar]
- 73.Jorgensen PM, Brundell E, Starborg M, Hoog C. A subunit of the anaphase-promoting complex is a centromere-associated protein in mammalian cells. Mol Cell Biol. 1998;18:468–476. doi: 10.1128/mcb.18.1.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang JY, Morley G, Li D, Whitaker M. Cdk1 phosphorylation sites on Cdc27 are required for correct chromosomal localisation and APC/C function in syncytial Drosophila embryos. J Cell Sci. 2007;120:1990–1997. doi: 10.1242/jcs.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bentley AM, Normand G, Hoyt J, King RW. Distinct sequence elements of cyclin B1 promote localization to chromatin, centrosomes, and kinetochores during mitosis. Mol Biol Cell. 2007;18:4847–4858. doi: 10.1091/mbc.E06-06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177:1005–1015. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto TG, Watanabe S, Essex A, Kitagawa R. SPDL-1 functions as a kinetochore receptor for MDF-1 in Caenorhabditis elegans. J Cell Biol. 2008;183:187–194. doi: 10.1083/jcb.200805185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan YW, Fava LL, Uldschmid A, Schmitz MH, Gerlich DW, Nigg EA, et al. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J Cell Biol. 2009;185:859–874. doi: 10.1083/jcb.200812167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barisic M, Sohm B, Mikolcevic P, Wandke C, Rauch V, Ringer T, et al. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol Biol Cell. 2010;21:1968–1981. doi: 10.1091/mbc.E09-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gassmann R, Holland AJ, Varma D, Wan X, Civril F, Cleveland DW, et al. Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 2010;24:957–971. doi: 10.1101/gad.1886810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21:6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang M, Li B, Tomchick DR, Machius M, Rizo J, Yu H, et al. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131:744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mapelli M, Filipp FV, Rancati G, Massimiliano L, Nezi L, Stier G, et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 2006;25:1273–1284. doi: 10.1038/sj.emboj.7601033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia G, Luo X, Habu T, Rizo J, Matsumoto T, Yu H. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wassmann K, Liberal V, Benezra R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 2003;22:797–806. doi: 10.1093/emboj/cdg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Sun H, Ball HL, Wassmann K, Luo X, Yu H. Phosphorylation of the spindle checkpoint protein Mad2 regulates its conformational transition. Proc Natl Acad Sci U S A. 2010;107:19772–19777. doi: 10.1073/pnas.1009000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palframan WJ, Meehl JB, Jaspersen SL, Winey M, Murray AW. Anaphase inactivation of the spindle checkpoint. Science. 2006;313:680–684. doi: 10.1126/science.1127205. [DOI] [PubMed] [Google Scholar]
- 90.Qi W, Yu H. KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J Biol Chem. 2007;282:3672–3679. doi: 10.1074/jbc.M609376200. [DOI] [PubMed] [Google Scholar]
- 91.Cui Y, Cheng X, Zhang C, Zhang Y, Li S, Wang C, et al. Degradation of the human mitotic checkpoint kinase Mps1 is cell cycle-regulated by APC-cCdc20 and APC-cCdh1 ubiquitin ligases. J Biol Chem. 2010;285:32988–32998. doi: 10.1074/jbc.M110.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan J, Chen RH. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 2004;18:1439–1451. doi: 10.1101/gad.1184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 95.Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 96.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]