Abstract
How a single fertilized cell generates diverse neuronal populations has been a fundamental biological problem since the 19th century. Classical histological methods revealed that post-mitotic neurons are produced in a precise temporal and spatial order from germinal cells lining the cerebral ventricles. In the 20th century DNA labeling and histo- and immuno-histochemistry helped to distinguish the subtypes of dividing cells and delineate their locations in the ventricular and subventricular zones. Recently, genetic and cell biological methods have provided insights into sequential gene expression and molecular and cellular interactions that generate heterogeneous populations of NSCs leading to specific neuronal classes. This precisely regulated developmental process does not tolerate significant in vivo deviation, making replacement of adult neurons by NSCs during pathology a colossal challenge. In contrast, utilizing the trophic factors emanating from the NSC or their derivatives to slow down deterioration or prevent death of degenerating neurons may be a more feasible strategy.
Here we present a view of neural stem cells (NSC) and their derivatives, which begins at their initial discovery and then moves forward to the time to their contemporary descriptions and classifications. We intend to highlight the significant diversity and complexity in this cellular population, and importance of timing as well as highlight the similarities and differences between NSC across mammalian species, as they pertain to promises and cautions associated with their potential use for therapeutic intervention.
Age of Rationalism: Origin of neural stem cell research
The realization that human brain development begins from the initially multipotent dividing cells did not start with the introduction of the term neural stem cell in the mid late 20th century, but at the second half of the 19th century. Old masters then recognized, with the use of histological methods, that dividing cells in the embryonic human brain are different from the similar cells in other organs. These cells, which they usually called matrix or germinal, divide close to the ventricular surface. Upon neuronal commitment, they stop dividing and migrate to a final position where they remain for the rest of the individual’s life. To our knowledge, this concept was first clearly formulated by Swiss neurologist Wilhelm His (1831–1904). He made a simple observation that mitotic figures (which signify cell division in histological preparation) are localized close to the surface of the human cerebral ventricles but are virtually absent in the overlying cortex that is forming below the outer, pial surface (His, 1874, 1886, 1904). He concluded that the germinal cells (which he called Kimzellen) produce all classes of neurons over time, which then migrate from the place of their origin to increasingly more distant locations. His’ concept that progenitors of the brain consist of two separate lines that generate neurons and glial cells was shared by Retzius (1893a, b), but opposed by the proponents of the pluripotential germinal cells (e.g. Kölliker, 1879). In addition, in spite of some recent claims to priority, he also recognized asymmetrical cell division, by which one daughter cell remains attached to the ventricular surface and her twin migrates away (Figure 1). For some of his discoveries, subsequently explained in more detail in his book published in 1904, His was a serious contender to co-share the Nobel Prize with Ramon y Cajal and Golgi had he not died before it was awarded in 1906. His’ absence on the awards stage may, in fact, have prevented some additional controversies, as some of his ideas, particularly the concept of spongioblasts as progenitors of glial cells, was contested and later proven incorrect.
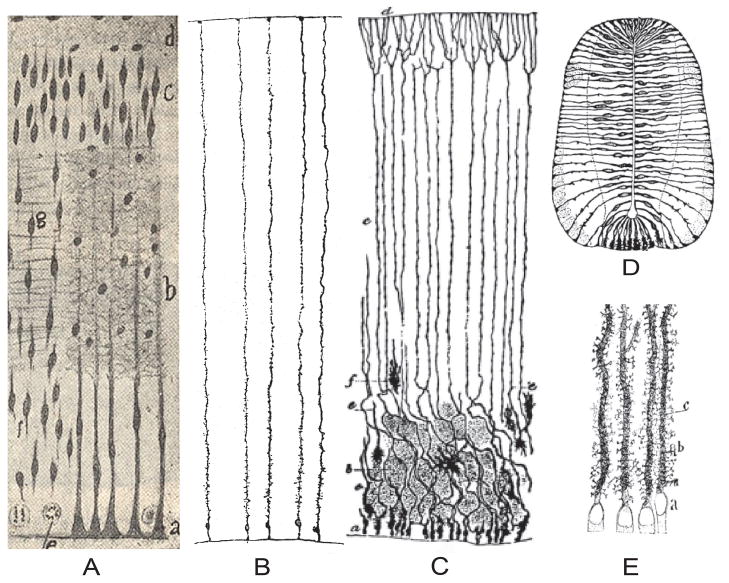
Figure 1. A Potpourri of Classical Depiction of Neural Glial Stem Cells.
A. Illustration taken from the work of Wilhelm His (1904) on the human embryonic forebrain. Notice the incredible detail and fidelity with which cell types [mitotic figures, “pongiobasts” (radial glia) migrating neurons, etc.] were depicted without the use of modern methods, including the horizontal and vertical (asymmetric)_division of the mitotic cells. B. The drawings of the “enedymal glial cells” in the human fetal cerebrum at 10 week old stand with Golgi method (Retzius, 1893b). C. Epithelial (radial glial) and neuroglial cells of the cerebral cortex at later stage of development in the neonatal rabbit stained with the Golgi method depicted by Ramón y Cajal (Ramon y Cajal, 1909). D. Primordial epithelium including trnsition to glial cells morphology in the spinal cord of the chick embryo-3rd day of incubation when, according to Ramón y Cajal, they become stainable by the Golgi method. E. Characteristic lamellate expansion on the radial shafts of epithelial (radial glial) cells. See the text for further explanation.
The introduction of the DNA replication marker 3H-thymidine in the mid-20th century increased interest in germinal cells and enabled a better delineation of their positions in the vertebrate embryonic brain. As a result, the Boulder Committee formed by the American Association of Anatomists in 1970 standardized the heterogeneous and confusing nomenclature for the developing vertebrate central nervous system and suggested that the proliferative ventricular and subventricular zones are source of all neurons and macroglia of the central nervous system (reviewed in Bystron et al., 2008). This framework, which was based on the human cerebrum, has been widely adopted as a generic description for development of the entire vertebrate central nervous system.
While the site of the active proliferative zones is not in question, the way they produce the diversity of neuronal and glial cells is. One of the dividing cell types in the developing brain that has a history of changes in its name and its role in development is the fetal glia, also discovered originally in the embryonic human brain by the old masters using the silver impregnation method (Golgi, 1885; Kölliker, 1879; Magini, 1888; Ramón y Cajal, 1899; Retzius, 1893a). Some of their conclusions, such as that the initial bipolar neuroepithelial cells (now considered NSCs) eventually transform into a fetal glioblast that produces astrocytes were by necessity based on morphological criteria (Figure.1A–E). The glial nature of these cells was confirmed a century later by the use of electron microscopy and GFAP immunohistochemistry (Levitt and Rakic, 1980; Rakic, 1972). More specifically, in the macaque fetal forebrain, radial glial shafts have utrastructurally distinct composition, including an abundance of GFAP and a difference in cytoplasmic density than the adjacent migrating neurons. In addition, they have numerous lamellate expansions that protrude at right angle from the main shaft that terminates with one to several endfeet at the pial surface. The studies in primates have lead to the concept that these elongated processes of fetal glial cells which span the thickness of the convoluted primate cerebrum serve as guides for migrating neurons (see Rakic, 1988 for review). The molecular characteristics, basic cell shape and radial orientation in structures ranging from the spinal cord to the large primate cerebrum has inspired the name “radial glial cells” (RGC) because it includes the term “glia”, favored by the old literature, as well as the term “radial” that refer to their basic orientation and connection between ventricular and pial surface, but avoids the term “fetal”, since they are not confined to the prenatal period (Rakic, 1972; Schmechel and Rakic, 1979b). This name has been generally accepted for all vertebrate species (Parnavelas and Nadarajah, 2001) in spite of the substantial species-specific differences in the timing of their transformation from the neuroepithelial cells (Kriegstein and Parnavelas, 2003, 2006; Rakic, 2003a, b). For example, in primates, some GFAP positive RGCs appear during early embryonic development (Choi, 1986; deAzevedo et al., 2003; Gadisseux and Evrard, 1985; Kadhim et al., 1988; Levitt et al., 1981; Levitt and Rakic, 1980; Rakic, 1972; Schmechel and Rakic, 1979b; Sidman and Rakic, 1973; Zecevic, 2004) and a subpopulation stop dividing transiently (Schmechel and Rakic, 1979a) to provide stable scaffolding for the formation of the large and convoluted cortex (Rakic and Zecevic, 2003a, b).
Age of Romantic Exuberance
The introduction of the new term Neural Stem Cell (NSC) about two decades ago and development of advanced methods to study cell lineages in vivo (Gage et al., 1995) and in vitro (Lendahl et al., 1990; Reynolds and Weiss, 1992; Temple, 1989) transformed the field and led to an unprecedented level of expectation that NSCs might be used to replace virtually any type of neuron lost from neurodegenerative disorders and brain trauma (e.g. Clarke et al., 2000; Horner and Gage, 2000). Since this time, neural stem cell research has also given us new insights into the regulation of cell division and programmed cell death, both of which determine neuron number. Patterns of gene expression have been elucidated, including the role of various transcription factors that influence regional differentiation and regulate broad aspects of mitotic activity, fate choice and differentiation. These aspects have been the subject of numerous reviews, including some articles in this Special Issue of Neuron, and will not be discussed here in any detail, except in cases where they may serve to help understand the history of the subject.
The terminology of various subtypes of dividing cells and their offspring, however, was never clearly defined, and each investigator now chooses the terms that they like, with the hope that others will understand what they mean. Heackel originated the term Stem Cell (“Stammzelle”) (Haeckel, 1868), the more specific name neural stem cell (NSC) become popular only in the early 1990’s (e.g. Chu-LaGraff and Doe, 1993; Mackay-Sim and Kittel, 1991) and though widely used it is not precisely defined (see Breunig et al., 2007 for discussion). Embryonically, the term usually refers to the early population of dividing cells, traditionally called neuroepithelial cells, which line the ventricular surface (VZ) and have the potential to give rise to both neurons and glial cells; it is also sometimes used, however, to describe the cells that translocate to the SVZ which are also called Intermediate Amplifying Progenitors (IAPs) or Intermediate Neuronal Progenitors (INPs). Recent studies have also provided more detail about the transient neurons and proliferative cells outside the classical neuroepithelium, and those in additional “abventricular” cellular compartments (Bielle et al., 2005; Bystron et al., 2006; Carney et al., 2007; Smart et al., 2002; Zecevic et al., 2005) (e.g. Subpial Granular Layer (SPG) (reviewed in Bystron et al., 2008)and Outer Subventricular Zone (OSVZ) in primate and rodent (Fietz et al., 2010; Hansen et al., 2010; Reillo et al., 2010; Shitamukai et al., 2011; Wang et al., 2011). Some of these latter cell types already have multiple names in the literature, so providing consistent definitions and labels for the many cells present in the developing system remains an important task.
Although developmental neurobiologists have spent the last decade increasingly finding that NSCs have intrinsic properties related to their spatial and temporal characteristics, adult NSCs, beginning more from a standing start, are only recently becoming progressively better characterized. It is now understood that the adult brain contains a large number of stem cells throughout virtually all regions of the brain(Gage, 2000). In addition, new neurons are produced in discrete sites—even in the human brain (Eriksson et al., 1998). Furthermore, human embryonic stem cell (hESC) and induced pluripotent stem cell (IPS) technology offers a potentially unlimited source of neural stem cells for clinical use (Mattis and Svendsen, 2011). A number of recent studies indicate that we will need to use our knowledge of neurodevelopment and cell specification to coax these seemingly pluripotent cells into more precise, differentiated cells for transplantation. The promise of this technology is great but so are the challenges that need to be surmounted prior to practical use.
Prior to studies by Reynolds and Weiss (Reynolds and Weiss, 1992) and Steve Goldman (Kirschenbaum et al., 1994), transplantation experiments largely involved grafting experiments using immortalized cell types or the transplantation of embryonic progenitors—both prospects having rather severe limitations for clinical use due to the potential for aberrant growth or limited source material, respectively (Gage and Fisher, 1991). With the finding of self-renewing adult neural stem cells came the realization that stem cells capable of producing all neural cell types could be potentially harvested (Clarke et al., 2000). Over the next decades, advancements in culturing and sorting techniques were made (Gage et al., 1995; Pastrana et al., 2009; Roy et al., 2000). Furthermore, embryonic stem cells derived from the blastocyst stage embryo provided a virtually unlimited source of neural stem cells for research and clinical usage (Thomson et al., 1998).
At approximately the same time, neural stem cells in the postnatal brain were beginning to be characterized in situ in a more comprehensive manner. New methods, predominantly centered on the combination of immunofluorescence, confocal microscopy and bromodeoxyuridine labeling led to a renaissance in the study of neurogenesis in the forebrain (Cameron and Gould, 1994; Kuhn et al., 1996). High profile but nonetheless isolated reports had existed prior to this, detailing the generation of new neurons in the postnatal SVZ and hippocampal dentate gyrus (Altman, 1962; Altman and Das, 1965). Quickly this area of research exploded, and was galvanized by the finding of evidence for neurogenesis in the hippocampus of relatively aged human cancer patients (Eriksson et al., 1998). Furthermore, methods were developed for culturing human neural progenitors which increased the potential that transplantation methods could be developed for widespread clinical use (Svendsen et al., 1998). Importantly, the precise nature and character of neural stem cells were characterized in vivo (Denise et al., 2004; Doetsch et al., 1999; Seri et al., 2001). While these emerging descriptions provided an initial compelling glimpse into NSCs in the rodent brain, questions began to arise regarding the similarities and or differences in cell types between different mammalian species.
The Middle Ages: Highlight of Diversity
The initial primate studies, which identified the components and basic rules of NSC neurogenesis, have been extended and elaborated using mainly the mouse and rat as model systems which allow use of modern techniques to study gene expression and the mechanisms by which specific types of neurons can be produced. The principle of early specification of neurons through diversification of neural stem cells applies also to other parts of the nervous system such as the spinal cord (e.g. Jessell, 2000). As explained below, the data collectively indicate that many aspects of NSC regulation and production are common across mammalian species but that certain cellular components of the developing system have been modified or expanded to increase neuronal production and formation of evolutionarily novel traits in primates (Smart et al., 2002). For example, there are types of NSC in the outer SVZ of the embryonic forebrain that are markedly expanded in primates(Bystron et al., 2008; Smart et al., 2002). Thus, our schema in Figure 2 includes data in human and non-human primates in addition to the data obtained in rodents which demonstrate large overlaps in cellular diversity. It is important to acknowledge, however, that the precise lineage relationships and lineage potential of these rodent and primate neural precursors have not yet been precisely identified. Future work to understand the mechanisms by which NSCs generate the diversity of their resulting progeny within and between species is critical before this important cellular resource can be controlled to mitigate developmental disorders or for clinical therapies in adults.
Figure 2. A Modern Depiction of Neural Stem Cells.
Schema of the heterogeneity of stem cells in the mammalian forebrain obtianed by modern methods based on evolutionarily most advanced status in human. Initially, neuroepithelial cells constitute the major class of neural stem cells. During the neurogenic phase, these give rise to radial glia (RG) which can self-renew or generate neurons directly or can generate classes of intermediate types such as intermediate neural progenitors (INP) which divide in the SVZ or short neural progenitors (SNP) which contact and divide at the VZ surface—both of which can generate neurons. RG transition into neurogenic SEZ astrocytes and SGZ radial astrocytes during the gliogenic phase. In addition, radial glia can give rise to ependymal (EL) cells, oligodendrocytes (OC) and astrocytes (AC) pre- and peri-natally and in the adjacent dentate gyrus (DG) into prolonged postnatal stage.
One longstanding assumption has been that modulation of NSC proliferation during embryogenesis is a key factor in specifying brain size, and for generating size differences between mammalian species. Increased understanding of how growth factors control NSC development and neuronal survival have enabled long-term cultures of brain tissue to discover how the kinetic properties of VZ cells are regulated. The duration of each integer cell cycle (Tc) in the NSC population is considered a critical factor in controlling the rate and extent of neocortical expansion (Caviness et al., 1995; Rakic, 1995). Several in vivo and in vitro studies indicated large differences in Tc between mouse and monkey, with the primate cell cycle up to 5 times longer at the comparable developmental period (Haydar et al., 2000a; Kornack and Rakic, 1998; Lukaszewicz et al., 2005; Takahashi et al., 1995). When integrating the results from these multiple studies, however, there are several caveats to consider. First, comparisons of Tc in the mouse VZ measured in vivo and in vitro (in organotypic slice cultures) have demonstrated that Tc lengthens as much as 200% in vitro. For example, while the Tc of the E13.5 mouse VZ is 11.4 hrs when measured in vivo, it lengthens to 22.4 hrs in an organotypic slice culture. Thus, despite the increased survival and support of brain slices engendered by the new-found appreciation of growth factors, important elements regulating proper cell cycle progression are likely not present in the culture medium surrounding the mouse slices. Since the Tc in human embryonic telencephalon can only be measured in vitro, determining the degree to which Tc is lengthened in primate slice cultures is critical.
To answer this key question, several studies using the cumulative BrdU labeling technique were compared to analyze VZ proliferation profiles in mouse, monkey and human embryonic telencephalon in vivo and in slice cultures (Takahashi et al., 1995; Kornack and Rakic, 1998; Haydar et al., 1999; Haydar et al., 2000b; Figure 3). This comparison reveals several key findings which support the conclusion that primate and rodent NSCs are fundamentally and intrinsically different. In particular, while Tc doubles when mouse brain slices are cultured, the non-human primate Tc is not appreciably lengthened in vitro when compared to age-matched in vivo measurements. Secondly, the Tc in comparably staged human and non-human primates is highly similar (Figure 3); moreover, Tc values from both primate species are substantially longer than in the comparably staged mouse VZ. It is well established that there are considerable differences between human and rodent NSCs (e.g. Jekel and Svendson, 2004), but it is not understood why duration of cell cycle can be measured in minutes in drosophila, in hours in rodents and in days in primates. Furthermore, it seems paradoxical that largest brain that need to produce more neurons has the longest cell cycle. One straightforward interpretation of these results is that primate NSCs retain specific intrinsic cues regulating their proliferation while rodent NSCs rely more heavily on diffusible signals within the extracellular milieu that are lost when slices are cultured in vitro. Nevertheles, these studies clearly demonstrate that particular mechanisms of primate VZ cell proliferation need to be taken into consideration.
Figure 3. Intrinsic Differences in Cell Cycle Length.
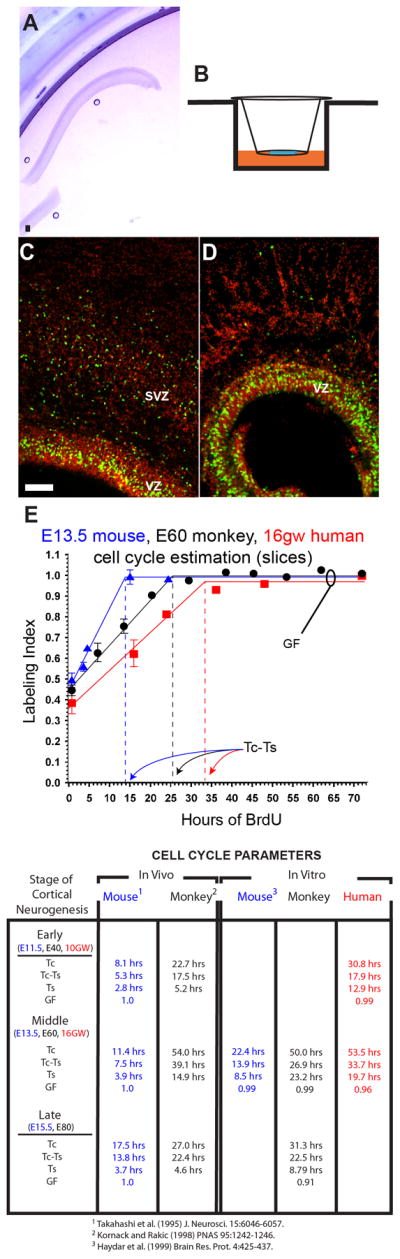
Representative examples of cultured E60 monkey (A) and stained 16 wg human (C and D) brain slices. Slices were cultured just underneath the media interface in the presence of 25mM BrdU (B). In C, notice the marked separation of the VZ from the SVZ and the band of S-phase cells in the VZ after 1 hour of BrdU application. After 16 hours of BrdU application (D), the VZ had nearly filled with BrdU-labeled nuclei. (E) Data from cumulative BrdU labeling studies on mouse (blue) monkey (black) and human (red) neocortical slice cultures. The slope of the rising phase of the plots = GF/Tc, where GF is growth fraction, or maximum number of proliferating cells in the VZ and Tc is the cell cycle duration. The level of labeling index (LI) saturation (maximum) at later time points defines the GF, or total population of proliferating cells. The time at which the maximum LI is reached is Tc-Ts, or the duration of the cell cycle minus the duration of S-phase. Bar, 100μM.
Apart from modulation of cell cycle progression, specialization in the neural precursor population was recognized to be one of the main strategies used to control the extent and complexity of brain growth in mammals. The evidence from the masters at the light and electron microscopy levels indicated that the constituency of the primate ventricular neuroepithelium is more heterogeneous than in the rodent. These classical studies have been confirmed recently by studies using contemporary labeling techniques to demonstrate a remarkable variety of RGCs in fetal human neocortex. Zecevic and colleagues have found that the fetal human VZ contains multiple types of precursors dividing at the surface of the ventricle, including RGCs stained with GLAST and GFAP as well as cells either singly expressing or co-expressing βIII-tubulin and phosphorylated neurofilaments (SMI-31), the latter two of which are thought to be neuronal-restricted progenitors (Howard et al., 2006; Zecevic, 2004). In addition, a dividing cell type expressing neither RGC nor neuronal-specific markers is abundant at the surface of the human VZ, indicating that additional precursor/stem cells have yet to be discovered (Howard et al., 2006). However, when RGC from human fetal VZ/SVZ were isolated at mid-term and genetically labeled in vitro, they generated all three neural cell types: neurons, including interneurons(Yu and Zecevic, 2011), astrocytes (Mo et al., 2007), and oligodendrocytes (Mo and Zecevic, 2009).
Although a plethora of dividing precursors have been identified in the developing primate VZ, molecular techniques initiated approximately a decade ago, including in vivo retroviral labeling and transgenic targeting, suggested that the rodent VZ contains a homogeneous population of RGCs. Rodent RGCs undergo self-renewal, generate neurons directly and give rise to the basal intermediate neural progenitors (INPs) in the SVZ (Noctor et al., 2004) expressing the transcription factor Tbr2 (Englund et al., 2005). Hence, rodent RGCs essentially perform all of the roles required for neocortical growth (Tbr2+ SVZ progenitors are distinguished by their transient localization in the SVZ and association with capillaries (Javaherian and Kriegstein, 2009; Stubbs et al., 2009) prior to terminal division and migration to the cortical plate). However, this RGC-centric model has been modified by several studies using retroviral labeling, in utero electroporation as well as other molecular methods such as cell sorting and time-lapse imaging, which indicate that RGCs can be antigenically and functionally separated into several groups (Hartfuss et al., 2001; Parnavelas et al., 1991; Pinto et al., 2008). In addition, the discovery of the short neural precursor cell (SNP), which is located in the neocortical VZ and divides at the ventricular surface to produce neurons, demonstrates that diversity of the dividing cell population in the VZ is important for proper neocortical growth - even in rodents (Gal et al., 2006; Stancik et al., 2010). The co-habitation of the rodent VZ by RGCs and SNPs closely resembles the arrangement of GFAP+ and GFAP− cells in the primate VZ. Unlike RGCs, the SNPs do not contact the pial surface and are molecularly distinct as they express the tubulin alpha-1 DNA promoter but not the Glast promoter expressed by RGCs.
Despite the power of the techniques used to highlight this newfound cell diversity, many of these cell types can be missed or misclassified even when using modern methods if they are not bolstered by more time-consuming studies at higher levels of resolution or by using more elemental identifiers, such as gene expression. For example, while the cell division of multiple types of dividing precursors in the VZ have been monitored with time-lapse imaging, the cells with even substantial differences in morphology can be missed or misclassified if they are not reconstructed using classical methods with higher resolution such as electron microscopy. In part to provide better resolution of cell type, recent studies have begun to use molecular analyses to highlight cell diversity. For example, SNPs and RGCs have been further differentiated by their use of the Notch signaling pathway; RGCs contain activated Notch while SNPs do not (Mizutani et al., 2007). This finding supports the distinction between the cell types and as well as the conclusion that SNPs undergo fewer self-renewal divisions compared to RGCs (Stancik et al., 2010). These data suggest a basic neurogenic theme in the VZ: NSCs in the VZ of the developing rodent and primate telencephalon divide to generate neurons as well as additional classes of dividing progenitors (such as Tbr2+ INPs, SNPs and OSVZ cells) which amplify the total neuron output. In primate VZ, this mechanism appears more robust and has likely been modified to include additional RGC and progenitor cell types which act in concert to produce the vastly larger pool of neurons during development. Beyond this, recent global transcriptome analysis of human brain development suggests that there has been rapid human-specific evolution of cis-regulatory elements leading to differentially regulated regional expression of genes in different cortical areas (Johnson et al., 2009). This may partially underlie some of the major species specific differences. Taken together, these studies indicate that NSC biology is extremely complex and that allocation into segregated stem and progenitor cell populations is a key element of proper brain development. These and other studies in rodents carefully set the stage for, and demarcate the limits of, endogenous stem cell activity and potential. These boundaries are now being tested in the burgeoning field of adult stem cell manipulation and therapeutic intervention.
Epoch Nouvelle – Back to Rationality
During the early part of this century, tissue specific stem cells continued to receive significant attention due to rapid methodological advances in viral labeling, mouse genetics, and development of culturing methods (Wagers and Weissman, 2004). As a result of new lineage studies, the boundaries that had seemingly existed for decades seemed to fall as reports of hematopoietic cells becoming brain cells, including neurons, appeared (Brazelton et al., 2000; Mezey et al., 2000). Furthermore, neuronal addition to areas beyond the hippocampus and olfactory bulb were suggested (Gould et al., 1999; Zhao et al., 2003). However, many of these claims have failed to hold up to scrutiny, seemingly due to methodological reasons (Ackman et al., 2006; Alvarez-Dolado et al., 2003; Breunig et al., 2007; Castro et al., 2002; Kornack and Rakic, 2001).
However, it did become evident that neural stem cells could produce functional neurons in vitro and in some areas in vivo in rodents as well as in some other mammals. For example, areas of adult neurogenesis such as the hippocampus and subventricular zone allowed neurogenesis from transplanted neural stem cells (Gage et al., 1995), including hESC-derived neural stem cells (Muotri et al., 2005). Other regions such as the substantia nigra were recalcitrant to neurogenesis (Lie et al., 2002). Endogenous neural stem cells in the hippocampus and olfactory bulb in young adult mice did proceed through the characteristic differentiation profile characteristic of embryonic neurons (Carleton et al., 2003; Song et al., 2002). These neurons integrated into existing circuits and produced action potentials (van Praag et al., 2002). Interestingly, in rodents, levels of neurogenesis are highly variable among strains and can be appreciably altered up or down by different types of environmental stimuli. For example, running (van Praag et al., 1999) and seizures (Parent et al., 1997) are among the most potent stimulators of neurogenesis. Ischemia has also been shown by several groups to provoke reactive neurogenesis in the hippocampus (Liu et al., 1998) and SVZ (Iwai et al., 2003). However, global ischemia has also been reported to stimulate the generation of interneurons from layer 1 progenitors (Ohira et al., 2010). Conversely, stress (Gould et al., 1997) and models of depression (Malberg and Duman, 2003) can decrease neurogenesis.
The extent and relevance of endogenous neurogenesis in the human brain remains unclear. The lack of definitive methods for tracking the birth of new cells in the brain of living humans or even post mortem has left us with more questions than answers. For example, while there is some evidence that there is neurogenesis in the adult human hippocampus, the existence of olfactory bulb neurogenesis remains controversial (Sanai et al., 2007; Sanai et al., 2004) and the existence of the rostral migratory stream after childhood has not been proven (Sanai, 2011; Sanai et al., 2004; Weickert et al., 2000). A novel technique based on retrospective 14C based dating has indicated that there is virtually no turnover of neocortical neurons, but other areas have not yet been examined (Bhardwaj et al., 2006). Histological methods indicate that the decrease in neurogenesis seen during rodent aging (Kuhn et al., 1996) is increasingly severe in primates (Jabes et al., 2010; Kempermann, 2011; Knoth et al., 2010; Leuner et al., 2007; Seress et al., 2001). Thus, despite the possible existence of neurogenesis in the adult human hippocampal dentate gyrus, the relative amount of newly generated neurons appears to be significantly smaller than those found in other mammals and even more so when compared to lower vertebrates.
However, just as we began to become comfortable again with the somewhat rigid natural bounds of cell fate and lineage potential, a remarkable discovery was made by Yamanaka and colleagues. Using four factors, Oct3/4, Sox2, c-Myc, and Klf4, they demonstrated that fibroblasts could be converted into pluripotent stem cells(Takahashi and Yamanaka, 2006). This was quickly followed by confirmation by several groups using human cells and refinement of the methods (Leuner et al., 2007; Meissner et al., 2007; Okita et al., 2007; Takahashi et al., 2007). In the very brief period since these findings, in the context of neurobiology in particular, many significant findings have been made. Notably, it was found the neural stem cells, which endogenously express Sox2, Klf4, and c-myc, can be reprogrammed by a single factor, indicating that they exist close to a pluripotent ground state (Kim et al., 2009). More recently, using a strategy aimed at avoiding the prolonged period required for reprogramming to pluripotency and then subsequent lineage commitment, Wernig and colleagues found that three factors, Ascl1, Brn2, and Myt1l had the capability of converting fibroblasts into functional neurons (Vierbuchen et al., 2010). Even more simply, it has been demonstrated that astroglia can be reprogrammed in vivo to become neurons by single proneural genes (Heinrich et al., 2010).
Nevertheless, clinical replacement of neurons is years if not decades away. However, IPS technology in particular has been employed for a novel purpose—disease modeling (Mattis and Svendsen, 2011). IPS technology allows for more rapid and faithful examination of the disease process in neural cells derived from patients with neurodegenerative disease. In the past, such neural cells could only be harvested post mortem and typically at the end stage of the disease (Jakel et al., 2004). Now, a virtually unlimited source of neural progenitors can be derived from reprogrammed fibroblasts derived from living patients and can be coaxed into becoming any cell of interest. IPS cells have so far been isolated from a great many neurodevelopmental and neurodegenerative diseases, including Rett’s syndrome (Hotta et al., 2009), Fragile X (Urbach et al., 2010), Spinal muscular atrophy (Ebert et al., 2009), Huntington’s disease (Zhang et al., 2010), and Amyotrophic Lateral Sclerosis (ALS) (Dimos et al., 2008). However, while these models open up exciting new avenues of study, they bring a host of new challenges such as designing cell type specific differentiation protocols, choosing proper controls, (Mattis and Svendsen, 2011) and the fact that human neurons in particular have a lengthy differentiation period. In addition, this technology promises patient-derived tissues for future transplantation (see Table 1 for a comparison of neural stem cell sources for clinical use). However, it is questionable whether this technically-demanding technology will reach the economies of scale and safety requirements necessary for such a promise.
Table 1.
| Cell Type | Fetal Human NSCs | Adult Human NSCs | hESC-derived NSCs | iPSC-derived NSCs | Induce Neuronal Stem Cell (iNSC) |
|---|---|---|---|---|---|
| Derivation procedure | Isolated after dissociation of Fetal CNS | Isolated from parenchymal NSCs post mortem using dissociation | Neural progenitors are enriched using embryoid body differentiation protocols after hESC derivation | IPSC reprogramming and then same as hESC-derived NSCs | Viral reprogramming of fibroblast (or other cell type?) |
| Ethical Issues? | Source is often aborted fetus | No significant issues beyond consent | hESC derivation-related ethical issues | No significant issues | No significant issues |
| Major Shortcoming | Primary source of tissue is limited and genetic abnormalities may be present | Progenitors are likely regionally specified and less potent | Source of cells is ethically contentious | Intrinsic aberrations may be associated with reprogramming | Potential and properties largely uncharacterized |
| Advantage | Well-characterized and easily derived | Source material is not ethically problematic | Can potentially provide any neural or neuronal cell type in the brain | Can be derived from patients own tissue and has many of the advantages of hESC-derived NSCs without ethical issues or need for immunosuppression | Very efficient compared with IPSC and may avoid abberations by shortening the reprogramming period |
NSCs - Neural Stem Cells
Recently a host of practical issues have arisen as aberrations which may be commonplace due to the selective pressures inherent to the reprogramming process These issues incluce chromosomal aberrations (Mayshar et al., 2010), somatic mutations (Gore et al., 2011), abnormal DNA methylation (Lister et al., 2011), and copy number variations(Hussein et al., 2011). It is important to note that these issues may not be specific to IPS cells as trisomy has been documented in human neural progenitors as well (Sareen et al., 2009). In any case, it is the pluripotency of these cell types and their extended derivation times that increase their potential for tumorigenicity (Ben-David and Benvenisty, 2011). This issue has already arisen clinically in the case of neural stem cell transplants. Notably, transplanted fetal neural stem cells were linked to tumor growths in the brain and spinal cord of a young ataxia telangiectasia patient (Amariglio et al., 2009). This particular case was unregulated and the numbers of cells injected were very large. However, even with proper oversight, this may in the end be one of the biggest safety hurdles to overcome.
In addition to making transplantation of reprogrammed cells affordable and safe, one of the major hurdles thus far left unsolved is to incorporate all of the sequential steps of neuronal differentiation and synaptic development. In particular, forming new projection neurons in the human brain will be a monumental challenge. Consider the case of a Betz cell which synapses in the lower spinal cord which is frequently lost in ALS (Udaka et al., 1986). If we were to imagine that the cell body was the size of a tennis ball, the axon would then extend several miles and would be roughly the diameter of a garden hose. Besides the tens of thousands of dendritic synapses that would need to be formed, the axon would need to find its target, starting as a growth cone a considerable distance way. This would all have to transpire within a milieu lacking the guidance cues which are normally present only during a limited window during development. Apart from these practical issues and the host of other intrinsic issues involved in neuronal regeneration and transplantation (accurate cell delivery, potential immune suppression, etc.), there is the growing appreciation that neural stem cells, whether in vitro or in vivo have intrinsic specification which may limit the cell types that can be produced upon differentiation (Gaspard et al., 2008; Hochstim et al., 2008; Merkle et al., 2007; Rakic et al., 2009). Indeed, transplanted hESC-derived neurons seem to obey the in vitro specification program when transplanted in vivo (Gaspard et al., 2008). Beyond this, there was a flurry of findings recently that a small proportion of transplanted cells acquired the pathology of the host tissue (Brundin et al., 2008; Kordower et al., 2008; Li et al., 2008). Thus, even if we can successfully coax stem cells to replace neurons in vivo, the battle may already be lost for some of them.
Others have taken advantage of the “bystander” or “chaperone” effect of neural stem cells in transplantation strategies aimed at preventing or ameliorating neurodegeneration (see Breunig et al., 2007 for review). Basically, it has been found that neural stem cells secrete neurotrophins, growth factors, and other beneficial proteins that promote neuronal health and function. For example, it was found that neural stem cells ameliorated cognitive functions in a model of Alzheimer’s disease not through neuronal replacement but due to their secretion of BDNF (Blurton-Jones et al., 2009). Other groups are taking these properties of transplanted cells and enhancing them with transgenes such as GDNF. In a rat model of ALS, such cells migrated to the sites of degeneration, differentiated into glia, and were able to preserve motor neurons at early and end stages of disease (Klein et al., 2005; Suzuki et al., 2007). Beyond their use as protein factories, many diseases characterized by primary glial deficiencies ((Ben-Hur and Goldman, 2008)e.g. myelin disorders) may provide more feasible clinical targets for neural stem cells and glial progenitors in the near-term (Windrem et al., 2008). Indeed, at this time a number of clinical trials aimed at using transplanted NSCs or NSC-derived glial cells are underway.
Our understanding of neural stem cells has progressed at an accelerating pace. Starting with the descriptions and insights of the old masters and continuing through the current era of genetic lineage tracing, a picture is beginning to emerge to explain the broad mechanisms of neurogenesis and brain development in general. Nevertheless, key questions remain regarding the generation of neuronal diversity and how this can be replicated in vitro. Neurodevelopmental findings and studies using cell culture and transplantation are coming to the conclusion that neural stem cell potency is directly related to the spatial and temporal location of neural stem cells in vivo or to the methods of their derivation and subsequent handling in vitro. In vivo studies indicate that the precise sequence of neurogenesis, neuronal fate determination and final positioning is essential for proper formation of functional connections. Thus, the development of protocols to direct differentiation of specific types of neurons and to place them in the appropriate location with the remaining cues necessary for their functional integration is the next major objective. Beyond this, challenges may arise in translating findings from lower organisms to human neural stem cells—which as we have documented appear to have unique properties. While neuronal replacement may not be among the ‘low-lying fruit’ of regenerative medicine, clinical uses of transplanted neural stem cells and glia may not be so far off as a number of clinical trials are underway. A great deal of basic neurodevelopmental questions remain to be answered from basic mechanisms of cell fate choices to the details of circuit formation. Many of these future insights will likely inform the clinical use of transplanted cells. Going forward, especially in terms of CNS protection and regeneration, a continued interplay between basic neurodevelopment and translational neuroscience will be necessary for the most efficacious progress.
Acknowledgments
We thank Clive Svendsen for a critical reading of the manuscript. J.J.B. was supported by the Connecticut Stem Cell Research Grant Program during the preparation of this manuscript and is currently supported by the Cedars-Sinai Regenerative Medicine Institute, Los Angeles, California, USA. T.F.H. is supported by grants from the US National Institutes of Health (NIH), including grants from NINDS and NICHD. P.R. is supported by grants from the US National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Drug Abuse (NIDA) and the Kavli Institute of Neuroscience at Yale University School of Medicine, New Haven, Connecticut, USA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ. Fusion of microglia with pyramidal neurons after retroviral infection. J Neurosci. 2006;26:11413–11422. doi: 10.1523/JNEUROSCI.3340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. ARE NEW NEURONS FORMED IN BRAINS OF ADULT MAMMALS. Science. 1962;135:1127. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Vardugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Goldman SA. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci. 2008;1142:218–249. doi: 10.1196/annals.1444.014. [DOI] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnar Z, Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Carney RS, Bystron I, Lopez-Bendito G, Molnar Z. Comparative analysis of extra-ventricular mitoses at early stages of cortical development in rat and human. Brain Struct Funct. 2007;212:37–54. doi: 10.1007/s00429-007-0142-4. [DOI] [PubMed] [Google Scholar]
- Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Choi BH. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J Neuropathol Exp Neurol. 1986;45:408–418. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Chu-LaGraff Q, Doe CQ. Neuroblast specification and formation regulated by wingless in the Drosophila CNS. Science. 1993;261:1594–1597. doi: 10.1126/science.8372355. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- deAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R. Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol. 2003;55:288–298. doi: 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- Denise A, Garcia R, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Gadisseux JF, Evrard P. Glial-neuronal relationship in the developing central nervous system. A histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Dev Neurosci. 1985;7:12–32. doi: 10.1159/000112273. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Fisher LJ. Intracerebral grafting: a tool for the neurobiologist. Neuron. 1991;6:1–12. doi: 10.1016/0896-6273(91)90116-h. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Golgi C. Sulla fina anatomia degli organi centrali del sistema nervoso (Giunti) 1885 [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Haeckel E. Natürliche Schöpfungsgeschichte. Berlin: G. Reimer; 1868. [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000a;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Kornack DR, Rakic P. Differences in the corticogenic cell cycle between primates and rodents are maintained in vitro: evidence for intrinsic regulation of neocortical diversity. Soc Neurosci Abst. 2000b;26:608. [Google Scholar]
- Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W. Unsere Körperform und das physiologische Problem ihrer Entstehung : Briefe an einen befreundeten Naturforscher. Leipzig: F.C.W. Vogel; 1874. [Google Scholar]
- His W. Zur Geschichte der menschlichen Rückenmarkes und der Nervenwurzeln. Vol. 13. Leipzig: 1886. [Google Scholar]
- His W. Die Entwicklung des menschlichen Gehirns wahrend der ersten Monate. Leipzig: Hirzel; 1904. [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Hotta A, Cheung AY, Farra N, Vijayaragavan K, Seguin CA, Draper JS, Pasceri P, Maksakova IA, Mager DL, Rossant J, et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6:370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53:57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–341. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- Jabes A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Schneider BL, Svendsen CN. Using human neural stem cells to model neurological disease. Nat Rev Genet. 2004;5:136–144. doi: 10.1038/nrg1268. [DOI] [PubMed] [Google Scholar]
- Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex. 2009;19(Suppl 1):i70–77. doi: 10.1093/cercor/bhp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim HJ, Gadisseux JF, Evrard P. Topographical and cytological evolution of the glial phase during prenatal development of the human brain: histochemical and electron microscopic study. J Neuropathol Exp Neurol. 1988;47:166–188. doi: 10.1097/00005072-198803000-00009. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Seven principles in the regulation of adult neurogenesis. Eur J Neurosci. 2011;33:1018–1024. doi: 10.1111/j.1460-9568.2011.07599.x. [DOI] [PubMed] [Google Scholar]
- Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Scholer HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–643. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker A. Grundriβ der Entwicklungsgeschichte des Menschen und der höheren Tiere. Leipzig: Aufl; 1879. [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Parnavelas JG. Changing concepts of cortical development. Cereb Cortex. 2003;13:541. doi: 10.1093/cercor/13.6.541-i. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Parnavelas JG. Progress in corticogenesis. Cereb Cortex. 2006;16(Suppl 1):i1–2. doi: 10.1093/cercor/bhk041. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel P. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991;11:979–984. doi: 10.1523/JNEUROSCI.11-04-00979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magini G. Nouvelles recherches histologiques sur le cerveau du foetus. Arch Ital Biol. 1888;13:1730–1750. [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mattis VB, Svendsen CN. Induced pluripotent stem cells: a new revolution for clinical neurology? Lancet Neurol. 2011;10:383–394. doi: 10.1016/S1474-4422(11)70022-9. [DOI] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–4145. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Human fetal radial glia cells generate oligodendrocytes in vitro. Glia. 2009;57:490–498. doi: 10.1002/glia.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb Cortex. 1991;1:463–468. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Nadarajah B. Radial glial cells are they really glia? Neuron. 2001;31:881–884. doi: 10.1016/s0896-6273(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Mader MT, Irmler M, Gentilini M, Santoni F, Drechsel D, Blum R, Stahl R, Bulfone A, Malatesta P, et al. Prospective isolation of functionally distinct radial glial subtypes--lineage and transcriptome analysis. Mol Cell Neurosci. 2008;38:15–42. doi: 10.1016/j.mcn.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003a;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003b;43:19–32. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia. 2003a;41:117–127. doi: 10.1002/glia.10140. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003b;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Ramon Y, Cajal S. Histologie du Système Nerveux de l’Homme et des Vertébrés. Vol. 1. Paris: 1909. [Google Scholar]
- Ramón Y, Cajal S. Textura del Sistema Nervioso del Hombre y de los Vertebrado. Madrid: Moya; 1899. [Google Scholar]
- Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- Retzius G. Die Cajal’schen Zellen der Grosshirnrinde beim Menschen und bei Säugetieren. 1893a. [Google Scholar]
- Retzius G. Studien uber Ependym and Neuroglia. Vol. 5 1893b. [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RAR, Couldwell WT, Kawaguchi A, Okano H, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on "Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension". Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. author reply 393. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H, Won M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Postnatal Development of the Human SVZ Reveals a Migratory Pathway of Young Neurons into Cortex. Under Review 2011 [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sareen D, McMillan E, Ebert AD, Shelley BC, Johnson JA, Meisner LF, Svendsen CN. Chromosome 7 and 19 trisomy in cultured human neural progenitor cells. PLoS One. 2009;4:e7630. doi: 10.1371/journal.pone.0007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature. 1979a;277:303–305. doi: 10.1038/277303a0. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979b;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Tornoczky T, Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105:831–843. doi: 10.1016/s0306-4522(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. Oblique Radial Glial Divisions in the Developing Mouse Neocortex Induce Self-Renewing Progenitors outside the Germinal Zone That Resemble Primate Outer Subventricular Zone Progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D, DeProto J, Nie K, Englund C, Mahmud I, Hevner R, Molnar Z. Neurovascular congruence during cerebral cortical development. Cereb Cortex. 2009;19(Suppl 1):i32–41. doi: 10.1093/cercor/bhp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJE, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Udaka F, Kameyama M, Tomonaga M. Degeneration of Betz cells in motor neuron disease. A Golgi study. Acta Neuropathol. 1986;70:289–295. doi: 10.1007/BF00686086. [DOI] [PubMed] [Google Scholar]
- Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Lamonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011 doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, Kleinman JE. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol. 2000;423:359–372. doi: 10.1002/1096-9861(20000731)423:3<359::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zecevic N. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. J Neurosci. 2011;31:2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, An MC, Montoro D, Ellerby LM. Characterization of Human Huntington’s Disease Cell Model from Induced Pluripotent Stem Cells. PLoS Curr. 2010;2:RRN1193. doi: 10.1371/currents.RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]