Abstract
Accumulating evidences suggest that serotonin (5-HT) contributes to the developmental programming of childhood- and adult-onset mental illnesses. This is thought to occur through the capacity of 5-HT to modulate developmental processes such as cell proliferation, migration and circuit wiring. For instance, genetic studies in mice show that disruption of 5-HT signaling during a restricted period of pre- and postnatal development results in long-term behavioral abnormalities such as increased anxiety in adulthood (Gaspar et al., 2003, Oberlander et al., 2009). The developmental programming of adult anxiety can be triggered either by a transient knockdown of a single 5-HT receptor [5-HT1A, (Gross et al., 2002)] or by a transient developmental exposure to specific serotonin reuptake inhibitors (SSRIs) (Ansorge et al., 2004, Ansorge et al., 2008). The recent discovery of a role for 5-HT in fetal forebrain wiring in utero (Bonnin et al., 2007, Bonnin et al., 2011) suggests a potential mechanism by which 5-HT signaling disruption can have long-term consequences on brain function. 5-HT signaling through two receptors (htr1b/1d; 5-HT1B/1D) switches the response of thalamic axons to netrin-1 from attraction to repulsion in vitro (Bonnin et al., 2007). Targeted disruption in vivo of 5-HT1B/1D receptors expression in the dorsal thalamus led to abnormal thalamocortical axons (TCAs) pathways in the internal capsule and cortex (Bonnin et al., 2007). Thus, the level 5-HT signaling through the number and/or type of 5-HT receptors activated is critical for normal brain development. The concentration of 5-HT available for receptors activation is likely another important parameter for normal brain development. We recently demonstrated that the fetal forebrain selectively accumulates placenta-derived 5-HT during the initial axon growth period (Bonnin et al., 2011). These data suggest that certain developing circuits in the fetal brain are sensitive to placental 5-HT during their initial ontogeny, which could be impacted by both genetic and environmental disturbances that are known to increase risk for mental illnesses. The role of 5-HT signaling in fetal brain wiring and the potential importance of placental metabolism in 5-HT-mediated fetal programing are discussed below.
1. Role of 5-HT in fetal forebrain wiring
Anatomical studies have shown the early appearance of serotonergic neurons in the developing hindbrain and suggested a role for 5-HT in fetal brain development (Lidov and Molliver, 1982, Aitken and Tork, 1988, Buznikov et al., 2001). These neurons differentiate in the fetal dorsal raphe (DR) as early as embryonic day (E) 10.5 in the mouse and send axons through the ventral forebrain via the medial forebrain bundle; by E14.5 some 5-HT axons are already reaching rostral regions, including the neocortex (Lidov and Molliver, 1982, Aitken and Tork, 1988). Since these early studies, 5-HT signaling has been shown to influence diverse processes of brain development such as cell migration, proliferation, maturation and axon growth [see (Lauder and Krebs, 1978, Rakic and Lidow, 1995, Gaspar et al., 2003, Vitalis and Parnavelas, 2003, Janusonis et al., 2004)]. Although the actual release of 5-HT synthesized by growing DR axons during early fetal period was never clearly demonstrated, it is assumed that they provide 5-HT to the fetal forebrain and serve its ontogenic roles. Regardless of the actual source of the ligand, the ontogenic effects of 5-HT depend on where and when its receptors are expressed.
A precise, systematic, spatiotemporal expression map of all 5-HT receptors would help understanding and proposing new hypotheses regarding 5-HT signaling role in fetal brain development. There is expression data of varying detail for some 5-HT receptors, either in mice or rats [see (Bonnin et al., 2006) and references therein], but the large number of receptor subtypes [>15 unique receptor genes, not including splice and editing variants, (Hannon and Hoyer, 2008)] has made systematic mapping challenging. We started to address this question by obtaining spatial and temporal expression mapping of all Gi/o-coupled 5-HT1 subfamily receptor transcripts in the mouse fetal forebrain, using in situ hybridization (Bonnin et al., 2006). These studies revealed complex patterns of 5-HT1 receptor subtype expression in the developing forebrain. Surprisingly, receptor transcripts are expressed in regions such as the dorsal thalamus (DT), hippocampal neuroepithelium or neocortex even before DR serotonergic axons reach the area. We noted that the expression patterns of 5-HT1B and 5-HT1D receptor transcripts in the DT were similar to guidance cue receptors expression [i.e. netrin-1 receptors Unc5c and DCC; (Braisted et al., 2000, Garel and Rubenstein, 2004)]. This suggested that 5-HT signaling could be important for thalamocortical axon (TCA) pathway formation, a major developmental process of the DT at these ages (E12.5 to E16.5). This hypothesis was tested in vitro, and in explant studies we showed that 5-HT signaling influences the netrin-1-mediated guidance of thalamic axons. More specifically, a 5-HT1B/1D-mediated decrease in intracellular cAMP level in DT neurons switches their axons response to netrin-1 from attraction to repulsion (Bonnin et al., 2007). Using in utero electroporation to knockdown or overexpress 5-HT1B/1D receptors simultaneously and specifically in the DT, we observed that 5-HT signaling is important for mediating the growth trajectory of the TCA pathway. This targeted genetic manipulation allowed us to decrease or increase both receptors expression simultaneously starting at E12.5, without affecting transcript expression in other regions of the developing brain. Changes in the path toward the cortex of axons originating from transfected DT neurons were followed and quantified by co-electroporation with a GFP plasmid and immnuolabelling of TCAs with specific markers [Netrin-G1a and L1; (Bonnin et al., 2007)]. We observed two opposing effects of decreasing or increasing 5-HT signaling in DT neurons in vivo. When 5-HT signaling was decreased by knocking down 5-HT1B/1D receptors expression with siRNAs, TCAs followed a ventrally-shifted, or expanded, path in the internal capsule before entering the cortex. Conversely, when 5-HT signaling was increased by overexpressing the receptors, TCAs followed an abnormally restricted dorso-medial path in the internal capsule (Bonnin et al., 2007). Based on 5-HT effects in vitro and on netrin-1 expression in this region, we proposed that changing 5-HT signaling in TCAs affects their positioning within netrin-1 gradient in the internal capsule (Bonnin et al., 2007). Thus altering 5-HT signaling in vivo can modulate the patterning of a major axonal pathway in the fetal brain (Fig. 1). Previous studies have shown that altered TCA pathway formation in SERT and MAOA knockout (MAOA−/−) mice, which have excess extracellular cortical 5-HT, was rescued in the double knockout (5-HT1B−/−/MAOA−/−) (Salichon et al., 2001). This suggested that a precise level of 5-HT1B-mediated signaling is important for TCA pathway formation. Curiously, 5-HT1B−/− mice do not exhibit obvious defects of TCA organization; however, based on the embryonic expression patterns we described (Bonnin et al., 2006), signaling through the 5-HT1D receptor, which presents similar pharmacology and cellular localization to 5-HT1B receptor may be sufficient for normal TCA pathway formation in either (5-HT1B−/−/MAOA−/−) or 5-HT1B−/− mice. The overlapping temporal and spatial expression of 5-HT1B and 5-HT1D receptors provided the rationale for our combinatorial disruption of both receptors by in utero electroporation (Bonnin et al, 2007).
Figure 1.
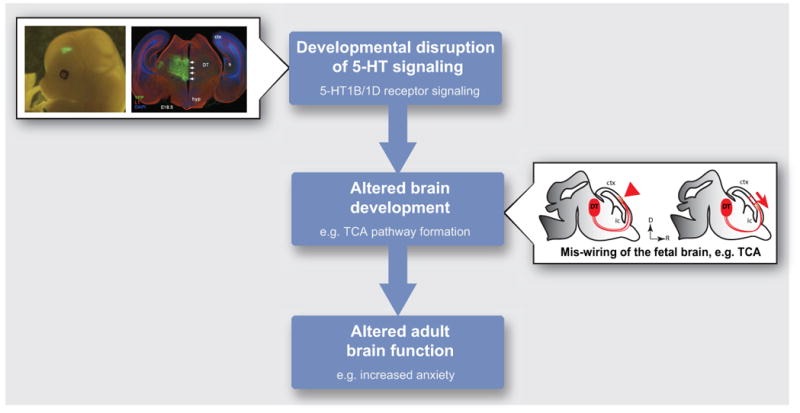
Developmental disruptions of 5-HT signaling in utero can lead to abnormal brain function at adult stages. Altering 5-HT signaling in vivo by in utero electroporation of 5-HT1B/1D receptors siRNAs or over-expression plasmids in the dorsal thalamus (DT) affects the formation of the thalamocortical axon (TCA) pathway in the fetal brain. Results suggest that this 5-HT-mediated disruption of fetal brain wiring mechanisms could contribute to long-term alteration of brain function. Abnormal 5-HT signaling in the fetal brain could result from altered function or expression of 5-HT receptors, as described in the text, or from altered availability of the ligand itself, 5-HT. Hyp, hypothalamus; ctx, cortex; ic, internal capsule.
As mentioned above, developmental disruptions of 5-HT signaling in utero can lead to abnormal brain function at adult stages. The results described above suggest that a 5-HT-mediated disruption of fetal brain wiring mechanisms could contribute to these long-term effects. Abnormal 5-HT signaling in the fetal brain could result from altered function or expression of 5-HT receptors, as described above, or from altered availability of the ligand itself, 5-HT. Therefore, determining the specific sources of fetal brain 5-HT in utero, which generally have been attributed to fetal and maternal sources, is crucial to understand how disrupted 5-HT signaling affects brain development and the fetal programing of adult disorders.
2. Sources of 5-HT in the fetal forebrain
Over the past 40 years, the field has embraced the concept that there are both fetal and maternal contributions of 5-HT during pregnancy. Serotonergic neurons appear early in the fetal hindbrain and could provide an endogenous source of 5-HT. Sources of 5-HT other than the fetal brain was assumed because of the observations that 5-HT receptors expression in the rostral forebrain, craniofacial and peripheral regions is evident several days before serotonergic axons even reach these regions (Buznikov et al., 2001). Moreover, an exogenous source of 5-HT was consistent with the finding that in Pet-1 knockout (Pet-1−/−) mice, in which 70–80% of raphe neurons fail to develop 5-HT synthetic capacity, thalamic neurons were nonetheless normally targeted (Bonnin and Levitt, unpublished). Thus, all these data were consistent with the existence of an alternative, exogenous source of 5-HT at early stages of development. There are claims regarding multiple sources of the neurotransmitter during fetal development, including the fetal gut and maternal circulation (Lauder et al., 1981, Yavarone et al., 1993), prior to blood-brain barrier formation. But a careful examination of the literature indicated to us that the exact source of 5-HT in the early fetal brain was not completely clear. Some studies have suggested that maternal blood 5-HT could be transferred to the fetal circulation after crossing the placenta (Cote et al., 2003, Cote et al., 2007). Yet direct or indirect transfer was never demonstrated.
Given the lack of altered axonal guidance problems in the Pet-1−/− mouse, and to take advantage of the deficiency of 5-HT levels in the adult brain (Hendricks et al., 2003), we measured 5-HT concentrations throughout embryogenesis in the Pet-1−/− and wild type littermate mice in order to gather direct evidence for source of 5-HT other than the fetal brain. We assayed different brain regions using high-pressure liquid chromatography (HPLC). The results uncovered a biphasic nature of the source of 5-HT specifically in the embryonic forebrain. In the hindbrain, the location of raphe neurons, we observed a consistent, massive reduction in 5-HT in the Pet-1−/− mouse throughout fetal development. However, at early ages (E10.5 to E15.5) 5-HT levels are normal in the Pet-1−/− mouse compared to wild type littermates. In contrast, from E16.5 on, 5-HT concentration is significantly decreased in the forebrain of Pet-1−/− embryos compared to littermate controls, indicating that forebrain 5-HT becomes exclusively provided by DR serotonergic neurons; this corresponds to the timing of raphe axons reaching the forebrain in large numbers (Bonnin et al., 2011). The differential accumulation of 5-HT in the forebrain, but not the hindbrain, in the Pet-1−/− embryos early in development indicates that there is a striking transition from an early exogenous source to a later endogenous (DR serotonergic neurons) source. This switch during development allows the forebrain during early- and mid-embryogenesis to become dependent progressively on its own production of 5-HT (Fig. 2A).
Figure 2.
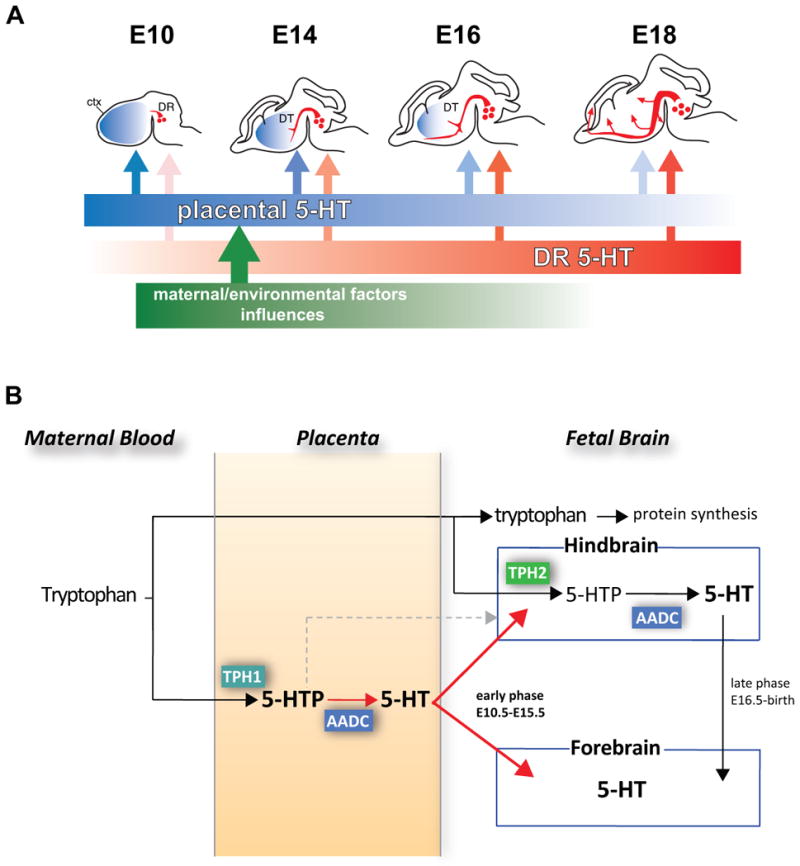
New model of placental contributions to the fetal brain and blood 5-HT. A, the source of 5-HT in the embryonic forebrain changes over time, from an early exogenous (placental) source to a later endogenous (dorsal raphe serotonergic neurons) source. This switch during development allows the embryo brain to progressively become dependent on its own production of 5-HT. DR, dorsal raphe; DT, dorsal thalamus; ctx, cortex. B, The placenta synthesizes 5-HT and 5-HTP from maternal blood tryptophan. This placental 5-HT (red color) is a source of 5-HT to the forebrain during an early phase of development (E10 to E15). Later (E16 to birth), the main source of 5-HT is provided by conversion of maternal tryptophan in hindbrain DR neurons and their axons throughout the forebrain.
From E10.5–15.5, there is no synthetic capacity of the gut to produce 5-HT (Branchek and Gershon, 1989). Myenteric plexus neurons express TPH2 and are able to uptake 5-HT at E12.5, and synthesize it at E18 (Rothman and Gershon, 1982, Cote et al., 2007). However, only enterochromaffin cells, which begin to express TPH1 at later stages (E15.5) (Cote et al., 2007), are able to provide a significant source of 5-HT for the fetal blood (Chen et al., 2001). We thus turned to the possibility that maternal 5-HT may be the source that is transferred to the fetal brain. We examined fetal brains of SERT knockout (SERT−/−) dams in which maternal there is no detectable blood and platelets 5-HT (Chen et al., 2001). Despite this status of the maternal blood, there was no impact on the concentration of 5- in the forebrain of SERT+/− E12.5 embryos from SERT−/− or wild type dams (Bonnin et al., 2011). These data indicated that in contrast to prevailing dogma in the field, maternal blood 5-HT in the mouse is not the main source of fetal blood and forebrain 5-HT at early stages of development. Similar observations were made with another mouse mutant [the pallid mice – (Li et al., 2004)], in which normal concentrations of fetal forebrain 5-HT were measured at E12.5, even though the maternal blood contains no 5-HT (Del Angelica and Bonnin, unpublished).
One additional source of 5-HT had not been investigated – the placenta. We designed studies to examine the possibility that the precursor and essential amino acid tryptophan, originating from the pregnant dam, is converted to 5-HT in the placenta and delivered to the fetal circulation. Immunocytochemistry, RNAseq and qRT-PCR confirmed that 5-HT biosynthetic enzymes TPH1 and AADC are expressed in the syncytiotrophoblastic cell layer of the placenta at E10.5-E18.5 (Bonnin et al., 2011). In vitro placental 5-HT neosynthesis was confirmed biochemically at E10.5, E14.5 and E18.5. This synthetic capacity was also observed in human placental fetal villi at 11 weeks of gestation, suggesting that a placental source of 5-HT is important for human fetal development.
In order to prove neosynthesis and transport of placental 5-HT from maternal tryptophan precursor, we developed an ex vivo technology for regulating live placental organ perfusion in the mouse (Bonnin et al., 2011). Briefly, a mouse placenta is harvested and kept alive in an oxygenated and thermostated organ chamber. On the maternal side of the placenta, the uterine artery is cannulated and connected to a low-flow peristaltic pump; on the fetal side, the umbilical artery and veins are cannulated and connected to independent ultra-low flow peristaltic pumps. These independent perfusions allow recreating artificial maternal and fetal blood circulations in intact, live isolated placentas (Bonnin et al., 2011). Effects of modifying the molecular composition of the artificial ‘maternal blood’ on 5-HT (or other molecules) release into the fetal blood through the umbilical vein are then quantified overtime. Within minutes after tryptophan injection through the maternal uterine artery, we observed large accumulation of newly synthesized 5-HT that was collected through the umbilical vein, demonstrating that the live placenta is able to convert tryptophan to 5-HT, and releases the neurotransmitter into the fetal circulation. In contrast, less than 1% of 5-HT injected on the maternal side could be transported to the fetal circulation. To establish placental synthetic capacity and transport of 5-HT to the fetus in vivo, we blocked TPH1 enzymatic activity by microinjecting small volumes of the TPH inhibitor p-chlorophenyalanine (PCPA) directly into the labyrinth zone of E14.5 placentas in utero (Bonnin et al., 2011). This pharmacological manipulation significantly reduced 5-HT levels in the placenta and fetal forebrain but not in the fetal hindbrain. These in vivo and ex vivo experiments demonstrate that an exogenous source of 5-HT produced in the placenta is required to maintain normal levels of forebrain 5-HT during early stages of forebrain development (Fig. 2B). Because the fetal blood brain barrier is not functional at early stages of brain development (Daneman et al., 2010), we proposed that placental 5-HT could reach TCAs and other axons as they grow and influence their response to guidance cues (Bonnin et al., 2007). This would be consistent with the diffusion of placenta-derived 5-HT within the fetal brain extracellular matrix, similar to bioactive molecules like growth factors and cytokines (see for instance (Li et al., 2009)). The extracellular accumulation would be analogous in nature with the adult brain, in which 5-HT released through ‘volume transmission’ from axonal varicosities can have long-range effects (De-Miguel and Trueta, 2005). Stability of the extracellular 5-HT accumulation might occur because of the requirement for uptake and degradation by cells that express MAOA (e.g. serotonergic DR and noradrenergic LC neurons); this is an unlikely route at early stages (<E15.5) of brain development, because serotonergic axons are just starting to reach the forebrain at that age, and they are few in number. Interestingly, SERT is expressed in TCAs (but MAOA is not) (Lebrand et al., 1996), and therefore growing thalamic axons could provide an additional mechanism by which placental 5-HT is taken up and protected from degradation specifically in the forebrain. However, at these early stages of development (E10–E16), 5-HT immunoreactivity is not detectable above background in growing TCAs. The possibility that placental 5-HT affects TCA formation remains to be fully demonstrated and the impact of a placental-specific TPH1 gene deletion on fetal forebrain 5-HT levels and TCA pathway formation is currently under investigation.
Although a direct maternal source of 5-HT may also be provided to the embryo before and during placentation (e.g. before E10.5 in the mouse), and may affect very early events of embryonic development (Cote et al., 2007), our data collectively provided the first evidence that a maternal precursor metabolized directly by the placenta influences fetal brain development. Because similar synthetic capability were observed in early human placenta, the extent to which there is a placental 5-HT influence on brain development during human pregnancy is key area for new investigations.
3. Conclusion
The results summarized here provide a mechanism through which alterations of tryptophan metabolic pathways in the placenta can affect placental 5-HT synthesis and fetal forebrain development. We showed that there is a progressive switch from an early dependence on an exogenous (placental) source of 5-HT to a later endogenous brain source. The placental source of 5-HT is provided to the forebrain during developmental periods that include cortical neurogenesis, migration and initial axon targeting [see (Gaspar et al., 2003)]. A variety of experiments from others and our laboratories show that that these events can be modulated by 5-HT signaling [for example, (Salichon et al., 2001, Janusonis et al., 2004, Bonnin et al., 2007, Homberg et al., 2010)]. Our proposed model of a progressive switch from placental to endogenous source of fetal forebrain 5-HT suggests that 5-HT-dependent developmental processes may be differentially sensitive to maternal and environmental factors depending on when they occur during pregnancy and also depending on the degree to which they impact placental metabolic pathways. Moreover, because the fetus and placenta are genetically identical, specific contributions of gene mutations and allelic variation to mental illness, which are typically assigned to direct fetal impact, must now be examined in the context of a placental contribution (McKay, 2011).
Most interestingly, earlier studies showed that chronic mild stress during pregnancy in rats not only increases the fraction of free tryptophan in the maternal blood but also 5-HT concentration in the fetal brain; this gestational stress also led to increased anxiety in the adult offspring (Peters, 1988b, a, 1990). This work was the first indication that maternal tryptophan blood level may be an important trigger or mediator of the fetal programing of adult brain disorders. The results summarized in this review suggest a potential mechanism by which this maternal tryptophan-mediated fetal programing can take place: maternal stress-induced increase in free plasma tryptophan availability could increase its metabolism in the placenta and the delivery of 5-HT to the developing forebrain, thereby affecting developmental processes such as axon pathways formation mentioned above. A complete demonstration that this proposed chain of events does lead to long-term effects on adult offspring brain function is currently under investigation. Because of naturally occurring fluctuations in maternal tryptophan blood level, its placental metabolism is likely tightly regulated in order to prevent random negative fetal programing effects. Future studies will focus on abnormal environmental (e.g. chronic stress) or genetic (e.g. MAOA or SERT polymorphisms) conditions that may affect this placental metabolic regulation and therefore generate 5-HT-mediated negative fetal programing events.
The phase of placental source of 5-HT in the mouse corresponds to the 1st and early 2nd trimesters in the human, prenatal periods that are associated with greater risk for mental illnesses due to maternal perturbations (Oberlander et al., 2009, Brown and Derkits, 2010). It will be important to examine fetal and placental tryptophan availability throughout normal and abnormal pregnancies. Studies in human and mice already showed that genetic conditions associated with altered tryptophan metabolism during pregnancy have long term consequences on the offspring; for example mutations in tryptophan 2,3-dioxygenase degrading enzymes (TDO1/2, which are important for tryptophan metabolism in the placenta (Suzuki et al., 2001)) affect neurogenesis, produce anxiety-related behavior in mice (Kanai et al., 2009) and in humans are associated with increased risk for schizophrenia, bipolar disorder and autism (Nabi et al., 2004, Miller et al., 2009). Interestingly, allelic variants of the Tph1 gene have been associated with schizophrenia (Zaboli et al., 2006). Although such association needs to be confirmed, it suggests that TPH1 activity in the placenta (and thus placental release of 5-HT to the fetus) could be an important trigger for fetal programming that may contribute to adult mental disorders such as schizophrenia.
Highlights.
Serotonin signaling affects fetal brain development.
During an early phase of pregnancy, the placenta provides serotonin to the fetal brain.
Placenta-derived serotonin may be important for normal fetal brain development.
Results suggest new maternal-fetal-placental contributions to the fetal programming of adult mental disorders.
Acknowledgments
We thank Nick Goeden for technical help. This work was supported by the NICHD (grant 5R21HD065287 to A.B.), NARSAD (to A.B.) and the NIMH (grant 1P50MH078280A1 to P.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alexandre Bonnin, Email: bonnin@usc.edu.
Pat Levitt, Email: plevitt@usc.edu.
References
- Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988;274:32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell and tissue research. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cellular and molecular neurobiology. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. doi: 10.1016/j.tins.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioural brain research. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends in pharmacological sciences. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, Nakamura T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Developmental neuroscience. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Sze PY, Krebs H. Maternal influences on tryptophan hydroxylase activity in embryonic rat brain. Developmental neuroscience. 1981;4:291–295. doi: 10.1159/000112768. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Li G, Kataoka H, Coughlin SR, Pleasure SJ. Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development. 2009;136:327–335. doi: 10.1242/dev.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. BioEssays: news and reviews in molecular, cellular and developmental biology. 2004;26:616–628. doi: 10.1002/bies.20042. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- McKay R. Developmental biology: Remarkable role for the placenta. Nature. 2011;472:298–299. doi: 10.1038/472298a. [DOI] [PubMed] [Google Scholar]
- Miller CL, Murakami P, Ruczinski I, Ross RG, Sinkus M, Sullivan B, Leonard S. Two complex genotypes relevant to the kynurenine pathway and melanotropin function show association with schizophrenia and bipolar disorder. Schizophr Res. 2009;113:259–267. doi: 10.1016/j.schres.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi R, Serajee FJ, Chugani DC, Zhong H, Huq AH. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:63–68. doi: 10.1002/ajmg.b.20147. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DA. Both prenatal and postnatal factors contribute to the effects of maternal stress on offspring behavior and central 5-hydroxytryptamine receptors in the rat. Pharmacol Biochem Behav. 1988a;30:669–673. doi: 10.1016/0091-3057(88)90081-0. [DOI] [PubMed] [Google Scholar]
- Peters DA. Effects of maternal stress during different gestational periods on the serotonergic system in adult rat offspring. Pharmacol Biochem Behav. 1988b;31:839–843. doi: 10.1016/0091-3057(88)90393-0. [DOI] [PubMed] [Google Scholar]
- Peters DA. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacol Biochem Behav. 1990;35:943–947. doi: 10.1016/0091-3057(90)90383-s. [DOI] [PubMed] [Google Scholar]
- Rakic P, Lidow MS. Distribution and density of monoamine receptors in the primate visual cortex devoid of retinal input from early embryonic stages. J Neurosci. 1995;15:2561–2574. doi: 10.1523/JNEUROSCI.15-03-02561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. J Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425–429. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Developmental neuroscience. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- Yavarone MS, Shuey DL, Sadler TW, Lauder JM. Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta. 1993;14:149–161. doi: 10.1016/s0143-4004(05)80257-7. [DOI] [PubMed] [Google Scholar]
- Zaboli G, Jonsson EG, Gizatullin R, Asberg M, Leopardi R. Tryptophan hydroxylase-1 gene variants associated with schizophrenia. Biol Psychiatry. 2006;60:563–569. doi: 10.1016/j.biopsych.2006.03.033. [DOI] [PubMed] [Google Scholar]


