Abstract
Background
Previous studies have demonstrated that intrathecal administration of the substance P amino-terminal metabolite substance P1-7 (SP1-7) and its C-terminal amidated congener induced antihyperalgesic effects in diabetic mice. In this study, we studied a small synthetic dipeptide related to SP1-7 and endomorphin-2, i.e. Phe-Phe amide, using the tail-flick test and von Frey filament test in diabetic and non-diabetic mice.
Results
Intrathecal treatment with the dipeptide increased the tail-flick latency in both diabetic and non-diabetic mice. This effect of Phe-Phe amide was significantly greater in diabetic mice than non-diabetic mice. The Phe-Phe amide-induced antinociceptive effect in both diabetic and non-diabetic mice was reversed by the σ1 receptor agonist (+)-pentazocine. Moreover, Phe-Phe amide attenuated mechanical allodynia in diabetic mice, which was reversible by (+)-pentazocine. The expression of spinal σ1 receptor mRNA and protein did not differ between diabetic mice and non-diabetic mice. On the other hand, the expression of phosphorylated extracellular signal-regulated protein kinase 1 (ERK1) and ERK2 proteins was enhanced in diabetic mice. (+)-Pentazocine caused phosphorylation of ERK1 and ERK2 proteins in non-diabetic mice, but not in diabetic mice.
Conclusions
These results suggest that the spinal σ1 receptor system might contribute to diabetic mechanical allodynia and thermal hyperalgesia, which could be potently attenuated by Phe-Phe amide.
Keywords: Allodynia, Antinociception, Diabetes, Hyperalgesia, Opioid receptors, Phe-Phe amide, σ1 receptor, Substance P1-7
Background
Diabetes is a global disease with an estimated worldwide prevalence of 2.8% in 2000, and this is predicted to climb to 4.4% in 2030 [1]. Diabetic neuropathy is seen in about 60% of all diabetic patients [2]. While the symptoms of diabetic polyneuropathy include hyperalgesia (hypersensitivity to noxious stimuli), hypoalgesia (loss of pain sensation) is also possible [3]. This pain is poorly relieved by opiates and the treatment regimen is usually based on the use of antiepileptics and antidepressants, which often have inadequate effects and are associated with a high prevalence of side effects [4]. There is a great need for new strategies for the treatment of diabetic neuropathy.
We recently demonstrated that substance P1-7 (SP1-7; H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH), administered spinally, could attenuate thermal hyperalgesia in diabetic mice [5]. SP1-7 is formed from substance P (SP; H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2). SP was discovered as a neuropeptide by Von Euler and Gaddum in 1931 [6] and is involved in pain signaling, peripheral inflammation and the maintenance of hyperalgesia [7]. SP is enzymatically degraded into several fragments, some of which retain their biological activity [8-10]. The main N-terminal fragment, SP1-7, exerts several effects that are opposite those of SP, e.g. the heptapeptide has antinociceptive [11], anti-inflammatory [12] and antihyperalgesic effects [5]. In addition, the heptapeptide attenuates several withdrawal signs in morphine-dependent rodents [13,14] and the development of morphine tolerance [13]. These effects are mediated through a specific receptor for SP1-7, which was detected in the rat and mouse spinal cord [15,16] and the rat ventral tegmental area [17], and is distinct from any of the known opioid and tachykinin receptors.
We previously reported that the σ1 receptor might be involved in the effect of SP1-7 and its amidated analogue, SP1-7-NH2 [5,18,19]. σ1 receptor ligands have poor affinity for the SP1-7 binding site [19] which may imply that SP1-7 has a downstream, rather than a direct, effect on the σ1 receptor. On the other hand, the effects of SP1-7 on σ1 receptors have been reported previously [20,21] and the σ1 receptor is interesting to study since it has been proposed to be involved in intracellular signaling cascades that lead to pain hypersensitivity [22].
We previously performed a thorough structure-activity relationship study of SP1-7 involving an alanine-scan and truncations as well as C- and N-terminal modification of the heptapeptide. Amidation of the heptapeptide increased its affinity for the SP1-7 binding sites [23] and produced a stronger effect than the native heptapeptide when used in behavioral tests [18,19]. The C-terminal part of SP1-7, especially the phenylalanine at position seven, is most essential for its binding. This finding, together with the knowledge that endomorphin-2 (EM-2; H-Tyr-Pro-Phe-Phe-NH2) binds to the SP1-7 binding site, resulted in the development of low-molecular, peptidergic ligands [24]. We discovered that the dipeptide H-Phe-Phe-NH2 (Phe-Phe amide) had the same affinity for the SP1-7 binding site as the endogenous heptapeptide. Therefore, the present study was designed to examine the ability of this dipeptide to attenuate allodynia and thermal hyperalgesia in diabetic mice.
Results
Effect of Phe-Phe amide on the thermal nociceptive threshold in diabetic and non-diabetic mice
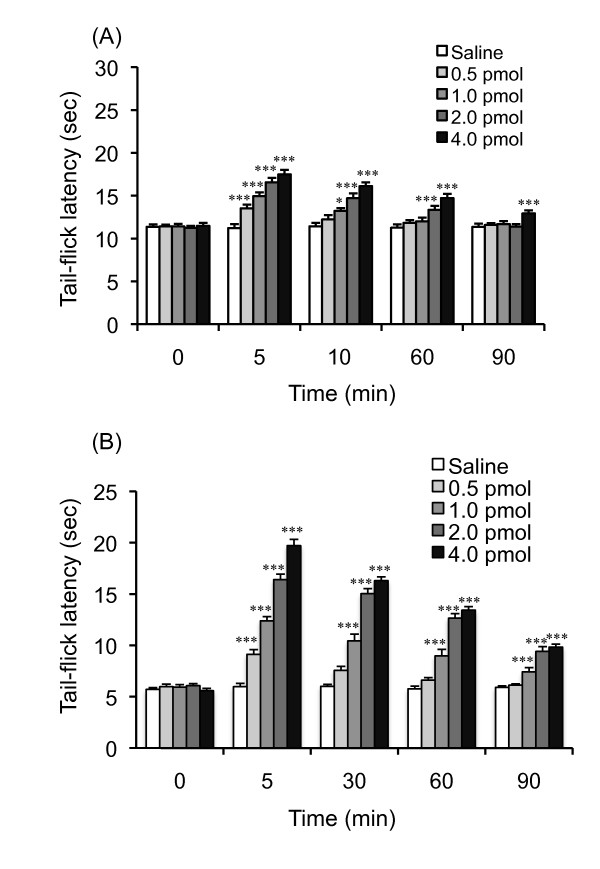
The baseline tail-flick latencies in diabetic mice were shorter than those in non-diabetic mice, indicating that diabetic mice have a reduced pain threshold (Figures 1A and 1B). As shown in Figure 1A, i.t. administration of Phe-Phe amide dose-dependently increased the tail-flick latencies in non-diabetic mice. Two-way ANOVA indicated a significant main effect of Phe-Phe amide treatment (F4, 250 = 46.59, P < 0.001), time (F4,250 = 58.8, P < 0.001), and the interaction between Phe-Phe amide treatment and time (F16,250 = 6.93, P < 0.001). A dose-dependent increase in tail-flick latency was seen in diabetic mice, but with greater potency (Figure 1B). Two-way ANOVA indicated a significant main effect of Phe-Phe amide treatment (F4,230 = 382.55, P < 0.001), time (F4,230 = 338.56, P < 0.001), and the interaction between Phe-Phe amide treatment and time (F16, 230 = 45.71, P < 0.001). Since the antinociceptive effect of Phe-Phe amide was more potent in diabetic mice, the ability of Phe-Phe amide to produce an increase in the tail-flick latency in diabetic mice is greater than that in non-diabetic mice.
Figure 1.
Time-response curve for the intrathecal injection of Phe-Phe amide on the tail-flick latency in non-diabetic (A) and diabetic (B) mice. The nociceptive threshold was determined by the tail-flick test 5, 30 60 and 90 min after injection of the dipeptide. Each point represents the mean with S.E.M. for 10-12 mice. *P < 0.05 and ***P < 0.001 vs. saline-treated group.
Effects of opioid receptor antagonists on Phe-Phe amide-induced antinociception in diabetic and non-diabetic mice
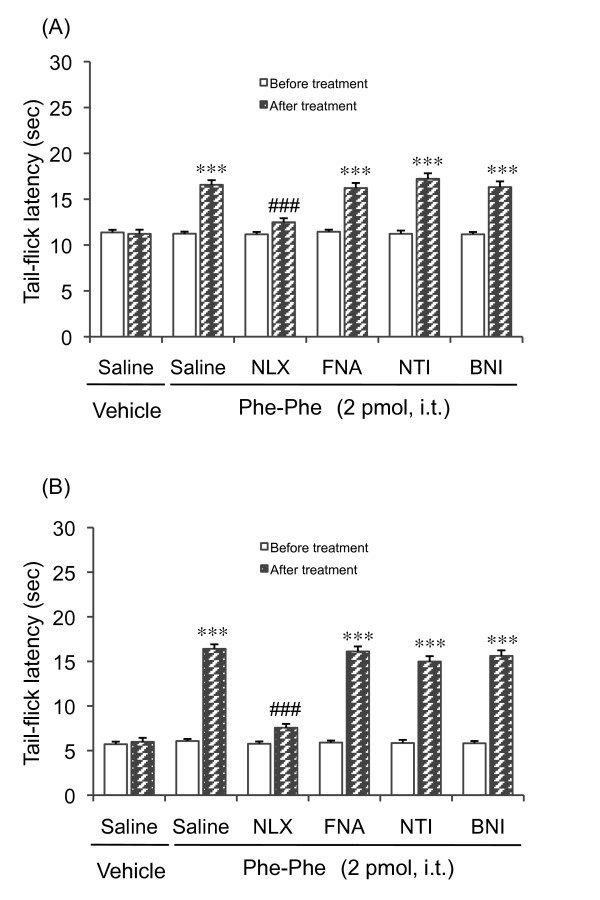
To investigate whether the opioid system is involved in the effect seen with Phe-Phe amide, we examined the effects of opioid receptor antagonists on the prolongation of the tail-flick latency seen after the administration of 2 pmol Phe-Phe amide. This dose was chosen according to the results of the dose-response study, where it was shown to produce an evident antinociceptive response in both diabetic and non-diabetic mice. Pretreatment with the non-selective opioid receptor antagonist naloxone (1 mg/kg, i.p.) inhibited the Phe-Phe amide-induced increase in the tail-flick latency in non-diabetic mice (Figure 2A) as well as in diabetic mice (Figure 2B). In contrast to naloxone, neither μ-, δ-, nor κ-opioid receptor antagonists had any effect on the Phe-Phe amide-induced prolongation of the tail-flick latency (Figure 2A and 2B).
Figure 2.
Effects of opioid receptor antagonists on Phe-Phe amide (2 pmol, i.t.)-induced antinociception in non-diabetic (A) and diabetic (B) mice. Naloxone (NLX, 1 mg/kg i.p) and naltrindole (NTI, 3 mg/kg, s.c.) were administered 30 min before the injection of Phe-Phe amide. β-Funaltrexamine (β-FNA, 20 mg/kg, s.c.) and nor-binaltorphimine (BNI, 20 mg/kg, s.c.) were administered 24 h before injection of the Phe-Phe amide. Each column represents the mean with S.E.M. for 6-11 mice. ***P < 0.001 vs. respective before Phe-Phe amide-treatment group; ###P < 0.001 vs. saline-pretreated Phe-Phe amide group (Bonferroni test).
Involvement of σ-receptors in Phe-Phe amide-induced antinociception in diabetic and non-diabetic mice
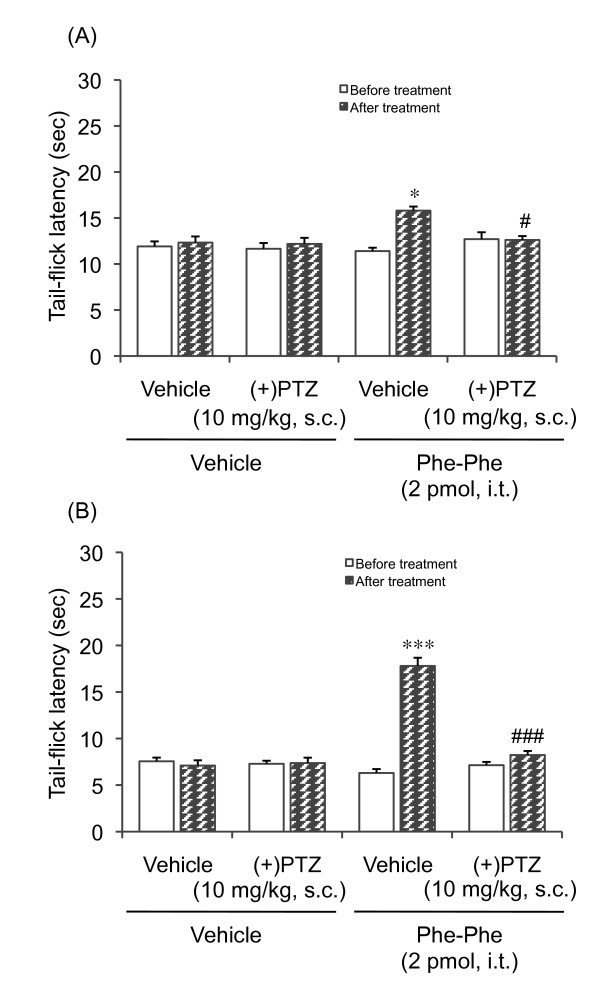
We recently demonstrated that the antihyperalgesic effects induced by both SP1-7 and its analogue SP1-7-NH2 in diabetic mice may involve σ1 receptors. Therefore, we also evaluated the possible involvement of the σ1 receptor by examining the effect of the agonist (+)-pentazocine on Phe-Phe amide-induced antinociception in diabetic and non-diabetic mice. Pretreatment with (+)-pentazocine attenuated the Phe-Phe amide-induced prolongation of the tail-flick latency in both non-diabetic (Figure 3A) and diabetic mice (Figure 3B).
Figure 3.
Effect of the σ1-receptor agonist (+)-pentazocine [(+)PTZ, 10 mg/kg, s.c] on the Phe-Phe amide (2 pmol, i.t.)-induced prolongation of the tail-flick latency in non-diabetic and diabetic mice. (+)PTZ was administered subcutaneously 30 min prior to the administration of Phe-Phe amide. Each column represents the mean with S.E.M. for 10 mice. *P < 0.05, ***P < 0.001 vs. respective before-treatment group; #P < 0.05, ###P < 0.001 vs. vehicle-pretreated group (Bonferroni test).
Effect of Phe-Phe amide on mechanical allodynia in diabetic mice
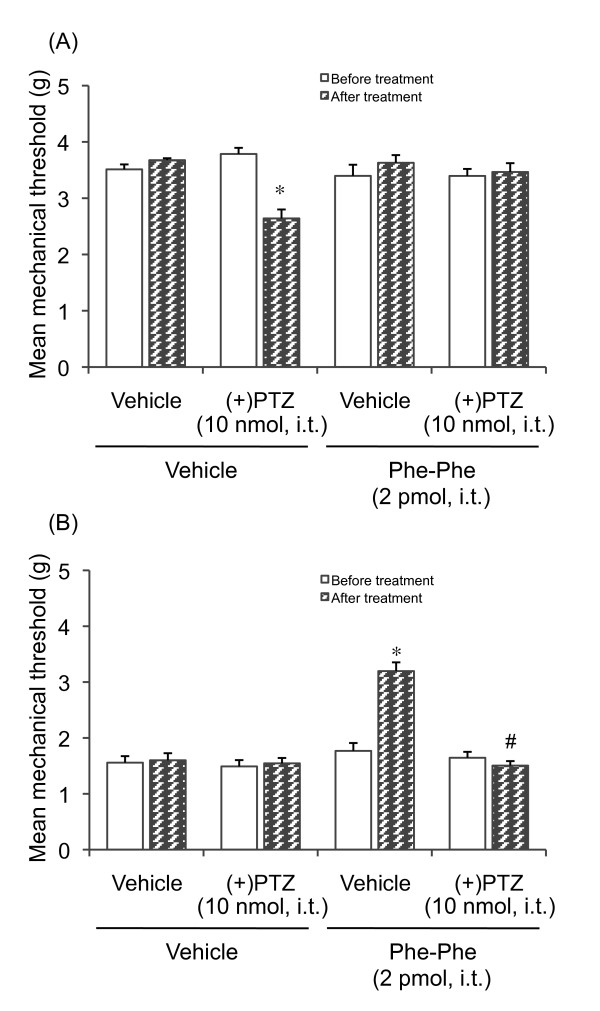
Phe-Phe amide produced a significant increase in the thermal threshold in diabetic and non-diabetic mice. In the next step of the experiment, we investigated the effect of Phe-Phe amide on the mechanical threshold in diabetic and non-diabetic mice. As shown in Figure 4, the mechanical threshold in diabetic mice was lower than that in non-diabetic mice, indicating that diabetic mice exhibit mechanical allodynia (Figure 4A and 4B). I.t. administration of Phe-Phe amide (2 pmol) did not affect the mechanical threshold in non-diabetic mice (Figure 4A). In contrast to non-diabetic mice, the decrease in the mechanical threshold in diabetic mice was reversed by treatment with Phe-Phe amide, an effect which in turn was attenuated by i.t. pretreatment with (+)-pentazocine (Figure 4B). Although i.t. treatment with Phe-Phe amide did not affect the mechanical threshold in non-diabetic mice, i.t. administration of (+)-pentazocine decreased the mechanical threshold in non-diabetic mice, which was reversed by i.t. treatment with Phe-Phe amide (Figure 4A).
Figure 4.
Effect of Phe-Phe amide (2 pmol, i.t.) on the mechanical threshold in diabetic and non-diabetic mice. The nociceptive threshold was determined by a von Frey filament test 30 min after dipeptide injection. (+)-Pentazocine [(+)PTZ, 10 nmol, i.t] was administered 10 min prior to the injection of Phe-Phe amide. Each point represents the mean with S.E.M. for 7-11 mice. *P < 0.05 vs. respective before-treatment group; #P < 0.001 vs. vehicle-pretreated group (Bonferroni test).
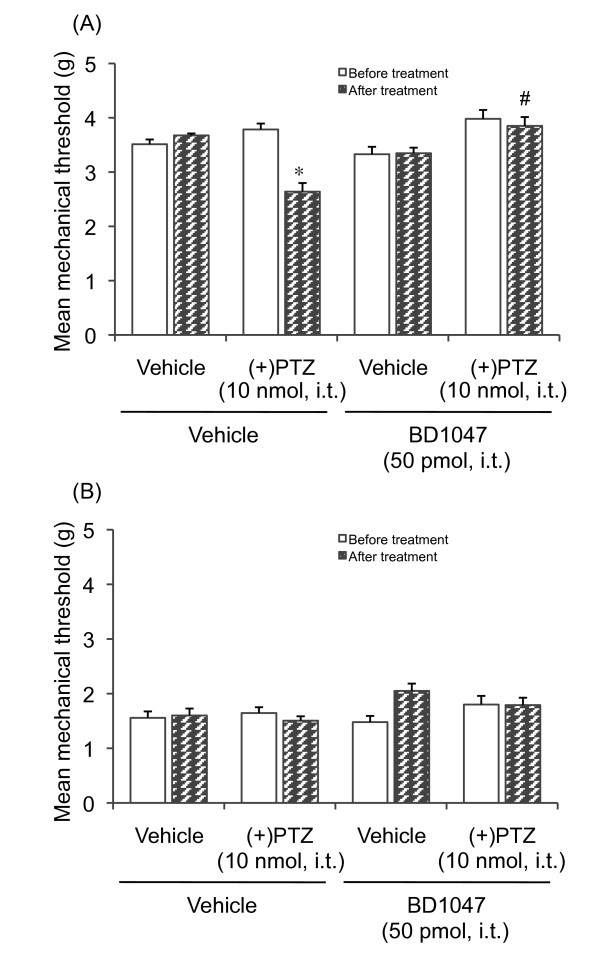
This i.t.-administered (+)-pentazocine-induced decrease in the mechanical threshold in non-diabetic mice suggests that the σ1 receptor may play a role in mechanical allodynia. To confirm this possibility, the effect of the σ1 receptor antagonist BD1047 on the (+)-pentazocine-induced decrease in the mechanical threshold in non-diabetic mice was examined. I.t. pretreatment with BD1047 completely reversed the decrease in the mechanical threshold in (+)-pentazocine-treated non-diabetic mice (Figure 5A). I.t. treatment with BD1047 slightly, but not significantly, increased the mechanical threshold in diabetic mice, while the mechanical threshold in non-diabetic mice was not affected (Figure 5B).
Figure 5.
Effect of the σ1-receptor antagonist BD1047 on (+)-pentazocine [(+)PTZ, 10 nmol, i.t.]-induced lowering of the mechanical threshold in non-diabetic and diabetic mice. BD1047 was injected i.t. 10 min prior to the administration of (+)PTZ. Each column represents the mean with S.E.M. for 10 mice. *P < 0.05 vs. respective before-treatment group; #P < 0.05 vs. vehicle-pretreated group (Bonferroni test).
Expression of σ1 receptor mRNA and proteins in the spinal cords of diabetic and non-diabetic mice
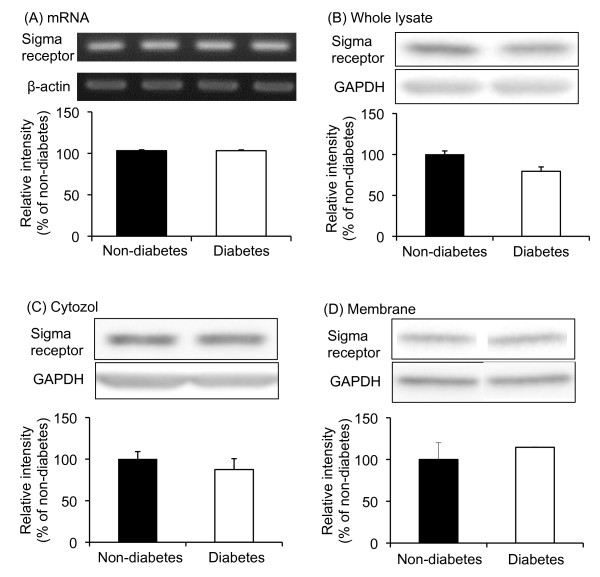
The behavioral studies strongly suggested that the spinal σ1 receptor system is enhanced in diabetic mice. Moreover, the potent antinociceptive and antiallodynic effect of Phe-Phe amide in diabetic mice might be correlated with enhanced activity in the spinal σ1 receptor system. To clarify this possibility, the expression of σ1 receptor mRNA and protein was examined in the spinal cords of diabetic and non-diabetic mice. Reverse-transcription semi-quantitative PCR indicated that the level of σ1 receptor mRNA in the spinal cord in diabetic mice was not different from that in non-diabetic mice (Figure 6A). Moreover, the expression of the σ1 receptor protein in the spinal cord was unchanged in diabetic mice (Figure 6B). σ1 receptors have been shown to translocate from the mitochondrion-associated endoplasmic reticulum membrane to the plasma membrane, which leads to functional activation [25]. Therefore, the expression of the σ1 receptor in the cytosol and membrane fractions in the spinal cords of diabetic and non-diabetic mice was examined. The expression of σ1 receptors in the cytosol and membrane fraction of the spinal cord in diabetic mice was not different from that in non-diabetic mice (Figure 6C and 6D).
Figure 6.
σ1 Receptor mRNA (A), and protein (B, C, D) expression in the spinal cords of diabetic and non-diabetic mice. Protein expression was evaluated in the whole lysate (B), cytosol fraction (C), and membrane fraction (D). Immunoblots are from an experiment that is of 4 similar experiments. Each column represents the mean ± S.E.M. of 4 separate experiments.
Effect of (+)-pentazocine on the phosphorylation of ERK1 and ERK2 proteins in the spinal cords of diabetic and non-diabetic mice
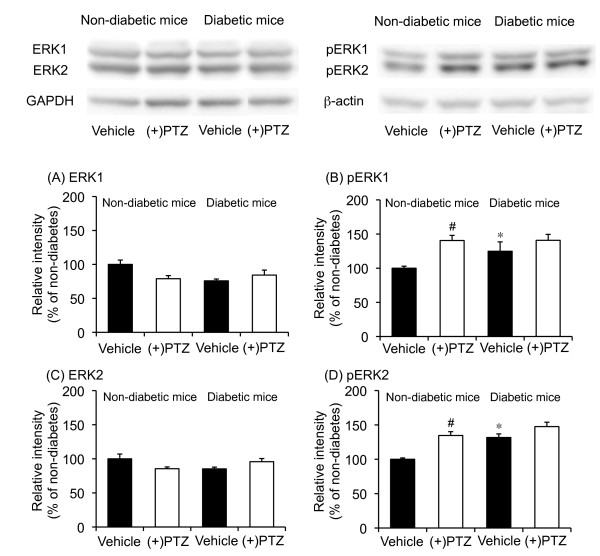
Since σ1 receptor expression was not changed in diabetic mice, the function of σ1 receptors in diabetic mice might be enhanced in the spinal cord. Therefore, we examined the effect of (+)-pentazocine on the phosphorylation of the extracellular signal-regulated protein kinase 1 (ERK1) and ERK2 proteins in the spinal cords of diabetic and non-diabetic mice. The expression of ERK1 and ERK2 proteins in the spinal cord of diabetic mice was not different than that in non-diabetic mice (Figure 7A and 7B). In contrast to the expression of ERK1 and ERK2 protein, the expression of phosphorylated ERK1 and ERK2 proteins was increased in the spinal cord of diabetic mouse compared to non-diabetic mice (Figure 7B and 7D). Treatment with (+)-pentazocine did not affect the expression of ERK1 and ERK2 protein in the spinal cords of diabetic and non-diabetic mice (Figure 7A and 7B). Treatment with (+)-pentazocine increased the phosphorylation of ERK1 and ERK2 in the spinal cord of non-diabetic mice (Figure 7A and 7B). In contrast to non-diabetic mice, phosphorylation of ERK1 and ERK2 was not observed in diabetic mouse spinal cord after treatment with (+)-pentazocine (Figure 7B and 7D).
Figure 7.
Effects of (+)-pentazocine [(+)PTZ] on the expression of ERK1 (A), ERK2 (C), phosphorylated ERK1 (B), and phosphorylated ERK2 (D) protein in the spinal cords of diabetic and non-diabetic mice. Spinal samples were collected 30 min after the i.t. administration of (+)PTZ. Immunoblots are from an experiment that is representative of 4 similar experiments. Each column represents the mean ± S.E.M of 4 separate experiments. *P < 0.05 vs. non-diabetic mice; #P < 0.05 vs. respective vehicle-treated group.
Discussion
The present results showed that the i.t. administration of Phe-Phe amide increased the tail-flick latency in diabetic and non-diabetic mice the same as larger SP-related peptides [5,18]. The Phe-Phe amide-induced increase in the tail-flick latency in diabetic mice was greater than that in non-diabetic mice. The antinociception elicited by the dipeptide in both diabetic and non-diabetic mice was completely blocked by the non-selective opioid receptor antagonist naloxone. However, none of the more selective μ-, δ- or κ- opioid receptor antagonists (β-funaltrexamine, naltrindole and nor-binaltorphimine, respectively) affected Phe-Phe amide-induced antinociception, suggesting that the antinociceptive effect of the dipeptide amide does not involve these receptors. Tsao and Su [26] previously reported that the (+)- stereoisomer of naloxone may interact with the σ1 receptor. We recently found that SP1-7 produced a σ1 receptor-sensitive antinociception in diabetic and non-diabetic mice [5], and that Phe-Phe amide exhibited high affinity for the specific binding site of SP1-7 [24]. We observed in the present study that i.t. pretreatment with (+)-pentazocine attenuated the Phe-Phe amide-induced antinociception in diabetic and non-diabetic mice, suggesting that the σ1 receptor may be involved in the antinociceptive effect of Phe-Phe amide.
The σ1 receptor system has previously been shown to influence the mechanical pain threshold [22]. We observed that the mechanical threshold in diabetic mice was lower than that in non-diabetic mice. Treatment with Phe-Phe amide increased the mechanical threshold in diabetic mice, but not non-diabetic mice. This Phe-Phe amide-induced antiallodynic effect in diabetic mice was attenuated by pretreatment with (+)-pentazocine, which again suggests that the spinal σ1 receptor system may be involved. Interestingly, (+)-pentazocine by itself decreased the mechanical threshold in non-diabetic mice. This (+)-pentazocine-induced mechanical allodynia was attenuated by pretreatment with the σ1 receptor antagonist BD1047 and Phe-Phe amide, suggesting that the activation of spinal σ1 receptors causes mechanical allodynia. It has been reported that spinal σ1 receptor activation enhances the N-methyl-D-aspartate (NMDA)-induced nociceptive response [27]. Moreover, mice that lack the σ1 receptor did not exhibit any mechanical allodynia after nerve ligation [22], which confirms that activation of the spinal σ1 receptor system might be involved in mechanical allodynia. On the other hand, i.t. administration of (+)-pentazocine in diabetic mice did not affect the mechanical threshold. A possible explanation for this finding is that the spinal σ1 receptor-mediated system is already activated in diabetic mice. This possibility is supported by the finding that i.t. pretreatment with the σ1 receptor antagonist BD1047 slightly, but not significantly, increased the mechanical threshold in diabetic mice.
I.t. administration of (+)-pentazocine produced mechanical allodynia in non-diabetic mice (see Figure 4A) but did not affect the thermal nociceptive threshold (Figure 3A). This suggests the differential modulation of nociceptive pathways by σ1 receptors depending on the stimulus quality and modality. This adds to previous findings that different receptor systems and mechanisms are involved in diverse sensory abnormalities, such as mechanical versus thermal pain [22,28-31].
We also examined the expression of the σ1 receptor gene transcript and protein in the spinal cord of both diabetic and non-diabetic mice, and did not find any differences. One of the few studies on the σ1 receptor in diabetic mice demonstrated that there were no differences in the expression of the σ1 receptor gene transcript and protein in retinal ganglion cells between diabetic and non-diabetic mice [32]. Furthermore, a receptor binding study has indicated a decrease in σ1 receptor density in the brains of long-term diabetic rats, while relatively short-term diabetic rats did not show any significant differences [33].
Since the expression of the spinal σ1 receptor mRNA and protein is not changed in diabetic mice, the function of σ1 receptors might be affected. Activation of the σ1 receptor increases the intracellular Ca2+ concentration by potentiating Ca2+ entry at the plasma membrane and Ca2+ mobilization from endoplasmic stores. Previously, it has been reported that σ1 receptors are functionally coupled to the NMDA receptor [34-38] and regulate intracellular Ca2+ concentration through phospholipase C and IP3 [39,40]. Ca2+ entry into neurons through the NMDA receptor or mobilization through IP3 may initiate the intracellular ERK signaling cascade in spinal dorsal horn neurons [41], thus contributing to central sensitization. The present study indicates that the expression levels of phosphorylated ERK1 and ERK2 are increased in diabetic mice compared to non-diabetic mice, while the expression levels of ERK1 and ERK2 protein are not changed. These results suggest that spinal ERK signaling is already activated in the spinal cord of diabetic mice. Moreover, i.t. treatment with (+)-pentazocine increased the phosphorylation of ERK1 and ERK2 proteins in the spinal cord of non-diabetic mice, but not diabetic mice. This observation suggests that the stimulation of spinal σ1 receptors produces the activation of ERK signaling. A previous report indicated that ERK on the ipsilateral side of the spinal cord dorsal horn was activated in nerve-ligated mice [42]. This ERK activation in the spinal cord after nerve ligation was not observed in σ1 receptor knockout mice [22], which supports our present finding that σ1 receptors are responsible for the regulation of ERK signaling in diabetic mice. Since (+)-pentazocine did not increase the phosphorylation of spinal ERK protein in diabetic mice, the increased phosphorylation of ERK1 and ERK2 protein in the spinal cord may be, at least in part, due to the enhancement of σ1 receptor-mediated functions.
The present results do not clarify the action site of Phe-Phe amide. Our recent findings clearly indicated that Phe-Phe amide has very high affinity for the SP1-7 binding site [24], which is distinct from the neurokinin receptors, NK1 and NK3 [15,24]. Our previous studies suggested that SP1-7 might be related to the σ receptor system, since a σ1 receptor agonist could reverse the effect of SP1-7 on hyperalgesia and naloxone-precipitated morphine withdrawal signs [5,19]. However, (+)-pentazocine has very low affinity for the SP1-7 binding site [19], suggesting that SP1-7 and its analogues modulate the effect of the σ1 receptor system rather than directly acting as ligands for σ1 receptors.
Conclusions
The present study suggests that the antinociceptive and antiallodynic effects induced by Phe-Phe amide occur through modulation of the spinal σ1 receptor system via the SP1-7 binding site. Furthermore, the spinal σ1 receptor system appears to contribute to the thermal hyperalgesia and mechanical allodynia seen in diabetic mice. Based on the present results, compounds that bind to a SP1-7-specific binding site, like Phe-Phe amide, might be attractive for the treatment of pain symptoms associated with diabetic neuropathy. Notably, this small synthetic dipeptide, with high affinity for the SP1-7 binding site, had a more pronounced in vivo effect than larger SP-related peptides. Since Phe-Phe amide has analgesic properties similar to those of SP1-7 as well as a smaller size, it is an interesting starting point for the development of new drugs to relieve neuropathic pain.
Methods
This study was carried out in accordance with the Declaration of Helsinki and/or with the guide for the committee on the care and use of laboratory animals of Hoshi University, Tokyo, which is accredited by the Ministry of Education, Science, Sports and Culture.
Animals
Male 4-week-old ICR mice (Tokyo Animal Laboratory Inc, Tokyo) weighing about 20 g at the start of the experiment were used. The mice had free access to food and water and were housed five per cage in an animal room that was maintained at 24 ± 1°C with a 12-h dark/light cycle. All behavioral experiments were performed between 10:00 and 19:00 each day.
Induction of diabetes with streptozotocin
Mice were rendered diabetic by an intravenous injection of streptozotocin (200 mg/kg) prepared in a 0.1 N citrate buffer at pH 4.5. Age-matched animals were injected with vehicle alone. Experiments were conducted two weeks after the administration of streptozotocin. Animals with a serum glucose level exceeding 400 mg/dl were considered diabetic.
Assessment of thermal hyperalgesia
The antihyperalgesic response was evaluated using the tail-flick test (KN-205E Thermal Analgesimeter, Natume, Tokyo, Japan) as described by D'amour and Smith [43]. Briefly, the mouse was gently restrained in a tube. The tail was positioned in a groove underneath a 50 W projection bulb with the dorsal part of the tail facing the light bulb. The light and timer were monitored with the same switch. Twitching or movement of the tail is a typical response elicited by heat. When this occurred, light reached a photocell and the light and timer were switched off. Latencies were determined as the mean of two trials. The voltage of a 50 W projection lamp was set to 50 V [44], which gave a baseline value in non-diabetic animals of 10-14 s. The cut-off time was set to 30 s to prevent injury to the tail. Tail-flick latencies were measured 5, 30, 60 and 90 min after i.t. injection of Phe-Phe amide.
Assessment of tactile allodynia
Mechanical sensitivity was determined by probing the plantar surface of the hind paw (von-Frey test) with a calibrated plastic filament of a dynamic plantar aesthesiometer purchased from Ugo Basile (Comerio, Italy). Force was applied to the hind paw at a rate of 0.25 g/s; the final force when paw withdrawal was observed was measured automatically (mechanical threshold). A maximal cut-off of 5 g was used to prevent tissue damage. A significant decrease in the mechanical threshold after the induction of diabetes compared with that in vehicle-treated animals was considered mechanical allodynia. The mechanical threshold was determined as the average of two measurements per mouse. Values were obtained before and 30 minutes after drug administration.
Intrathecal injections
Phe-Phe amide and σ ligands were administered by i.t. injection as described by Hylden and Wilcox [45] using a 25 μl Gastight® syringe (Hamilton, USA) and a BD Precisionglide® 30G 1/2 inch needle (Becton Dickinson, USA). The mouse was restrained manually and the needle was inserted between the L5 and L6 vertebrae. This site is near the end of the spinal cord and minimizes the risk of spinal damage [45].
Western blot
The spinal cord was quickly removed following decapitation to evaluate σ1 receptor proteins. To measure the phosphorylation of ERK protein, decapitation was performed 30 min after treatment with (+)-pentazocine. The spinal cord was homogenized in ice-cold buffered sucrose solution containing 20 mM Tris-HCl (pH 7.4), 2 mM EDTA, 0.5 mM EGTA, and 1 mM phenylmethylsulfonyl fluoride plus 250 μg/ml leupeptin, 250 μg/ml aprotinin, and 0.32 M sucrose. The homogenate was then centrifuged at 1,000 × g for 10 min at 4°C, and the resulting supernatant was centrifuged at 9000 × g for 20 min at 4°C. To separate the cytosol and membrane fractions, the supernatant was ultracentrifuged at 100,000 × g for 60 min at 4°C. The resulting supernatant was retained as the cytosolic fraction. The pellet was resuspended in ice-cold Tris buffer (ice-cold buffer without sucrose) and ultracentrifuged at 100,000 × g for 60 min. The supernatant was discarded and the pellet was resuspended in ice-cold Tris buffer. The protein concentration was measured using a Bradford assay kit (Thermo Fisher Scientific Inc., Suwannee, GA). Cytosol and plasma membrane samples with the same amounts of protein were diluted with an equal volume of 2x electrophoresis sample buffer containing 2% SDS and 10% glycerol with 0.2 M dithiothreitol. Proteins (20 μg) were separated by size on 5-20% SDS-polyacrylamide gradient gel using the buffer system and transferred to nitrocellulose membranes in Tris-glycine buffer containing 25 mM Tris and 192 mM glycine. For immunoblot detection, the membranes were blocked in Tris-buffered saline (TBS) containing 5% non-fat dried milk or 1% non-fat dried milk with 0.1% Tween 20 (Bio-Rad Laboratories, Hercules, CA, USA) for 1 hr at room temperature with agitation. The membrane was immunoblotted overnight at 4°C with antibodies against σ1 receptor (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), ERK1 (1:1000; Cell Signaling Technology Inc., Danvers, MA, USA), ERK2 (1:1000; Cell Signaling Technology Inc.), phosphorylated ERK1 (Cell Signaling Technology Inc.), and phosphorylated ERK2 (Cell Signaling Technology Inc.). The membrane was washed in TBS containing 0.05% Tween 20 (TTBS), and then incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG or rabbit anti-goat IgG (Southern Biotechnology Associates, Inc., Birmingham, AL, USA) diluted 1:10,000 in TBS containing 5% nonfat dried milk or 1% non-fat dried milk with 0.1% Tween 20. The antigen-antibody peroxidase complex was finally detected by enhanced chemiluminescence (Pierce, Rockford, IL, USA) and visualized using a Light-Capture II imaging system (Atto Co., Tokyo, Japan). The intensity of the band was analyzed and semi-quantified by computer-assisted densitometry using the NIH imaging system. Each value for σ1 receptors in diabetic and non-diabetic mice was normalized by the respective value for the internal control GAPDH.
Semi-quantification of reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the mouse spinal cord using a FastPure RNA isolation kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Total RNA was quantified by a spectrophotometer at A260. Next, cDNA was synthesized by a PrimeScript RT reagent kit (Takara Bio) with oligo dT primers. The mouse sigma1 receptor cDNA was amplified in 25 μl of PCR solution containing 0.8 mM MgCl2, 250 nM NTP mixture, and 0.5 units of platinum Taq DNA polymerase with synthesized primers (0.5 μM) corresponding to the mouse σ1 receptor cDNA (GenBankTM accession number XM136229). The primers used were as follows: 5'-primer, 5'-CAT TCG GGG CGA TAC TGG GC-3' (1-22); 3'-primer 5'-CCT GGG TAG AAG ACC TCA CTT TT -3'(311-332). Samples were heated to 94°C for 2 min, 94°C for 30 sec, 55°C for 1 min, and 72°C for 2 min for 35 cycles. The final incubation was at 72°C for 10 min. The mixture was run on 1.5% agarose gel electrophoresis with the indicated markers and primers for the internal standard β-actin. The agarose gel was stained with ethidium bromide and photographed with UV transillumination. The intensity of the band was analyzed and semi-quantified by computer-assisted densitometry using Image J. Data are expressed as a ratio to β-actin.
Chemicals
The non-selective opioid receptor antagonist naloxone, the σ1 receptor agonist (+)-pentazocine, and the σ1 receptor antagonist BD1047 were purchased from Sigma Chemical Co (St. Louis, MO, USA). Phe-Phe amide was prepared by using solid-phase peptide synthesis. Standard Fmoc conditions were used and the protecting group was removed by 20% piperidine in DMF. The coupling procedure was performed in N, N-dimethylformamide (DMF), using N-[(1H-benzotriazole-1-yl)-(dimethylamino)methylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HBTU) as a coupling reagent and N, N-diisopropylethylamine (DIEA) as a base. The dipeptide was cleaved from the resin by the addition of triethylsilane and 95% aqueous trifluoroactic acid (TFA), and purified by RP-HPLC to give Phe-Phe amide with purity above 99%. The selective μ-opioid receptor antagonist β-funaltrexamine, the selective δ-opioid receptor antagonist naltrindole, and the selective κ-opioid receptor antagonist nor-binaltorphimine were gifts from Toray Industries, Inc. (Kanagawa, Japan). (+)-Pentazocine was dissolved in a vehicle solution of 90% sterile saline (0.9% NaCl), 5% dimethylsulfoxide (DMSO), and 5% cremophore EL (Sigma), whereas all other drugs were dissolved in saline. Naloxone and naltrindole were injected intraperitoneally (i.p.) and subcutaneously (s.c), respectively, 30 min before injection of the peptide. (+)-Pentazocine was injected i.t. 10 min before the injection of Phe-Phe amide. β-Funaltrexamine and nor-binaltorphimine were injected s.c. 24 h before injection of the peptide. I.t. pretreatment with BD1047 was performed 10 min before the administration of (+)-pentazocine. The doses and routes for the administration of each antagonist were according to previous reports [5,46,47].
Statistical analysis
Data are presented as the mean ± S.E.M. Differences between treatment groups were evaluated using one-way or two-way analysis of variance (ANOVA) followed by the Bonferroni-Dunn test. At all times, a level of probability of 0.05 or less (P < 0.05) was considered significant.
List of abbreviations
Phe-Phe amide: Phenylalanine-phenylalanine amide; SP: substance P; EM-2: endomorphin-2; ANOVA: analysis of variance; i.p: intraperitoneal; i.t.: intrathecal; BD1047: N'-[2-(3,4-dichlorophenyl)ethyl]-N, N, N'-trimethylethane-1,2-diamine; ERK: extracellular signal-regulated protein kinase; NMDA: N-methyl D-aspartate; IP3: inositol 1,4,5-trisphosphate; NK: neurokinin
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MO carried out the behavioral and molecular studies, performed the statistical analysis, and participated in drafting the manuscript. AC, TK and YN carried out the behavioral studies. MA carried out the western blot study. RF and AS prepared Phe-Phe amide. MH edited the manuscript. FN supervised the project, coordinated the studies, and edited the manuscript. JK conceived of the project, designed and coordinated the studies, and drafted and edited the manuscript. All authors contributed to data interpretation, and have read and approved the final manuscript.
Contributor Information
Masahiro Ohsawa, Email: ohsawa@phar.nagoya-cu.ac.jp.
Anna Carlsson, Email: anna.carlsson@farmbio.uu.se.
Megumi Asato, Email: m-asato@hoshi.ac.jp.
Takayuki Koizumi, Email: painpainpainpain079@gmail.com.
Yuki Nakanishi, Email: s062520@hoshi.ac.jp.
Rebecca Fransson, Email: rebecca.fransson@orgfarm.uu.se.
Anja Sandström, Email: Anja.Sandstrom@orgfarm.uu.se.
Mathias Hallberg, Email: Mathias.Hallberg@farmbio.uu.se.
Fred Nyberg, Email: fred.nyberg@farmbio.uu.se.
Junzo Kamei, Email: kamei@hoshi.ac.jp.
Acknowledgements
This study was supported by the Swedish Medical Research Council (Grant 9459) and Uppsala Berzelii Technology Centre for Neurodiagnostics.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999;12:553–563. doi: 10.1097/00019052-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Dyck PJ, Larson TS, O'Brien PC, Velosa JA. Patterns of quantitative sensation testing of hypoesthesia and hyperalgesia are predictive of diabetic polyneuropathy: a study of three cohorts. Nerve growth factor study group. Diabetes Care. 2000;23:510–517. doi: 10.2337/diacare.23.4.510. [DOI] [PubMed] [Google Scholar]
- Kingery WS. A critical review of controlled clinical trrials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1990;73:123–139. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Ohsawa M, Hallberg M, Nyberg F, Kamei J. Substance P(1-7) induces antihyperalgesia in diabetic mice through a mechanism involving the naloxone-sensitive sigma receptors. Eur J Pharmacol. 2010;626:250–255. doi: 10.1016/j.ejphar.2009.10.006. [DOI] [PubMed] [Google Scholar]
- von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J Int Med. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- Hall ME, Stewart JM. Substance P and Behavior: Opposite effects of N-terminal and C-terminal fragments. Peptides. 1983;4:763–768. doi: 10.1016/0196-9781(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Hallberg M, Nyberg F. Neuropeptide conversion to bioactive fragments - an important Pathway in neuromodulation. Curr Prot Pept Sci. 2003;4:31–44. doi: 10.2174/1389203033380313. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Le Grevès P, Stewart J, Terenius L. Measurement of Substance P metabolites in rat CNS. J Neurochem. 1985;44:718–722. doi: 10.1111/j.1471-4159.1985.tb12874.x. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Hall ME, Harkins J, Frederickson RCA, Terenius L, Hökfelt T, Krivoy WA. A fragment of substance P with specific activity: SP(1-7) Peptides. 1982;3:851–857. doi: 10.1016/0196-9781(82)90027-4. [DOI] [PubMed] [Google Scholar]
- Wiktelius D, Khalil Z, Nyberg F. Modulation of peripheral inflammation by the substance P N-terminal metabolite substance P1-7. Peptides. 2006;27:1490–1497. doi: 10.1016/j.peptides.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kreeger JS, Larson AA. The Substance P amino-terminal metabolite Substance P(1-7), administered peripherally, prevents the development of acute morphine tolerance and attenuates the expression of withdrawal in mice. J Pharmacol Exp Ther. 1996;279:662–667. [PubMed] [Google Scholar]
- Zhou Q, Nyberg F. Injection of substance P (SP) N-terminal fragment SP(1-7) into the ventral tegmental area modulates the levels of nucleus accumbens dopamine and dihydroxyphenylacetic acid in male rats during morphine withdrawal. Neurosci Lett. 2002;320:117–120. doi: 10.1016/S0304-3940(01)02564-2. [DOI] [PubMed] [Google Scholar]
- Botros M, Hallberg M, Johansson T, Zhou Q, Lindeberg G, Frändberg PA, Tömböly C, Tóth G, Le Grevès P, Nyberg F. Endomorphin-1 and endomorphin-2 differentially interact with specific binding sites for substance P (SP) aminoterminal SP1-7 in the rat spinal cord. Peptides. 2006;27:753–759. doi: 10.1016/j.peptides.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Igwe OJ, Kim DC, Seybold VS, Larson AA. Specific binding of substance P aminoterminal heptapeptide [SP(1-7)] to mouse brain and spinal cord membranes. J Neurosci. 1990;10:3653–3663. doi: 10.1523/JNEUROSCI.10-11-03653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros M, Johansson T, Zhou Q, Lindeberg G, Tömböly C, Tóth G, Le Grevès P, Nyberg F, Hallberg M. Endomorphins interact with the substance P (SP) aminoterminal SP1-7 binding in the ventral tegmental area of the rat brain. Peptides. 2008;29:1820–1824. doi: 10.1016/j.peptides.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Carlsson A, Asato M, Koizumi T, Nakanishi Y, Fransson R, Sandström A, Hallberg M, Nyberg F, Kamei J. The effect of substance P1-7 amide on nociceptive threshold in diabetic mice. Peptides. 2011;32:93–98. doi: 10.1016/j.peptides.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Carlsson A, Botros M, Fransson R, Sandström A, Gordh T, Hallberg M, Nyberg F. The C-terminal amidated analogue of the substance P (SP) fragment SP(1-7) attenuates the expression of naloxone-precipitated withdrawal in morphine dependent rats. Peptides. 2009;30:2418–2422. doi: 10.1016/j.peptides.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Hornfeldt CS, Kitto KF, Larson AA. Evidence that the NH2-terminus of substance P modulates N-methyl-D-aspartate-induced activity by an action involving σ receptors. Eur J Pharmacol. 1996;306:15–22. doi: 10.1016/0014-2999(96)00199-9. [DOI] [PubMed] [Google Scholar]
- Larson AA, Sun X. Modulation of kainic acid-induced activity in the mouse spinal cord by the amino terminus of substance P: sensitivity to opioid antagonists. J Pharmacol Exp Ther. 1993;265:159–165. [PubMed] [Google Scholar]
- de la Puente B, Nadal X, Portillo-Salido E, Sánchez-Arroyos R, Ovalle S, Palacios G, Muro A, Romero L, Entrena JM, Baeyens JM, López-García JA, Maldonado R, Zamanillo D, Vela JM. Sigma-1 receptors regulate activity-induced spinal sensitization and neuropathic pain after peripheral nerve injury. Pain. 2009;145:294–303. doi: 10.1016/j.pain.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Fransson R, Botros M, Nyberg F, Lindeberg G, Sandström A, Hallberg M. Small peptides mimicking substance P (1-7) and encompassing a C-terminal amide functionality. Neuropeptides. 2008;42:31–37. doi: 10.1016/j.npep.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Fransson R, Botros M, Sköld C, Nyberg F, Lindeberg G, Hallberg M, Sandström A. Discovery of dipeptides with high affinity to the specific binding site for substance P1-7. J Med Chem. 2010;53:2383–2389. doi: 10.1021/jm901352b. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao LI, Su TP. Naloxone-sensitive, haloperidol-sensitive, [3H](+)SKF-10047-binding protein partially purified from rat liver and rat brain membranes: an opioid/sigma receptor? Synapse. 1997;25:117–124. doi: 10.1002/(SICI)1098-2396(199702)25:2<117::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Mechanisms of disease: neuropathic pain-a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- Meller ST, Dykstra CL, Gebhart GF. Investigations of the possible role for carbon monoxide (CO) in thermal and mechanical hyperalgesia in the rat. Neuroreport. 1994;5:2337–2341. doi: 10.1097/00001756-199411000-00032. [DOI] [PubMed] [Google Scholar]
- Nieto FR, Entrena JM, Cendán CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137:520–531. doi: 10.1016/j.pain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-C. [DOI] [PubMed] [Google Scholar]
- Ola MS, Moore P, Maddox D, El-Sherbeny A, Huang W, Roon P, Agarwal N, Ganapathy V, Smith SB. Analysis of sigma receptor (sigmaR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res. 2002;107:97–107. doi: 10.1016/s0169-328x(02)00444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon K, Kassiou M, Donald A. Effects of streptozotocin-induced diabetes on neuronal sigma receptors in the rat brain. Life Sci. 1999;65:PL281–PL286. doi: 10.1016/S0024-3205(99)00521-4. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kagaya A, Takebayashi M, Shimizu M, Uchitomi Y, Motohashi N, Yamawaki S. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-D-aspartate in primary culture of rat frontal cortical neurons. J Pharmacol Exp Ther. 1995;275:207–214. [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, De Montigny C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-A. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- D'amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Ohsawa M, Kamei J. Possible involvement of spinal protein kinase C in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 1999;372:221–228. doi: 10.1016/S0014-2999(99)00228-9. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Misawa M, Nagase H, Kasuya Y. Antinociceptive effect of L-arginine in diabetic mice. Eur J Pharmacol. 1994;254:113–117. doi: 10.1016/0014-2999(94)90377-8. [DOI] [PubMed] [Google Scholar]
- Wu HE, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (-)-morphine in attenuating the (-)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharmacol. 2007;571:145–151. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]