SUMMARY
Motility of the sleeping sickness parasite, Trypanosoma brucei, impacts disease transmission and pathogenesis. Trypanosome motility is driven by a flagellum that harbors a canonical 9 + 2 axoneme, together with trypanosome-specific elaborations. Trypanosome flagellum biology and motility have been the object of intense research over the last two years. These studies have led to the discovery of a novel form of motility, termed social motility, and provided revision of long-standing models for cell propulsion. Recent work has also uncovered novel structural features and motor proteins associated with the flagellar apparatus and has identified candidate signaling molecules that are predicted to regulate flagellar motility. Together with earlier inventories of flagellar proteins from proteomic and genomic studies, the stage is now set to move forward with functional studies to elucidate molecular mechanisms and investigate parasite motility in the context of host-parasite interactions.
Keywords: Flagellum, Cilium, Motility, Axoneme, Trypanosome, Social Behavior
INTRODUCTION
The African trypanosome1, Trypansoma brucei, causes human African trypanosomiasis and related trypanosomiases in wild and domestic animals. These protozoan parasites are responsible for significant human mortality and present a barrier to sustained economic development in sub-Saharan Africa. Motility of T. brucei is central to parasite development and disease pathogenesis. In the tsetse fly vector, the parasite must complete an ordered series of directional migrations to complete developmental transformation into mammalian infectious forms in the salivary gland [1, 2]. In the mammalian host, trypanosome penetration of the blood brain barrier represents a critical and defining step of disease pathogenesis. African trypanosomes are extracellular at all stages of infection and rely on their own flagellum for motility. As such, understanding mechanisms of flagellum-dependent cell motility in these pathogens presents opportunities for fundamental discoveries in microbial cell propulsion and has the potential to identify targets for therapeutic intervention in African trypanosomiasis. The current review covers recent advances in understanding flagellum-mediated motility in T. brucei. The period of the review spans from January 2008 to January 2010. Several comprehensive reviews of the trypanosome flagellum and flagellum motility have been completed recently [3-5] and the reader is directed to these resources for details not covered in the current review, which concentrates on only the most recent developments. Likewise, the focus here is on motility, while other important and exciting aspects of trypanosome flagellum biology are covered elsewhere in the current volume.
Tryps rock
The classical view of trypanosome cell motility has remained largely unchanged since the genus, Trypanosoma, or “auger body” was described nearly 160 years ago [6]. The basic view has been that the parasite moves in an auger-like fashion, with a twisted cell body rotating around its long axis as it moves forward, flagellum tip leading [3, 5]. Trypanosome motility has generally been considered to be driven by a flagellum that wraps around the cell body in a left-handed helix, thereby causing the entire cell to rotate as the flagellum beats (Figure 1a) [3, 5]. Recently, Rodriguez and colleagues provided a revision of this model based on high-speed (1000 frames/sec) video microscopy analysis [7]. Their studies revealed that, rather than continuously rotating, the T. brucei cell body rocks back and forth about its long axis as it moves forward. This rocking seems at first paradoxical, since reciprocal motion at low Reynold’s numbers, the scale that describes microbial cell movement, would not drive cell propulsion [8]. Rather, the parasite would simply move forward and backward as it switched between counter-clockwise and clockwise rotations. Remarkably, trypanosomes overcome this paradox by changing the handedness of the helical flagellum simultaneously with changing the direction of helix rotation (Figure 1). Therefore, cell propulsion in T. brucei is driven by a bihelical waveform, in which helical waves of alternating handedness propagate along the flagellum and are separated by a distinctive topological feature called a “kink” (Figures 1, S1) [7]. A consequence is that the anterior end of the cell is rotating in one direction, while the posterior end is rotating in the other direction (Figure 1b).
Figure 1. Tryps Rock.
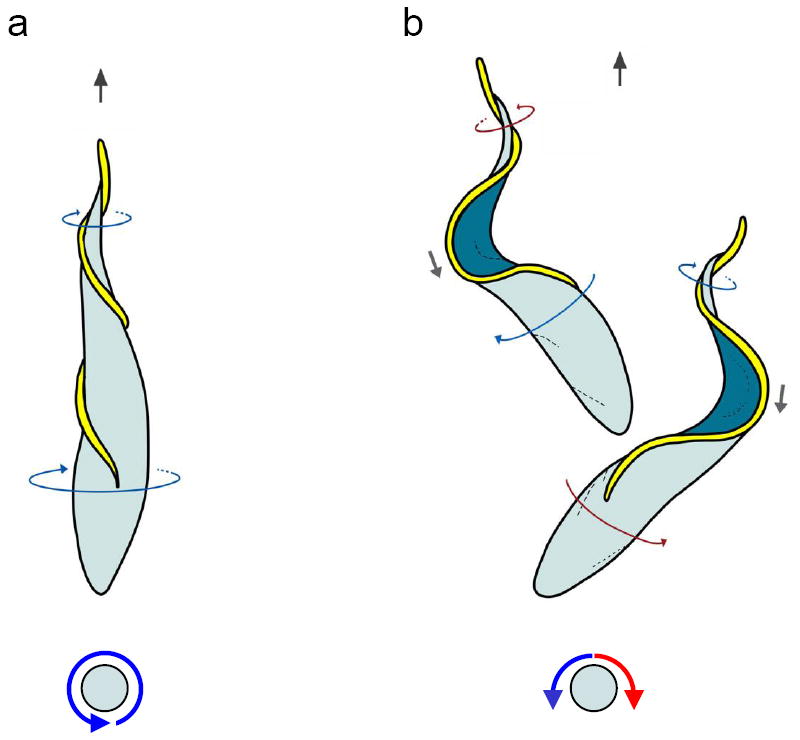
Cartoons of T. brucei cells show the classical (a) and bihelical (b) model for T. brucei motility. Direction of cell propulsion is with the flagellum tip leading (black arrows, top). Top panels show view from above the cell and bottom panels show the end-on view, looking at the cell posterior. In panel (a), classical model, a left-handed helical flagellum (yellow) drives rotation of the cell body counterclockwise, looking from the cell posterior toward anterior (blue arrows). In panel (b), bihelical model, the flagellum (yellow) alternately generates right-handed (red arrows) and left-handed (blue arrows) helical waves that propagate from tip to base and drive cell motion with the flagellum tip leading. Between helical segments of opposite handedness the flagellum forms a kink (gray arrows) that propagates in the direction opposite cell movement. The direction of helix rotation changes simultaneously with the change in helix handedness, causing the cell to rock back and forth along its long axis (red and blue arrows), while continuing to move forward. A helix is a chiral form, meaning that a mirror-image of the form cannot be superimposed on the original. Your hand is an example of a chiral object. The handedness of a helix can be determined by wrapping one’s hand around the helix with the thumb aligned along the long axis, then tracing the helix with your finger tips in the direction the thumb is pointing (Supplemental Figure 1). For a given helix, this is possible only with the right or left hand, not both, thus defining the “handedness”. In the examples shown, thin blue arrows indicate flagellum sections with left-handed chirality and thin red arrows indicate flagellum sections having a right-handed chirality. Adapted from [7], with permission.
Bihelical motility is also used by Spiroplasma, a cell wall-less bacterial pathogen, where it is proposed that recoil against the fluid carried backward by traveling kinks provides propulsive force [9, 10], but bihelical motility has not previously been described in eukaryotes. What is the advantage of this form of motility for T. brucei? Rodriguez and colleagues point out that a bihelical mechanism allows the cell to generate propulsive force by rotating in either direction and allows the leading end of the cell to maintain a faster rotation rate than would be allowed for a rotating helix of uniform handedness. These features could facilitate tissue penetration and migration through viscous, inhomogeneous environments encountered in the insect and mammalian hosts. The authors also suggest the intriguing possibility that build up and release of torsion in the cell body may provide a form of elastic energy to aid in cell propulsion. These studies offer insights into hydrodynamics of microbial cell propulsion and provide a quantitative and theoretical foundation for analysis of trypanosome flagellum beating, which will aid efforts to exploit flagellum motility as a drug target.
Structures, molecules and mechanisms
As evidenced by the studies of Rodriguez and coworkers [7] (see above), trypanosome flagellum motility exhibits several distinctive features. It is important to identify the structures, proteins and molecular mechanisms that underlie flagellar motility, not only as a matter of academic curiosity and understanding microbial cell propulsion, but also as a means to identify trypanosome proteins that might be exploited for therapeutic intervention. Previous genomic and proteomic studies have provided a solid inventory of flagellar proteins [11-13] and it is now important to define key regulatory proteins and trypanosome-specific functions. One of the most striking and distinctive features of the trypanosome flagellum is the paraflagellar rod, PFR [14].
The PFR is a paracrystaline filament that runs parallel to the axoneme and is essential for normal motility [15, 16]. Aside from two major PFR proteins, PFR1 and PFR2, the molecular composition of the PFR has remained largely unknown since it was described nearly fifty years ago [17]. Portman and colleagues [18] have now made major inroads to defining PFR protein composition. The authors used a combination of RNAi and quantitative mass spectrometry to identify a PFR-component, “PFC”, proteome. The authors identified thirty proteins missing in flagella from PFR-deficient cells and went on to substantiate a PFR location/function for all seven PFC proteins analyzed directly using RNAi and epitope tagging. Of particular note, the PFC dataset is enriched for proteins with calcium signaling signatures, suggesting a major role for the PFR in Ca-mediated signaling. The work supports earlier findings that the PFR harbors cAMP and Ca-signaling proteins [19, 20], thus strengthening the emerging idea that the PFR provides a platform for signaling and regulatory functions, rather than just imposing a structural influence on axoneme motility [21]. The work of Portman and colleagues suggests a place to focus efforts at elucidating the signaling capacity of the PFR.
Another recent proteomic study also provides insight into potential regulatory activities that may control T. brucei flagellar motility. Although flagellar motility requires coordinate regulation of thousands of dynein motors, the underlying mechanisms and proteins are largely unknown. Nett and colleagues [22] used phospho-tyrosine antibody affinity purification coupled with mass spectrometry to identify T. brucei tyrosine-phosphorylated proteins. The resulting proteomic dataset was highly enriched for signaling proteins, such as MAP kinases, and phospho-mapping indicated that many of these kinases were phosphorylated at residues that are key for kinase activation. Intriguingly, the authors found that immunofluorescence labeling with the anti-phosphotyrosine antibodies used for the proteomic study prominently labeled flagellum structures, including the basal body, axoneme and flagellum attachment zone. The phosphoproteins and kinases identified by Nett and colleagues therefore present an excellent starting point for elucidating regulatory mechanisms that control T. brucei flagellum motility.
In addition to the above proteomic studies pointing to potential regulatory proteins, a recent study by Demonchy and colleagues demonstrated a requirement for two kinesin motor proteins, KIF9A and KIF9B, in T. brucei flagellum motility [23]. Both proteins were found to be localized to the flagellum, but knockdown studies revealed distinct functions. The motility defect of KIF9B knockdowns likely reflects the requirement for this protein in PFR assembly [23]. Loss of KIF9A on the other hand caused motility defects without gross ultrastructural changes visible in transmission electron microscopy thin sections. KIF9A is most closely related to the KLP1 protein of Chlamydomonas reinhardtii. KLP1 functions in the central pair apparatus to control flagellar motility [24] and the KIF9A knockdown data support a conserved role for this kinesin motor in T. brucei motility. In addition to identifying motors required for flagellar assembly and motility, the work by Demonchy and colleagues illustrates the utility of T. brucei for investigating functional diversity within flagellar protein families.
Is motility required in vivo?
T. brucei is an extracellular parasite and it is therefore reasonable to expect that trypanosome motility is required for parasite development and disease pathogenesis in vivo. However, this hypothesis has not yet been tested empirically and this is a deficit that needs to be remedied. Reliance on RNAi for assessing protein function and the potential for emergence of RNAi resistance represent technical limitations. However, since gene knockouts are readily achieved in T. brucei, limitations of RNAi should not present a barrier to in vivo studies, particularly for the tsetse fly life cycle stage, where motility-deficient cells have been described with little or no growth deficit. The situation is different for mammalian-infectious forms, since RNAi knockdown of flagellum proteins most often results in a rapid and catastrophic cytokinesis defect and no viable motility mutants have been identified [4, 12, 25, 26]. Qualitatively similar lethal phenotypes are observed following knockdown of several independent flagellum proteins in bloodstream-form cells, leading to speculation that flagellum motility is essential in this life cycle stage [12, 25, 26]. However, recent reviews of the existing data point out caveats to this interpretation [4], including the presence of structural defects in the flagellum of many knockdowns and a lack of correlation between severity of beat defect and the lethal phenotype. As such, a direct connection between flagellum motility defects and a lethal phenotype has not been established and further effort needs to be applied to this question. Toward this end, there is a critical need for more sophisticated approaches to examine flagellum protein function, because RNAi knockdown ablates protein expression and often compromises structures associated with the target protein, thereby clouding phenotype interpretation. This problem is particularly relevant for proteins in the flagellum, which is comprised of thousands of interconnected protein complexes [27]. Recent development of systems to replace endogenous proteins with non-functional mutants, thereby minimizing gross structural consequences [28, 29] should allow resolution of these problems.
What happens to parasite motility when exposed to surfaces?
Current studies of trypanosome motility focus almost exclusively on parasites in suspension cultures. However, as is the case for most pathogens, trypanosomes in their natural environment are intimately associated with host tissue surfaces [30]. A large body of literature demonstrates that exposure to surfaces has a major impact on motility and behavior of bacterial pathogens [31-34]. Oberholzer and colleagues recently discovered that exposure of procyclic-form T. brucei to semisolid agarose surfaces induces a novel form of motility, termed social motility, in which the parasites assemble into multicellular communities with emergent properties that are not evident in single cells (Figure 2) [35]. Parasites in these groups undergo polarized migrations and cooperate to divert their movements in response to external signals. T. brucei social motility requires flagellum motility and resembles social motility and other surface-induced behaviors in bacterial pathogens. These findings reveal a level of complexity and cooperativity to trypanosome behavior that was not previously appreciated and therefore offer new paradigms for considering host-parasite interactions. The authors point out that social motility also provides a defined in vitro assay for extracellular sensing and might offer mechanisms for navigating through host tissues. In bacteria, social motility provides several advantages, such as enhanced tissue colonization, genetic exchange and increased resistance to host defense mechanisms [31-34]. The key next step will be to identify molecules and genes required for social motility in T. brucei, so that the role of this behavior can be tested in vivo.
Figure 2. Social motility reveals that trypanosomes sense, communicate and cooperate.
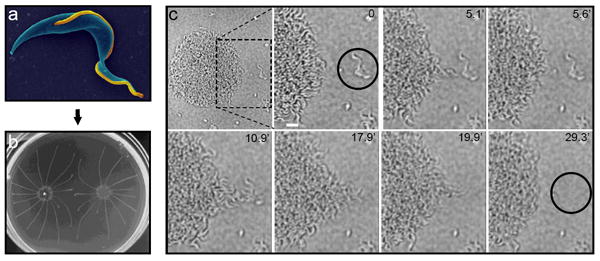
Panel (a) shows an electron micrograph of a procyclic T. brucei cell from suspension culture, pseudo-colored with the flagellum in gold. Panel (b) shows social motility colonies six days post-inoculation on semisolid agarose plates, with characteristic radial projections that migrate outward and avoid other parasites. Panel (c) shows a time-lapse series of the early stages of social motility. Parasites at the colony perimeter, termed “scouts” migrate out and back from the colony. When scouts identify external parasites (black circle in second panel), they return to the colony and direct coordinate movement of cells in the colony outward at this position to recruit the external parasites into the colony. Scale bar is 20 um. Timestamps are indicated in each panel. See also Supplemental Movie 1. Panels a and c are adapted from [5] and [35], respectively, with permission.
Efforts to understand host influences on parasite motility and biology will benefit from in vivo imaging and recent work on this front shows promise. Technical advances with in vivo imaging are revealing novel and important aspects of parasite-host interactions in other systems [36]. Building on their earlier work tracking fluorescent parasite movements in tsetse flies [37], Gibson and colleagues [38, 39] have now used in vivo imaging of fluorescent trypanosomes in tsetse flies to provide new understandings of genetic exchange in the insect vector. The work provides striking views of individual parasites in the fly and holds great promise for elucidating details of parasite motility and host-parasite interactions in the insect host. By applying bioluminescent imaging to study T. brucei infection in live mice, Claes and colleagues [40] uncovered an unexpected testis tropism. The sensitivity and resolution of the bioluminescent system that was employed need to be improved, but the work represents an important step toward imaging trypanosomes in live mammalian hosts. An ultimate, and lofty, goal will be to determine when, where and how trypanosomes exit the bloodstream and penetrate the blood brain barrier.
Non-motility functions for flagellum motility
The trypanosome flagellum emerges from the flagellar pocket near the posterior end of the cell. The flagellar pocket is the sole site for endocytosis and secretion in T. brucei, thereby making it a critical interface between parasite and host. Access to the flagellar pocket is restricted by tight apposition of the flagellar and plasma membranes (Figure 3) and motility of the flagellum has long been considered to influence access to the flagellar pocket. Previous work provided strong evidence in support of this hypothesis [41] and two papers within the current review period offer insight into this non-motility function of flagellum motility [42, 43]. Hanrahan and colleagues [43] use antibody and fluorescent protein tagging to demonstrate that GPI-specific phospholipase C, GPI-PLC, is localized on the cell exterior, within the flagellum attachment zone, FAZ, that interfaces between the flagellum and cell body. GPI-PLC participates in clearance of VSG from the trypanosome surface and is therefore considered important for immune evasion, as well as differentiation of bloodstream-form to procyclic form cells [44]. The restricted localization of GPI-PLC to the FAZ is consistent with the postulated role of flagellum beating in facilitating VSG clearance [41]. In separate work, Gadelha and colleagues [42] used electron tomography combined with freeze-fracture and transmission electron microscopy to determine the three-dimensional architecture of the flagellar pocket and associated membrane structures. Their studies identified a channel linking the flagellar pocket lumen and the extracellular environment that is postulated to provide a conduit for transport of endocytosed material. This channel lies precisely between the flagellum membrane and plasma membrane and corresponds to the site of accumulation of endocytic markers en-route to the flagellar pocket. It is hard to imagine flagellum beating not influencing the form or function of this endocytic channel, though direct tests of this idea remain to be done. In addition to identifying novel flagellum-associated structures, these studies illustrate the utility of combining existing and emerging structural imaging modalities for providing mechanistic insight into trypanosome biology.
Figure 3. The trypanosome flagellar pocket.
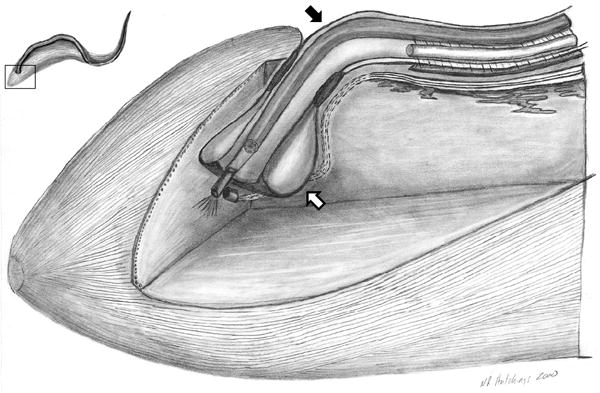
Cut-away, three-dimensional view of the posterior region of the T. brucei cell, illustrating the flagellar pocket (white arrow) and flagellum (black arrow). The flagellar pocket forms from an invagination of the cell surface membrane at the position where the flagellum (black arrow) emerges from the cytoplasm. The flagellum and flagellar pocket are intimately connected and flagellum motility is hypothesized to influence traffic into and out of the flagellar pocket [41]. The inset, upper left, shows a schematic of the T. brucei cell, with a boxed region to indicate the position of the cutaway view. Adapted from [45], with permission.
CONCLUSION
Recent advances in field of trypanosome flagellum motility have provided insight into mechanisms of cell motility, revealed novel forms of motility and identified candidate regulatory proteins. It will now be important to apply functional approaches to elucidate molecular mechanisms responsible for conserved and unique aspects of motility in these pathogens. Another critical need for future studies is to determine the role of parasite motility in vivo, including determining whether normal motility is actually essential in the bloodstream life cycle stage. A third area of focus should be determining how motility is modulated in response to cues provided by host tissues and how the parasite uses these cues for navigation and developmental differentiation. Advances in approaches for in vivo imaging and flagellum membrane biology will facilitate these efforts. Finally, application of electron tomography and other ultrastructural imaging approaches will undoubtedly provide insight into the structural mechanics of flagellum motility.
Supplementary Material
Video corresponds to the time-lapse images in Figure 2c and shows high magnification of a trypanosome community recruiting neighboring cells. The elapsed time is 29.3 minutes. Parasites at the colony perimeter, termed “scouts” migrate out and back from the colony. When scouts identify external parasites, they return to the colony and direct coordinate movement of cells in the colony outward at this position to recruit the external parasites into the colony. Adapted from [35], with permission.
Panel (a) is a cartoon depiction of a trypanosome cell showing the bihelical cell propulsion mechanism [7], with graphical aids to illustrate helix handedness. Panel (b) shows a kinked telephone cable as a commonly observed bihelical object to illustrate a kink between helices of alternate handedness. In panel (a) the top images show the view from above the cell and the bottom panel shows the end-on view looking at the cell posterior. Direction of cell propulsion is toward the top of the figure (small black arrow). The flagellum (yellow) alternates between right-handed (red arrows) and left-handed (blue arrows) helical waves that propagate from tip to base and drive cell motion with the flagellum tip leading. Between helical segments of opposite handedness the flagellum forms a kink that propagates in the direction opposite cell movement (gray arrows). The direction of helix rotation changes simultaneously with change in helix handedness, causing the cell to rock back and forth along its long axis (red and blue arrows), while continuing to move forward. A helix is a chiral form, meaning that a mirror-image of the form cannot be superimposed on the original. Your hand is an example of a chiral object. The handedness of a helix can be determined by wrapping one’s hand around the helix with the thumb aligned along the long axis, then tracing the helix with your finger tips in the direction the thumb is pointing. For a given helix, this is possible only with the right or left hand, not both, thus defining the “handedness”. In the examples shown, thin blue arrows indicate flagellum sections with left-handed chirality and thin red arrows indicate flagellum sections having a right-handed chirality. Modified from [7], with permission.
Box 1. Main topics discussed in this review.
Mechanism of cell propulsion and potential regulators of flagellar motility.
-
Trypanosome motility in the host.
What is the role of parasite motility in vivo?
How is parasite motility influenced by host tissues?
Non-motility functions for flagellum motility.
Acknowledgments
KH is a Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease. Thanks to Michael Oberholzer and Michelle Shimogawa for careful reading of the manuscript and to colleagues and members in my lab for helpful discussions. Work in the author’s laboratory is supported by grants from the National Institutes of Health and the Burroughs Wellcome Fund.
Footnotes
The term trypanosome is used to refer specifically to Trypanosoma brucei throughout the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Den Abbeele J, et al. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118(Pt 5):469–78. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- 2.Vickerman K, et al. Biology of African trypanosomes in the tsetse fly. Biol Cell. 1988;64(2):109–19. doi: 10.1016/0248-4900(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 3.Ginger ML, Portman N, McKean PG. Swimming with protists: perception, motility and flagellum assembly. Nat Rev Microbiol. 2008;6(11):838–50. doi: 10.1038/nrmicro2009. [DOI] [PubMed] [Google Scholar]
- 4.Ralston KS, Hill KL. The flagellum of Trypanosoma brucei: new tricks from an old dog. Int J Parasitol. 2008;38(8-9):869–84. doi: 10.1016/j.ijpara.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralston KS, et al. The Trypanosoma brucei flagellum: moving parasites in new directions. Annual Review of Microbiology. 2009;63:335–62. doi: 10.1146/annurev.micro.091208.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruby M. Recherches et observations sur une nouvelle espèce d’hématozoaire, Trypanosoma sanguinis. Comptes rendus hebdomadaire des séances de l’Académie des Sciences, Paris. 1843;17:1134–1136. [Google Scholar]
- 7.Rodriguez JA, et al. Propulsion of African trypanosomes is driven by bihelical waves with alternating chirality separated by kinks. Proc Natl Acad Sci U S A. 2009;106(46):19322–7. doi: 10.1073/pnas.0907001106. The authors use high-speed video microscopy to demonstrate that T. brucei motility is driven by a bihelical mechanism. These studies offer insights into hydrodynamics of microbial cell propulsion and provide a quantitative and theoretical foundation for analysis of trypanosome flagellum beating and efforts to exploit flagellum motility as a drug target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell EM. Life at low Reynolds number. Am J Phys. 1977;45:3–11. [Google Scholar]
- 9.Shaevitz JW, Lee JY, Fletcher DA. Spiroplasma swim by a processive change in body helicity. Cell. 2005;122(6):941–5. doi: 10.1016/j.cell.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Wolgelmuth CW, Huber G. Kinematics of the swiming of spiroplasma. Phys Rev Lett. 2009;102(21):218102–218105. doi: 10.1103/PhysRevLett.102.218102. [DOI] [PubMed] [Google Scholar]
- 11.Baron DM, et al. Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J Cell Sci. 2007;120(Pt 3):478–91. doi: 10.1242/jcs.03352. [DOI] [PubMed] [Google Scholar]
- 12.Broadhead R, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440(7081):224–7. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 13.Pazour GJ, et al. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170(1):103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cachon J, et al. The paraflagellar rod: a structure in search of a function. Biol Cell. 1988;63:169–181. [Google Scholar]
- 15.Bastin P, Sherwin T, Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391(6667):548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- 16.Santrich C, et al. A motility function for the paraflagellar rod of Leishmania parasites revealed by PFR-2 gene knockouts. Mol Biochem Parasitol. 1997;90(1):95–109. doi: 10.1016/s0166-6851(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 17.Vickerman K. The mechanism of cyclical development in trypanosomes of the Trypanosoma brucei sub-group: an hypothesis based on ultrastructural observations. Trans R Soc Trop Med Hyg. 1962;56:487–95. doi: 10.1016/0035-9203(62)90072-x. [DOI] [PubMed] [Google Scholar]
- 18.Portman N, et al. Combining RNA interference mutants and comparative proteomics to identify protein components and dependences in a eukaryotic flagellum. Journal of Biological Chemistry. 2009;284(9):5610–9. doi: 10.1074/jbc.M808859200. The authors combine RNAi and quantitative mass spectrometry to identify a proteome of predicted PFR candidate proteins, “PFC”. Independent tests validate several PFC proteins as bona fide PFR proteins. The studies provide a systematic analysis of PFR molecular composition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberholzer M, et al. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. Faseb J. 2007;21(3):720–31. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 20.Ridgley E, et al. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol Biochem Parasitol. 2000;109(2):195–201. doi: 10.1016/s0166-6851(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 21.Portman N, Gull K. The paraflagellar rod of kinetoplastid parasites: from structure to components and function. International Journal for Parasitology. 2010;40(2):135–48. doi: 10.1016/j.ijpara.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nett IR, et al. Identification and specific localization of tyrosine-phosphorylated proteins in Trypanosoma brucei. Eukaryot Cell. 2009;8(4):617–26. doi: 10.1128/EC.00366-08. The authors identify a dataset of phoshpotyrosine-containing proteins from T. brucei. The studies identify several proteins that are candidates for regulating flagellar motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demonchy R, et al. Kinesin 9 family members perform separate functions in the trypanosome flagellum. Journal of Cell Biology. 2009;187(5):615–22. doi: 10.1083/jcb.200903139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama R, et al. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci U S A. 2004;101(50):17398–403. doi: 10.1073/pnas.0406817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branche C, et al. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119(Pt 16):3443–55. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- 26.Ralston KS, Hill KL. Trypanin, a Component of the Flagellar Dynein Regulatory Complex, Is Essential in Bloodstream Form African Trypanosomes. PLoS Pathogens. 2006;2(9):873–882. e101. doi: 10.1371/journal.ppat.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heuser T, et al. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. Journal of Cell Biology. 2009;187(6):921–33. doi: 10.1083/jcb.200908067. The authors use cryoelectron tomography of flagella from Chlamydomonas reinhardtii to provide unprecedented three-dimensional structures of outer doublet microtubules and associated structures in the flagellar axoneme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aphasizheva I, Aphasizhev R. RET1-catalyzed uridylylation shapes the mitochondrial transcriptome in Trypanosoma brucei. Molecular and Cellular Biology. 2010;30(6):1555–67. doi: 10.1128/MCB.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberholzer M, et al. Approaches for functional analysis of flagellar proteins in African trypanosomes. Methods in Cell Biology. 2009;93:21–57. doi: 10.1016/S0091-679X(08)93002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roditi I, Lehane MJ. Interactions between trypanosomes and tsetse flies. Curr Opin Microbiol. 2008;11(4):345–51. doi: 10.1016/j.mib.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125(2):237–46. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–73. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 33.Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36(4):478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zusman DR, et al. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol. 2007;5(11):862–72. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 35.Oberholzer M, et al. Social motility in african trypanosomes. PLoS Pathog. 2010;6(1):e1000739. doi: 10.1371/journal.ppat.1000739. The authors discover surface-induced social motility in procyclic-form trypanosomes, whereby parasites assemble into multicellular communities that migrate en masse in response to external signals. These studies reveal that trypanosomes sense, communicate and cooperate, exposing a level of complexity and cooperativity to trypanosome behavior that was not previously appreciated and offering new paradigms for considering host-parasite interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amino R, et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3(2):88–96. doi: 10.1016/j.chom.2007.12.007. Using intravital imaging of Plasmodium berghei in mice, the authors show that traversal of host cells by parasites in the dermis is important for evading destruction by host defenses later during infection. These studies revealed novel aspects of host-parasite interaction and represent stat-of-the art in vivo imaging and quantitative analysis of host-parasite interactions. [DOI] [PubMed] [Google Scholar]
- 37.Gibson W, Bailey M. The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis. 2003;2(1):1. doi: 10.1186/1475-9292-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson W, et al. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors. 2008;1(1):4. doi: 10.1186/1756-3305-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock L, et al. Intraclonal mating occurs during tsetse transmission of Trypanosoma brucei. Parasit Vectors. 2009;2(1):43. doi: 10.1186/1756-3305-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claes F, et al. Bioluminescent imaging of Trypanosoma brucei shows preferential testis dissemination which may hamper drug efficacy in sleeping sickness. PLoS Negl Trop Dis. 2009;3(7):e486. doi: 10.1371/journal.pntd.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engstler M, et al. Hydrodynamic Flow-Mediated Protein Sorting on the Cell Surface of Trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 42.Gadelha C, et al. Membrane domains and flagellar pocket boundaries are influenced by the cytoskeleton in African trypanosomes. Proc Natl Acad Sci U S A. 2009;106(41):17425–30. doi: 10.1073/pnas.0909289106. The authors use electron tomography and freeze fracture electron microscopy to generate a three-dimensional model of the T. brucei flagellar pocket. They identify a novel channel that links the flagellar pocket to the extracellular environment and is hypothesized to function in endocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanrahan O, et al. The glycosylphosphatidylinositol-PLC in Trypanosoma brucei forms a linear array on the exterior of the flagellar membrane before and after activation. PLoS Pathog. 2009;5(6):e1000468. doi: 10.1371/journal.ppat.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandgenett PM, et al. A function for a specific zinc metalloprotease of African trypanosomes. PLoS Pathog. 2007;3(10):1432–45. doi: 10.1371/journal.ppat.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill KL, et al. T Lymphocyte triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275(50):39369–39378. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video corresponds to the time-lapse images in Figure 2c and shows high magnification of a trypanosome community recruiting neighboring cells. The elapsed time is 29.3 minutes. Parasites at the colony perimeter, termed “scouts” migrate out and back from the colony. When scouts identify external parasites, they return to the colony and direct coordinate movement of cells in the colony outward at this position to recruit the external parasites into the colony. Adapted from [35], with permission.
Panel (a) is a cartoon depiction of a trypanosome cell showing the bihelical cell propulsion mechanism [7], with graphical aids to illustrate helix handedness. Panel (b) shows a kinked telephone cable as a commonly observed bihelical object to illustrate a kink between helices of alternate handedness. In panel (a) the top images show the view from above the cell and the bottom panel shows the end-on view looking at the cell posterior. Direction of cell propulsion is toward the top of the figure (small black arrow). The flagellum (yellow) alternates between right-handed (red arrows) and left-handed (blue arrows) helical waves that propagate from tip to base and drive cell motion with the flagellum tip leading. Between helical segments of opposite handedness the flagellum forms a kink that propagates in the direction opposite cell movement (gray arrows). The direction of helix rotation changes simultaneously with change in helix handedness, causing the cell to rock back and forth along its long axis (red and blue arrows), while continuing to move forward. A helix is a chiral form, meaning that a mirror-image of the form cannot be superimposed on the original. Your hand is an example of a chiral object. The handedness of a helix can be determined by wrapping one’s hand around the helix with the thumb aligned along the long axis, then tracing the helix with your finger tips in the direction the thumb is pointing. For a given helix, this is possible only with the right or left hand, not both, thus defining the “handedness”. In the examples shown, thin blue arrows indicate flagellum sections with left-handed chirality and thin red arrows indicate flagellum sections having a right-handed chirality. Modified from [7], with permission.


