Abstract
Lennox–Gastaut syndrome (LGS) is a devastating childhood epilepsy syndrome characterized by the occurrence of multiple types of seizures and cognitive decline. Most children suffer from frequent seizures that are refractory to current medical management. Recent clinical trials have suggested that addition of clobazam may improve the clinical outcome for some LGS patients. Although clobazam has been available for over five decades, it has only recently been approved by the US Food and Drug Administration for this indication. As a 1,5-benzodiazepine, clobazam is structurally related to the widely used 1,4-benzodiazepines, which include diazepam. Clobazam has been shown to modulate GABAergic neurotransmission by positive allosteric modulation of GABAA receptors, and to increase expression of transporters for both GABA and glutamate. The active metabolite n-desmethylclobazam (norclobazam) also modulates GABAA receptors, and the relative importance of these two compounds in the clinical effectiveness of clobazam remains an open question. Clinical trials involving clobazam as an addon therapy in a variety of pediatric epilepsy populations have found a significant improvement in seizure control. In patients with LGS, clobazam may have greatest efficacy for drop seizures. Longstanding clinical experience suggests that clobazam is a safe and well tolerated antiepileptic drug with infrequent and mild adverse effects. These results suggest that adjunctive treatment with clobazam may be a reasonable option for LGS patients, particularly those who are treatment-resistant.
Keywords: benzodiazepine, epilepsy, gamma aminobutyric acid, pediatric, pharmacoresistance
Lennox–Gastaut syndrome
Lennox–Gastaut syndrome (LGS) is a catastrophic epileptic encephalopathy with a poor prognosis and limited treatment options. Although rare, LGS constitutes 3%–10% of childhood epilepsies, due to its intractable nature.1–5 Generally, LGS onset occurs before 8 years of age, with a peak at 3–5 years,5–7 and is more common in males.1,6,8 LGS is identified by its characteristic triad of symptoms, including multiple generalized seizure types, a slow spike and wave (≤2.5) pattern in the awake electroencephalogram, and cognitive decline. The types of seizures most commonly associated with LGS are tonic, atypical absence, myoclonic, and atonic seizures,5,6 but many LGS patients also experience generalized tonic-clonic and focal seizures.2,4,6,9,10 In addition to the slow spike and wave pattern, bursts of paroxysmal fast activity during sleep are also classically present on the electroencephalogram and may be associated with subtle tonic seizures.1,5,6 Up to 90% of patients with LGS have mental retardation and experience cognitive deterioration,2,3 and many children also develop behavioral and psychological problems, including aggression, hyperactivity, and characteristics of autism.3,5,11
LGS often results from an underlying neurological injury or disorder, such as hypoxic-ischemic encephalopathy, cerebral palsy, tuberous sclerosis complex, or cortical dysplasia, but approximately 30% of LGS cases are cryptogenic, having no clear cause.1,5–7 Diagnosis is difficult and may take years because in addition to the various etiologies of LGS, the syndrome lacks a uniform clinical presentation,6 and often patients do not have all of the diagnostic elements at the onset of epilepsy.5 LGS is resistant to treatment and often, in part due to the multiple seizure types, a combination of antiepileptic drugs is required.3,6–8,12 LGS is considered an epileptic encephalopathy, in which the degree of cognitive deterioration present is thought to be related to seizure frequency and burden of epileptic discharges.4,5,11
Current treatment options
A broad spectrum antiepileptic drug or combination of antiepileptic drugs is frequently necessary to treat the multiple seizure types associated with LGS.3,6–8,12 Valproate is often used as a first-line treatment for LGS by many physicians because it is effective for both generalized and focal seizures and is not known to worsen any seizure types associated with LGS.13,14 However, valproate is rarely effective as monotherapy3 and has not been approved by the US Food and Drug Administration for this purpose.3,6,12,15 Adverse events related to valproate use can be serious, including hepatic toxicity and pancreatitis, and there are many potential drug interactions.16
Felbamate, lamotrigine, topiramate, and rufinamide are all approved by the Food and Drug Administration as adjunctive treatments for LGS. Each of these antiepileptic drugs has been tested in randomized, double-blind, placebo-controlled clinical trials demonstrating their efficacy for seizures associated with LGS.3,7,12 There have been no studies comparing approved treatment options for LGS patients,15 and comparing results from different trials is complicated by variations in study populations, concurrent use of other antiepileptic drugs, and differences in the types of reported data.3,7 A Cochrane database review of treatment options for LGS included seven randomized, controlled studies that evaluated rufinamide, lamotrigine, cinromide, felbamate, thyrotropin-releasing hormone, and topiramate in children and adults with LGS.7 In each of these studies, the drug being evaluated was compared with placebo, with the exception of a study evaluating thyrotropin-releasing hormone, which compared low-dose and high-dose efficacy. In their review, Hancock and Cross7 concluded that an optimum treatment option could not be identified from these studies, but that lamotrigine, rufinamide, topiramate, and felbamate may be useful as adjunctive therapies.
Felbamate was the first antiepileptic drug approved for use as addon therapy for LGS.3 Since its approval in 1993, felbamate has been associated with aplastic anemia and hepatic failure, and due to these severe adverse events, its use has been limited to patients who have not responded to other antiepileptic drugs.17–19 Lamotrigine, approved as an adjunctive treatment for LGS in 1998, is a broad spectrum antiepileptic drug that is effective against multiple seizure types.20–22 The most common side effect of lamotrigine is a mild skin rash,21,23,24 but Stevens–Johnson syndrome and toxic epidermal necrolysis have occurred in rare cases.22,23 Drug interactions with lamotrigine are common, complicating its use in combination therapy.25 Topiramate, approved for use in LGS in 2001, lacks the risk of life-threatening adverse events, like those associated with lamotrigine and felbamate,3 but has been associated with cognitive impairment,26–28 although this can often be minimized by slow titration.3,10,15 Rufinamide, approved in 2011, may be particularly effective for drop seizures (due to either tonic or atonic events) in children with LGS.29 Rufinamide has been associated with somnolence and vomiting,29 which can be mitigated by slowed titration.30
If pharmacological treatment fails, other options include the ketogenic diet, vagus nerve stimulation, corpus callosotomy, and resective surgery.3,6,12 The ketogenic diet, ie, a high-fat, low-protein, and low-carbohydrate diet, has been shown to decrease drop seizure frequency in patients who do not respond to antiepileptic drugs, including patients with LGS.31–34 In studies of the ketogenic diet in the treatment of children with refractory epilepsy including LGS, the diet provided complete seizure control for more than 50% of patients.34 Common side effects include gastrointestinal symptoms, such as nausea, vomiting, and constipation, which may be improved by decreasing the nonlipid to lipid ratio. Compliance with the diet may also be difficult to maintain in patients with cognitive and behavioral problems.15 Although vagus nerve stimulation is not as effective in patients with LGS as it is in patients with partial epilepsy,35 it has been demonstrated to decrease seizure frequency with minimal adverse effects.35–37 Corpus collosotomy is used to decrease the spread of epileptic discharges between hemispheres and can be helpful for patients with intractable drop attacks.38 There may be a seizure focus in symptomatic cases of LGS such as those caused by tuberous sclerosis or cortical dysplasia, in which case resective surgery may be effective.12,39
Each of the approved antiepileptic drugs is effective for some patients, but many LGS patients continue to have seizures even with the use of multiple antiepileptic drugs, and combination therapy puts these patients at increased risk for experiencing side effects.1,7,8 It is clear that new options are necessary for these treatment-resistant patients. One such option is the use of clobazam, a 1,5-benzodiazepine, which may be particularly effective in pediatric populations.
Clobazam, a 1,5-benzodiazepine
Clobazam was initially proposed as an effective anticon-vulsant and anxiolytic with an improved side effect profile compared with the 1,4-benzodiazepines, which include diaz-epam and clonazepam (Figure 1).2,40,41 The original report42 found clobazam to be effective in several animal models of acute seizures, and it was first reported to have therapeutic activity in patients with a variety of seizure disorders by Gastaut and Low in 1979.43 Clobazam (marketed under the brand names Frisium®, Urbanyl®, Onfi, and Mystan®) is now available in many countries as adjunctive therapy for several types of seizures.
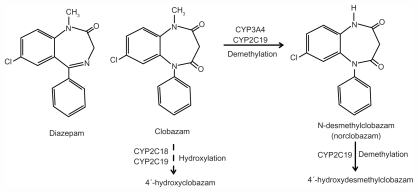
Figure 1.
Clobazam (7-chloro-1-methyl-5-phenyl-1,5-benzodiazepine-2,4-dione). Structures of diazepam, clobazam, and norclobazam and major metabolic pathways for clobazam and norclobazam.
Note: Clobazam is primarily metabolized by demethylation to n-desmethylclobazam (norclobazam). Dashed line indicates a minor pathway by hydroxylation. Norclobazam is hydroxylated to an inactive compound mainly through the activity of CYP2C19. Adapted with permission from Giraud C, Tran A, Rey E, Vincent J, Tréluyer JM, Pons G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004;32:1279–1286.44
Clobazam acts primarily through positive allosteric modulation of GABAA receptors, a mechanism of action shared by all clinically useful benzodiazepines. These ligandgated chloride channels are responsible for fast inhibitory neurotransmission throughout the central nervous system, and drugs that enhance their activity are often effective anxiolytics, sedatives, and anticonvulsants. While the benzodiazepines are widely considered to be safe and effective for the treatment of acute seizures, their clinical utility for long-term therapy is often limited by side effects and the development of tolerance.41
Metabolism of clobazam
The primary pathway for metabolism of clobazam is demethylation by cytochrome P450 (CYP)3A4 and CYP2C19 to its active metabolite n-desmethylclobazam (norclobazam, Figure 1).44 Clobazam can be hydroxylated to an inactive form, but this appears to be a minor pathway. CPY2C19 also acts on norclobazam, inactivating it through hydroxylation.44,45 Since norclobazam itself is an anticonvulsant, an increase in its levels through inhibition of CYP2C19 can greatly increase the duration of therapeutic effect. Mutations in CYP2C19 that reduce its activity are relatively common, with nearly 3% of Caucasians and up to 20% of Asians characterized as “poor metabolizers”.46 In epileptic patients treated with clobazam, the norclobazam to clobazam ratio was found to be dramatically higher in those with mutations in CYP2C19.44,47,48 Interestingly, one study found that clobazam therapy was more effective in patients with defective CYP2C19 alleles, with no correlation to adverse side effects,49 which may suggest a prominent role for norclobazam in determining the therapeutic benefits. However, others have reported an increased occurrence of side effects, primarily sedation, with clobazam administration in patients carrying CYP2C19 mutations, and clobazam doses may need to be reduced for some in this patient population.47
Studies in animal models of seizure and epilepsy
Clobazam has demonstrated effectiveness in a wide variety of animal models, including acute and chronic seizures and genetic forms of epilepsy.2,50 In recent studies, clobazam generally showed efficacy similar to that of the 1,4- benzodiazepines, albeit with lower potency when compared with diazepam or clonazepam.51–54 The side effect profile, development of tolerance, and withdrawal hyperexcitability produced by clobazam were all similar to that seen with diazepam in these animal models.51 However, the activity of clobazam was not identical to the 1,4-benzodiazepines in all cases, because clobazam was found to be more effective in a model of inherited epilepsy,53 and had a distinct profile of activity against acute seizures.51
Activity at GABAA receptors
A variety of studies have clearly shown that, like the 1,4-benzodiazepines, clobazam is a positive allosteric modulator of GABAA receptors. Direct enhancement of the response by clobazam to applied GABA was demonstrated in cultured cortical55 and cerebellar56 neurons, and clobazam was also found to slow the decay of miniature inhibitory post-synaptic currents in brain slices from rat hippocampus.57
Neuronal GABAA receptors are structurally heterogeneous, and the pentameric channel can be assembled from a combination of at least 16 different subunit subtypes (including α1–6, β1–3, γ1–3, δ, ɛ, π, and θ).58 These subunits show different patterns of expression throughout the brain, and their levels change throughout development and in response to pathological conditions, including seizure activity.59 The subunit composition of the receptor greatly influences its pharmacological properties.
Benzodiazepine agonists do not modulate receptors containing α4 or α6 subunits, and require the presence of a γ subunit.60 Clobazam appears to share the same binding site as other benzodiazepine agonists, because a mutation in the γ subunit had a similar effect on potency of both diazepam and clobazam.61 However, clobazam and norclobazam have been tested directly on very few GABAA receptor isoforms, and no comprehensive studies of the subunit dependence of their activity have been reported for either compound.
The modulatory activity of clobazam, norclobazam, and diazepam was compared at recombinant α3β3γ2 receptors,62 which is likely to be a significant isoform in the developing brain.59 In that study, clobazam had efficacy similar to that of diazepam, but lower potency, consistent with the higher clobazam doses required in animal studies. Relative to one another, norclobazam and clobazam had similar potency at these receptors, although norclobazam showed lower efficacy. 62 Few other studies have been performed to examine the possible subunit dependence of clobazam or norclobazam activity at GABAA receptors. Clobazam was shown to enhance the response of α1β2γ1 receptors modestly63 and to bind to an α5-containing receptor population from rat hippocampus with characteristics similar to those of diazepam. 64 It is important to understand whether clobazam or norclobazam show a different pattern of subunit selectivity compared with other benzodiazepines, which might explain the distinct characteristics associated with clobazam. Drugs that modulate different receptor populations would be expected to have unique effects on seizure activity, sedation, and anxiety,65,66 and could also produce different levels of tolerance development and abuse potential.67
In addition to direct modulation of GABAA receptor activity, clobazam was shown to cause a region-specific increase in expression of transporters for GABA (GAT3) and glutamate (GLT-1) in an animal model of temporal lobe epilepsy. 68 This alteration may have been an indirect effect from the reduction in seizure activity, because clobazam had no effect on transporter levels in control (seizure-free) animals. The impact of these changes in the clinical effectiveness of clobazam is not known, but an increase in GLT-1 could potentially reduce the high hippocampal glutamate levels associated with epileptogenesis in animal models of temporal lobe epilepsy.69 Modulation of voltage-gated Na+ and Ca2+ channels by clobazam has also been suggested by some authors,2,70 although no studies have demonstrated a direct action at these channels.
Does norclobazam have a therapeutic role?
It is clear that norclobazam is an active metabolite of clobazam, with direct anticonvulsant activity both in animal models of epilepsy and in patients with refractory epilepsy.70 Less clear are the relative roles of each of these compounds in the therapeutic and side effect profiles of clobazam. In epilepsy patients, the degree of seizure control was correlated with blood levels of norclobazam rather than clobazam.72 Studies with both neurons and recombinant expression systems have shown that norclobazam acts as a positive allosteric modulator of GABAA receptors, with a similar potency but lower efficacy than clobazam.55,62 It has been suggested that partial agonists at modulatory sites might have improved side effect profiles compared with full agonists,73 and indeed, norclobazam was associated with reduced development of tolerance compared with clobazam in a mouse seizure model.71 However, very few studies have directly examined the properties of norclobazam. If norclobazam is a primary mediator of the anticonvulsant effects of clobazam, further studies into its mechanism(s) of action are warranted.
Clinical trials in Lennox–Gastaut syndrome and other pediatric epilepsies
Clobazam was first synthesized in the 1960s and is approved for use as an antiepileptic drug in over 100 countries.2 Thus, longstanding clinical experience indicates that clobazam is a safe and effective addon therapy for many patients (Table 1). In many countries, clobazam has been used as a first-line antiepileptic drug in pediatric epilepsy, and in spite of other options becoming available, it continues to be used as an adjunctive therapy for patients with treatment-resistant epilepsy.74–76 In the US, until its October 2011 approval by the Food and Drug Administration, clobazam was only obtained from foreign pharmacies and paid for out-of-pocket by patients. Thus, the use of clobazam in the US was typically limited to patients with severe epilepsy that had proven refractory to multiple medication options.30,77 However, clobazam is anticipated to be more widely available from early 2012 for LGS patients. Clobazam was granted orphan drug status by the Food and Drug Administration in December 2008, and a new drug application submitted in March 2011 for use in children with LGS was approved in October 2011.
Table 1.
Clobazam as addon therapy in refractory pediatric epilepsy
| Study | Trial design | Participants and included diagnoses | Dosage | Results |
|---|---|---|---|---|
| Conry et al8 | Phase II, multicenter, randomized, double-blind, dose-ranging | 68 patients (42 males, 26 females) 2–26 years LGS |
0.25 mg/kg/day or 1.0 mg/kg/day | 0.25 mg/kg/day: 38% of patients had a ≥50% decrease in drop seizure frequency 1.0 mg/kg/day: 83% of patients had a ≥50% decrease in drop seizure frequency |
| Conry et al87 | Phase III, multicenter, randomized, double-blind, dose-ranging, placebo-controlled | 238 patients 2–54 years LGS |
0.25 mg/kg/day, 0.5 mg/kg/day, or 1.0 mg/kg/day | 0.5 mg/kg/day: 58% of patients had a ≥50% decrease in drop seizure frequency 1.0 mg/kg/day: 77% of patients had a ≥50% decrease in drop seizure frequency |
| da Silveira et al84 | Retrospective | 100 patients (61 males, 39 females) 1–18 years Refractory focal epilepsy |
5–60 mg/day | 33% of patients had a ≥75% decrease in seizure frequency |
| Farrell78 | Open-label, prospective | 50 patients, 33 with LGS2, 16 years Refractory epilepsy |
5–40 mg/day | 54% of patients had a ≥50% decrease in seizure frequency |
| Jan and Shaabat79 | Open-label, prospective | 31 patients (21 males, 10 females), 14 with LGS 2 months to 15 years Intractable childhood epilepsy |
5–40 mg/day | 80% of patients had a ≥50% decrease in seizure frequency |
| Kalra et al81 | Open-label, prospective | 88 patients (59 males, 29 females) 7 months to 12 years refractory epilepsy |
0.3–2.0 mg/kg/day | 85% of patients had a ≥50% decrease in seizure frequency |
| Keene et al86 | Double-blind, placebo-controlled, crossover | 21 patients (11 males, 10 females) 2–19 years Refractory epilepsy |
0.25–1.0 mg/kg/day | 54% of patients had a ≥50% decrease in seizure frequency |
| Munn and Farell74 | Open-label, prospective | 115 patients (68 males, 47 females), 25 with LGS 15 months to 17 years refractory epilepsy | 0.36–3.8 mg/kg/day | 62% of all patients had a ≥50% decrease in seizure frequency 64% of LGS patients had a ≥50% decrease in seizure frequency |
| Silva et al85 | Retrospective | 97 patients (58 males, 39 females), 26 with LGS, 2 with LGS and West syndrome 1–17 years Epileptic encephalopathy |
5–60 mg/day | 37% of patients had a ≥50% decrease in seizure frequency |
| Sheth et al82 | Open-label, prospective | 63 patients (30 males, 33 females), 14 with LGS 3–20 years Intractable epilepsy |
Average 0.8 mg/kg/day | 65% of patients had ≥50% decrease in seizure frequency |
| Sugai80 | Open-label, prospective | Short term: 55 patients, 8 with LGS Long-term: 31 patients, 4 with LGS Refractory epilepsy |
0.28–1.25 mg/kg/day | Short term: 71% of all patients and 62% of LGS patients had a ≥50% decrease in seizure frequency Long-term: 81% of all patients and 50% of LGS patients had a ≥50% decrease in seizure frequency |
| Vadja et al83 | Open-label, prospective or double-blind, placebo-controlled, crossover | 14 patients* (5 males, 9 females), 7 with LGS 6–38 years Refractory epilepsy |
15–60 mg/day | 40% of patients had a ≥50% decrease in seizure frequency |
Note: Results not reported for four patients.
Abbreviation: LGS, Lennox–Gastaut syndrome.
In six open-label prospective studies evaluating the efficacy of clobazam as addon therapy for pediatric patients with refractory epilepsy, 54%–85% of patients experienced at least a 50% decrease in seizure frequency74,78–83 (Table 1). These studies included a total of 423 patients, with 98 patients identified as having LGS. Most studies did not provide distinct data for the LGS population, but Sugai80 reported that 62% of LGS patients in the group evaluated for short-term efficacy and half of LGS patients followed for at least 6 months had a 50% or greater decrease in seizure frequency on clobazam. In another study, 64% of 25 patients with LGS achieved at least a 50% decrease in seizure frequency.74 Jan and Shaabat79 noted that three of the 14 LGS patients included in their study continued to have daily seizures while taking clobazam, a higher proportion than in the rest of the study population.
Two retrospective studies on the efficacy of clobazam as addon therapy for pediatric patients also reported significant reductions in seizure frequency84,85 (Table 1). Da Silveira et al84 evaluated 100 patients who received clobazam as addon therapy for refractory focal epilepsy, and 33% of these patients had a 75% or greater decrease in seizure frequency. Silva et al85 reviewed the efficacy of clobazam as addon therapy for 97 pediatric patients with epileptic encephalopathies. Of the patients in this study, 28 had LGS. Thirty-seven percent of all patients had a 50% or greater decrease in seizure frequency, and complete seizure control was achieved in nine patients.
In 1990, Keene et al86 reported the results of a double-blind, placebo-controlled, crossover study evaluating clobazam as addon therapy in 21 patients aged 2–19 years with refractory epilepsy. Fifty-two percent of patients in the clobazam arms had a 50% or greater decrease in seizure frequency. More recently, Conry et al8 reported the results of a multicenter, double-blind Phase II study evaluating low-dose (0.25 mg/kg/day) or high-dose (1 mg/kg/day) clobazam in 68 patients with LGS, aged 2–26 years. Eighty-three percent of patients in the high-dose group had a 50% or greater reduction in seizure frequency compared with baseline. In addition to decreased seizure frequency, patients had improved global assessments on both high-dose and low-dose clobazam,8 consistent with prior work suggesting improved cognitive and behavioral performance on clobazam.74,78 Following the encouraging results of the Phase II study, a multicenter, randomized, double-blind, Phase III study was performed.87 This study evaluated the efficacy of low-dose (0.25 mg/kg/day), medium-dose (0.5 mg/kg/day), and high-dose (1 mg/kg/day) clobazam versus placebo as addon therapy for 238 patients with LGS, aged 2–54 years. Fifty-eight percent of patients in the medium-dose group and 77% of patients in the high-dose group had a 50% or greater decrease in seizure frequency. Improved global assessments were reported for patients in all dosage groups compared with placebo.
Adverse effects in epilepsy patients
Adverse effects from clobazam are generally similar to those of the other benzodiazepines, but perhaps less frequent. Conry et al8 reported little difference in the occurrence of side effects in patients receiving clobazam 0.25 mg/kg/day and those receiving 1.0 mg/kg/day. The most common adverse effect is somnolence, reported by 9%–19% of patients.8,78,81,84 Other common side effects include behavioral abnormalities, irritability, ataxia, and drooling, each occurring in under 10% of patients.8,81,84 Notably, in spite of the efficacy observed in many patients, an increase in seizures, worsening of seizures, or development of new seizure types have been reported in up to 5%–13% of patients.8,74,80,84 In the only double-blind trial, Conry et al8 reported 13% with adverse events related to seizures, each mild or moderate in severity, and more common in the low-dose clobazam group than in the high-dose group. Other studies reporting adverse seizure-related events were open-label, prospective,74,80 or retrospective84 in nature without randomization or control arms. Because the rate and types of seizures often fluctuate over several weeks or months in patients with LGS, it remains unclear whether these episodes of seizure worsening are related to clobazam administration.3,8
Tolerance is an issue with many antiepileptic drugs, especially benzodiazepines, and the loss of efficacy of clobazam in some patients has been noted. Reports of the development of tolerance among published studies varied from as few as 10% to as many as 87% of patients.81,84 For up to 70% of patients who developed tolerance, efficacy returned after stopping and reintroducing clobazam after 2–3 months or after increasing the dosage.74,80,81 Others noted persistent efficacy for more than one year in as many as 85% of patients who experienced improved seizure control, and some patients maintained complete seizure control during this time.85
Clobazam is typically initiated at a low dose, often 5 mg/ day or 0.1 mg/kg/day for smaller patients, and increased at 5–7 day intervals until a minimum effective dose is reached or side effects occur.74,79 Studies have suggested that slow titration may help avoid adverse effects and that when present, side effects may be reduced or eliminated with dose reduction.79,80 Doses of 0.2–3.8 mg/kg/day2,73 have been used in trials evaluating the use of clobazam (Table 1). In our experience, doses up to 2 mg/kg/day divided into twice daily doses are often required. Rarely, higher doses up to 3 mg/kg/day are required and tolerated.
Interactions with stiripentol and other antiepileptic drugs
Clobazam has been coadministered with a wide variety of other antiepileptic drugs, with few reported harmful drug– drug interactions. Any inhibitors or inducers of CYP2C19 can have an impact on clobazam and norclobazam levels, and coadministration of CYP2C19 inhibitors has been successfully used to enhance the duration and efficacy of clobazam treatment, possibly by increasing levels of norclobazam. This interaction seems particularly beneficial when clobazam is coadministered with stiripentol (Diacomit®), an antiepileptic drug, which is both a GABAA receptor modulator and a potent CYP2C19 inhibitor.88–90 Clobazam and stiripentol act via separate mechanisms at the GABAA receptor57,62 and stiripentol can dramatically increase norclobazam levels.45 Animal studies have demonstrated a significant positive interaction between clobazam and stiripentol, with both an additive pharmacodynamic interaction and a large increase in the brain concentration of clobazam.54 The combination of clobazam with stiripentol is widely used in the treatment of patients with Dravet syndrome (severe myoclonic epilepsy of infancy).88 In contrast with its possibly beneficial interaction with stiripentol, clobazam has also been reported to inhibit the metabolism of valproate, so has the potential to increase valproateassociated toxicity.91 Overall, clobazam is generally well tolerated when combined with most of the antiepileptic drugs commonly used in clinical practice.
Summary
LGS is an epileptic encephalopathy with childhood onset that is characterized by multiple seizure types and an intractable nature. LGS is also associated with a number of cognitive and behavioral problems that progress over time, often even after seizure control has improved. Clobazam has been demonstrated to decrease the overall rate of seizures in patients with LGS, with a significant reduction in the frequency of drop seizures, often considered to be the most disabling type of seizure associated with the syndrome.3,6,10,12 Improved global assessments for patients on clobazam have been noted, which may warrant further investigation. Hancock and Cross7 reported that the behavioral and cognitive deterioration associated with LGS are the symptoms that are hardest to cope with for many families. Further work needs to be done to characterize fully the activity of both clobazam and norclobazam, its active metabolite, at different GABAA receptor populations and to optimize the incorporation of clobazam into a treatment plan.
Clobazam has been used as a first-line treatment in many countries, and is now frequently used as an adjunctive therapy for patients with refractory epilepsy. Its recent approval by the Food and Drug Administration will now allow its use for LGS patients in the US. The antiepileptic drugs currently approved for the treatment of LGS are not effective for all patients and each is associated with significant side effects. Other safe and effective options for treatment-resistant patients are needed, and recent studies of clobazam suggest that it may be an effective and well tolerated option for patients with LGS.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Markand O. Lennox-Gastaut syndrome (childhood epileptic encephalopathy) J Clin Neurophysiol. 2003;20(6):426–441. doi: 10.1097/00004691-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Ng YT, Collins SD. Clobazam. Neurotherapeutics. 2007;4(1):138–144. doi: 10.1016/j.nurt.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rijckevorsel K. Treatment of Lennox-Gastaut syndrome: overview and recent findings. Neuropsychiatr Dis Treat. 2008;4(6):1001–1019. doi: 10.2147/ndt.s1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michoulas A, Farrell K. Medical management of Lennox-Gastaut syndrome. CNS Drugs. 2010;24(5):363–374. doi: 10.2165/11530220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Camfield P. Definition and natural history of Lennox-Gastaut syndrome. Epilepsia. 2011;52(Suppl 5):3–9. doi: 10.1111/j.1528-1167.2011.03177.x. [DOI] [PubMed] [Google Scholar]
- 6.Arzimanoglou A, French J, Blume W, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82–93. doi: 10.1016/S1474-4422(08)70292-8. [DOI] [PubMed] [Google Scholar]
- 7.Hancock EC, Cross HH. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev. 2009;8(3):CD003277. doi: 10.1002/14651858.CD003277.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Conry JA, Ng YT, Paolicchi JM, et al. Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia. 2009;50(5):1158–1166. doi: 10.1111/j.1528-1167.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 9.Shields WD. Catastrophic epilepsy in childhood. Epilepsia. 2000;41(Suppl 2):S2–6. doi: 10.1111/j.1528-1157.2000.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 10.Trevathan E. Infantile spasms and Lennox-Gastaut syndrome. J Child Neurol. 2002;17(Suppl 2):2S9–2S22. doi: 10.1177/08830738020170021201. [DOI] [PubMed] [Google Scholar]
- 11.Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 3):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- 12.Montouris G. Rational approach to treatment options for Lennox-Gastaut syndrome. Epilepsia. 2011;52(Suppl 5):10–20. doi: 10.1111/j.1528-1167.2011.03178.x. [DOI] [PubMed] [Google Scholar]
- 13.Wheless JW, Clark DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol. 2005;20(Suppl 1):S1–56. doi: 10.1177/088307380502000101. [DOI] [PubMed] [Google Scholar]
- 14.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Discord. 2007;9(4):353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt D, Bourgeois B. A risk-benefit assessment of therapies for Lennox-Gastaut syndrome. Drug Saf. 2000;22(6):467–477. doi: 10.2165/00002018-200022060-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bryant AE, Dreifuss FE. Valproic acid hepatic fatalities. III. US experience since 1986. Neurology. 1996;46(2):465–469. doi: 10.1212/wnl.46.2.465. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil MG, Perdun CS, Wilson MB, McGown ST, Patel S. Felbamate-associated fatal acute hepatic necrosis. Neurology. 1996;46(5):1457–1459. doi: 10.1212/wnl.46.5.1457. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman DW, Kelly JP, Anderson T, Harmon DC, Shapiro S. Evaluation of case reports of aplastic anemia among patients treated with felbamate. Epilepsia. 1997;38(12):1265–1269. doi: 10.1111/j.1528-1157.1997.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 19.Pellock JM, Faught E, Leppik IE, Shinnar S, Zupanc ML. Felbamate: consensus of current clinical experience. Epilepsy Res. 2006;71(2–3):89–101. doi: 10.1016/j.eplepsyres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson JA, Glauser TA, Olberding LS. Lamotrigine adjunctive therapy in childhood epileptic encephalopathy (the Lennox Gastaut Syndrome) Epilepsia. 1997;38(1):68–73. doi: 10.1111/j.1528-1157.1997.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 21.Farrell K, Connolly MB, Munn R, Peng S, MacWilliams LM. Prospective, open-label, add-on study of lamotrigine in 56 children with intractable generalized epilepsy. Pediatr Neurol. 1997;16(3):201–205. doi: 10.1016/s0887-8994(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 22.Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. N Engl J Med. 1997;337(25):1807–1812. doi: 10.1056/NEJM199712183372504. [DOI] [PubMed] [Google Scholar]
- 23.Dooley J, Camfield P, Gordon K, Camfield C, Wirrell E, Smith E. Lamotrigine-induced rash in children. Neurology. 1996;46(1):240–242. doi: 10.1212/wnl.46.1.240. [DOI] [PubMed] [Google Scholar]
- 24.Schlumberger E, Chavez F, Placios L, Rey E, Pajot N, Dulac O. Lamotrigine in treatment of 120 children with epilepsy. Epilepsia. 1994;35(2):359–367. doi: 10.1111/j.1528-1157.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 25.Kanner AM, Frey M. Adding valproate to lamotrigine: a study of their pharmacokinetic interaction. Neurology. 2000;55(4):588–591. doi: 10.1212/wnl.55.4.588. [DOI] [PubMed] [Google Scholar]
- 26.Faught E, Wilder BJ, Ramsay RE, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, and 600-mg daily dosages. Neurology. 1996;46(6):1684–1690. doi: 10.1212/wnl.46.6.1684. [DOI] [PubMed] [Google Scholar]
- 27.Martin R, Kuzniecky R, Ho S, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52(2):321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- 28.Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636–641. doi: 10.1136/jnnp.69.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70(21):1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 30.Chu-Shore CJ, Thiele EA. New drugs for pediatric epilepsy. Semin Pediatr Neurol. 2010;17(4):214–223. doi: 10.1016/j.spen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly MT. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102(6):1358–1363. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 32.Freeman JM, Vining EP, Kossoff EH, Pyzik PL, Ye X, Goodman SN. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia. 2009;50(2):322–325. doi: 10.1111/j.1528-1167.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 33.Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55(11):1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 34.Seo JH, Lee YM, Lee JS, Jang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios – comparison of 3:1 with 4:1 diet. Epilepsia. 2007;48(4):801–805. doi: 10.1111/j.1528-1167.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 35.Rychlicki F, Zamponi N, Trignani R, Ricciuti RA, Iacoangeli M, Scerrati M. Vagus nerve stimulation: clinical experience in drug-resistant pediatric epileptic patients. Seizure. 2006;15(6):483–490. doi: 10.1016/j.seizure.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Frost M, Gates J, Helmers SL, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia. 2001;42(9):1148–1152. doi: 10.1046/j.1528-1157.2001.23900.x. [DOI] [PubMed] [Google Scholar]
- 37.Rossignol E, Lortie A, Thomas T, et al. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure. 2009;18(1):34–37. doi: 10.1016/j.seizure.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Cukiert A, Burattini JA, Mariani PP, et al. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia. 2006;47(2):371–374. doi: 10.1111/j.1528-1167.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 39.You SJ, Lee JK, Ko TS. Epilepsy surgery in a patient with Lennox-Gastaut syndrome and cortical dysplasia. Brain Dev. 2007;29:167–170. doi: 10.1016/j.braindev.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Hanks GW. Clobazam: pharmacological and therapeutic profile. Br J Clin Pharmacol. 1979;7(Suppl 1):151S–155S. doi: 10.1111/j.1365-2125.1979.tb04685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008;118(2):69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 42.Barzaghi F, Fournex R, Mantegazza P. Pharmacological and toxicological properties of clobazam (1-phenyl-5-methyl-8-chloro-1,2,4,5-tetrahydro-2,4-diketo-3H-1,5-benzodiazepine), a new psychotherapeutic agent. Arzneimittelforschung. 1973;23(5):683–686. [PubMed] [Google Scholar]
- 43.Gastaut H, Low MD. Antiepileptic properties of clobazam, a 1–5 benzodiazepine, in man. Epilepsia. 1979;20(4):437–446. doi: 10.1111/j.1528-1157.1979.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 44.Giraud C, Tran A, Rey E, Vincent J, Tréluyer JM, Pons G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004;32(11):1279–1286. [PubMed] [Google Scholar]
- 45.Giraud C, Treluyer JM, Rey E, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34(4):608–611. doi: 10.1124/dmd.105.007237. [DOI] [PubMed] [Google Scholar]
- 46.Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochrome P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29(3):192–209. doi: 10.2165/00003088-199529030-00005. [DOI] [PubMed] [Google Scholar]
- 47.Contin M, Sangiorgi S, Riva R, Parmeggiani A, Abani F, Baruzzi A. Evidence of polymorphic CYP2C19 involvement in the human metabolism of n-desmethylclobazam. Ther Drug Monit. 2002;24(6):737–741. doi: 10.1097/00007691-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Kosaki K, Tamura K, Sato R, Samejima H, Tanigawara Y, Takahashi T. A major influence of CYP2C19 genotype on the steady-state concentration of N-desmethylclobazam. Brain Dev. 2004;26(8):530–534. doi: 10.1016/j.braindev.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Seo T, Nagata R, Ishitsu T, et al. Impact of CYP2C19 polymorphisms on the efficacy of clobazam therapy. Pharmacogenomics. 2008;9(5):527–537. doi: 10.2217/14622416.9.5.527. [DOI] [PubMed] [Google Scholar]
- 50.Meldrum BS, Chapman AG, Horton RW. Clobazam: anticonvulsant action in animal models of epilepsy [proceedings] Br J Clin Pharmacol. 1979;7(Suppl 1):59S–60S. doi: 10.1111/j.1365-2125.1979.tb04666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Löscher W, Rundfeldt C, Hönack D, Ebert U. Long-term studies on anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. I. Comparison of diazepam, clobazam and abercarnil. J Pharm Exp Ther. 1996;279(2):561–572. [PubMed] [Google Scholar]
- 52.Šlamberová R, Mareš P, Vorlíček J. Clobazam exerts an anticonvulsant action in immature rats. Physiol Res. 1998;47(4):301–305. [PubMed] [Google Scholar]
- 53.Miura Y, Amano S, Torii R, Ihara N. Clobazam shows a different antiepileptic action profile from clonazepam and zonisamide in Ihara epileptic rats. Epilepsy Res. 2002;49(3):189–202. doi: 10.1016/s0920-1211(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 54.Luszczki JJ, Trojnar MK, Ratnaraj N, Patsalos PN, Czuczwar SJ. Interactions of stiripentol with clobazam and valproate in the mouse maximal electroshock-induced seizure model. Epilepsy Res. 2010;90(3):188–198. doi: 10.1016/j.eplepsyres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura F, Suzuki S, Nishimura S, Yagi K, Seino M. Effects of clobazam and its active metabolite on GABA-activated currents in rat cerebral neurons in culture. Epilepsia. 1996;37(8):728–735. doi: 10.1111/j.1528-1157.1996.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 56.Gatta E, Cupello A, Di Braccio M, et al. New 1,5-benzodiazepine compounds: activity at native GABA(A) receptors. Neuroscience. 2010;166(3):917–923. doi: 10.1016/j.neuroscience.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47(4):704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 58.Whiting PJ, Bonnert TP, McKernan RM, et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 59.Galanopoulou AS. GABA(A) receptors in normal development and seizures: friends or foes? Curr Neuropharmacol. 2008;6(1):1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korpi ER, Gründer G, Lüddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67(2):113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 61.Ogris W, Pöltl A, Hauer B, et al. Affinity of various benzodiazepine site ligands in mice with a point mutation in the GABA receptor gamma2 subunit. Biochem Pharmacol. 2004;68(8):1621–1629. doi: 10.1016/j.bcp.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Fisher JL. Interactions between modulators of the GABA(A) receptor: Stiripentol and benzodiazepines. Eur J Pharmacol. 2011;654(2):160–165. doi: 10.1016/j.ejphar.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khom S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABAA receptors containing gamma1 subunits. Mol Pharmacol. 2006;69(2):640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- 64.Li M, Szabo A, Rosenberg HC. Evaluation of native GABA(A) receptors containing an alpha 5 subunit. Eur J Pharmacol. 2001;413(1):63–72. doi: 10.1016/s0014-2999(01)00751-8. [DOI] [PubMed] [Google Scholar]
- 65.Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22(4):188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 66.Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6(1):18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102(3):915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doi T, Ueda Y, Tokumaru J, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by clobazam during epileptogenesis in Fe(+++)-induced epileptic rats. Brain Res Mol Brain Res. 2005;142(2):91–96. doi: 10.1016/j.molbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Ueda Y, Doi T, Tokumaru J, et al. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. J Neurochem. 2001;76(3):892–900. doi: 10.1046/j.1471-4159.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 70.Mehndiratta MM, Krishnamurthy M, Rajesh KN, Singh G. Clobazam monotherapy in drug naïve adult patients with epilepsy. Seizure. 2003;12(4):226–228. doi: 10.1016/s1059-1311(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 71.Haigh JR, Pullar T, Gent JP, Dailley C, Feely M. N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy? Br J Clin Pharmacol. 1987;23(2):213–218. doi: 10.1111/j.1365-2125.1987.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kinoshita M, Ikeda A, Begum T, Terada K, Shibasaki H. Efficacy of low-dose, add-on therapy of clobazam (CLB) is produced by its major metabolite, N-desmethyl-CLB. J Neurol Sci. 2007;263(1–2):44–48. doi: 10.1016/j.jns.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 73.Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6(1):24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Munn R, Farell K. Open study of clobazam in refractory epilepsy. Pediatr Neurol. 1993;9(6):465–469. doi: 10.1016/0887-8994(93)90026-9. [DOI] [PubMed] [Google Scholar]
- 75.Canadian Study Group for Childhood Epilepsy. Clobazam has equivalent efficacy to carbamazepine and phenytoin as monotherapy for childhood epilepsy. Epilepsia. 1998;39(9):952–959. doi: 10.1111/j.1528-1157.1998.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 76.Mills JK, Lewis TG, Mughal K, Ali I, Ugur A, Whitehouse WP. Retention rate of clobazam, topiramate, and lamotrigine in children with intractable epilepsy at 1 year. Seizure. 2011;20(5):402–405. doi: 10.1016/j.seizure.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Montenegro MA, Arif H, Nahm EA, Resor SR, Jr, Hirsch LJ. Efficacy of clobazam as add-on therapy for refractory epilepsy: experience at a US epilepsy center. Clin Neuropharmacol. 2008;31(6):333–338. doi: 10.1097/WNF.0b013e31815cd960. [DOI] [PubMed] [Google Scholar]
- 78.Farrell K. Benzodiazepines in the treatment of children with epilepsy. Epilepsia. 1986;27(Suppl 1):S45–52. doi: 10.1111/j.1528-1157.1986.tb05733.x. [DOI] [PubMed] [Google Scholar]
- 79.Jan MM, Shaabat AO. Clobazam for the treatment of intractable childhood epilepsy. Saudi Med J. 2000;21(7):622–624. [PubMed] [Google Scholar]
- 80.Sugai K. Clobazam as a new antiepileptic drug and clorazepate dipotassium as an alternative antiepileptic drug in Japan. Epilepsia. 2004;45(Suppl 8):S20–25. doi: 10.1111/j.0013-9580.2004.458005.x. [DOI] [PubMed] [Google Scholar]
- 81.Kalra V, Seth R, Mishra D, Saha NC. Clobazam in refractory childhood epilepsy. Indian J Pediatr. 2010;77(3):263–266. doi: 10.1007/s12098-010-0035-z. [DOI] [PubMed] [Google Scholar]
- 82.Sheth RD, Ronen GM, Goulden KJ, Penney S, Bodensteiner JB. Clobazam for intractable pediatric epilepsy. J Child Neurol. 1995;10(3):205–208. doi: 10.1177/088307389501000306. [DOI] [PubMed] [Google Scholar]
- 83.Vadja FJ, Bladin PF, Parsons BJ. Clinical experience with clobazam: a new 1,5 benzodiazepine in the treatment of refractory epilepsy. Clin Exp Neurol. 1985;21:177–182. [PubMed] [Google Scholar]
- 84.da Silveira MR, Mentenegro MA, Franzon RC, Guerreiro CA, Geurreiro MM. Effectiveness of clobazam as add-on therapy in children with refractory focal epilepsy. Arq Neuropsiquiatr. 2006;64(3B):705–710. doi: 10.1590/s0004-282x2006000500001. [DOI] [PubMed] [Google Scholar]
- 85.Silva RC, Montenegro MA, Geurreiro CA, Geurreiro MM. Clobazam as add-on therapy in children with epileptic encephalopathy. Can J Neurol Sci. 2006;33(2):209–213. [PubMed] [Google Scholar]
- 86.Keene DL, Whiting S, Humphreys P. Clobazam as an add-on drug in the treatment of refractory epilepsy of childhood. Can J Neurol Sci. 1990;17(3):317–319. doi: 10.1017/s0317167100030651. [DOI] [PubMed] [Google Scholar]
- 87.Conry JA, Ng Y, Drummond R, Stolle J, Sagar S. Efficacy and safety of clobazam in the treatment of seizures associated with Lennox-Gastaut syndrome: results of a phase III trial [abstract]. Proceedings of the 64th Annual Meeting of the American Epilepsy Society; December 3–7, 2010; San Antonio, Tx. 2010. p. 1.283. [Google Scholar]
- 88.Trojnar MK, Wojtal K, Trojnar MP, Czuczwar SJ. Stiripentol. A novel antiepileptic drug. Pharmacol Rep. 2005;57(2):154–160. [PubMed] [Google Scholar]
- 89.Chiron C, Dulac O. The pharmacological treatment of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):72–75. doi: 10.1111/j.1528-1167.2011.03007.x. [DOI] [PubMed] [Google Scholar]
- 90.Fisher JL. The effects of stiripentol on GABA(A) receptors. Epilepsia. 2011;52(Suppl 2):76–78. doi: 10.1111/j.1528-1167.2011.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Theis JG, Koren G, Daneman R, et al. Interactions of clobazam with conventional antiepileptics in children. J Child Neurol. 1997;12(3):208–213. doi: 10.1177/088307389701200311. [DOI] [PubMed] [Google Scholar]