Abstract
Background
Effectiveness of a drug is a key concept dependent on efficacy, safety, and tolerability. Time to discontinuation of treatment is also representative of effectiveness. We investigated differences in treatment discontinuation among newly started second-generation antipsychotics in the clinical setting.
Methods
Using a retrospective cohort study design, we screened all outpatients (n = 7936) who visited the Shioiri Mental Clinic between July 1, 2008 and June 30, 2010. We identified a cohort of patients (n = 703) diagnosed with schizophrenia or schizoaffective disorder and calculated the time to discontinuation of each second-generation antipsychotic.
Results
Of the 703 patients, 149 were newly treated with aripiprazole, 67 with blonanserin, 95 with olanzapine, 36 with quetiapine, 74 with perospirone, and 120 with risperidone. The time to discontinuation for all causes was significantly longer for aripiprazole than for blonanserin, olanzapine, and risperidone. In addition, aripiprazole tended to be continued for longer than quetiapine and perospirone, but these differences were not significant.
Conclusion
Aripiprazole may be considered the best available option for long-term treatment of patients with schizophrenia or schizoaffective disorder.
Keywords: retrospective study, second-generation antipsychotics, effectiveness, treatment continuation, schizophrenia, aripiprazole
Introduction
Second-generation (atypical) antipsychotics are first-line treatment for schizophrenia in the clinical setting at present because they are better tolerated than first-generation (typical) antipsychotics and are subjectively preferred by patients.1,2
Treatment effectiveness has become a key concept for evaluating long-term pharmacotherapy in schizophrenia.3–5 Effectiveness is considered when integrating the parameters of efficacy, safety, and tolerability by both the patient and the clinician. Rates of continuation or discontinuation of treatment serve as appropriate surrogate parameters of effectiveness.4–7 There are several reports of time to treatment discontinuation among antipsychotics. The Clinical Antipsychotics Trials of Intervention Effectiveness (CATIE) schizophrenia study is the most famous of the comparative studies of antipsychotic effectiveness.4,5 However, few naturalistic studies have evaluated differences in effectiveness between the available second-generation antipsychotics. We undertook this retrospective study to investigate treatment continuation for the second-generation antipsychotics in the clinical setting.
Materials and methods
Study design
The second-generation antipsychotics investigated were aripiprazole, blonanserin, olanzapine, perospirone, quetiapine, and risperidone. These were the agents available in Japan between July 1, 2008 and June 30, 2010, when we conducted this study. Using a retrospective cohort design, we screened all outpatients who visited the Shioiri Mental Clinic during this time period. Shioiri Mental Clinic is a psychiatric outpatient office in Yokosuka in Japan that employs six psychiatrists. All patients were diagnosed using the International Classification of Diseases, Tenth Revision (ICD-10) by the treating psychiatrists in a routine clinical setting and not on the basis of a structured interview. The treating psychiatrists also judged whether treatment with the antipsychotic should be continued on the basis of patient response.
From medical records we formed a cohort of patients diagnosed with schizophrenia (F20) or schizoaffective disorder (F25). We then evaluated groups of patients who were newly treated with each second-generation antipsychotic in the cohort. We included not only patients who were newly started on a single drug but also those undergoing adjunctive treatment with a second-generation antipsychotic drug.
To assess rates of continuation or discontinuation and adherence or nonadherence, we retrospectively reviewed medical records between the date of treatment initiation and the latest visit in June 2011. Treatment discontinuation was deemed to have occurred if the following criteria were met: the antipsychotic drug was discontinued because of all causes; the patient did not visit the clinic as a result of dropout, being admitted to the hospital, moving to another hospital, or death; and duration without medication were over 14 consecutive days. Treatment continuation was defined as continuation of the antipsychotic drug until the latest visit day in June 2011. We counted the time (days) until discontinuation of treatment due to all causes until the latest visit day in June 2011.
Statistical analysis
Time until discontinuation of treatment was estimated using a Kaplan–Meier survival analysis. Treatment groups were compared using the Cox’s proportional hazards regression model. P values <0.05 were considered significant. All analyses were performed using Excel Tokei 2010 for Windows, Version 1.04 (Social Survey Research Information Company Ltd, Tokyo, Japan).
Results
Of the 7936 outpatients who visited Shioiri Mental Clinic between July 1, 2008 and June 30, 2010, 703 were diagnosed with schizophrenia or schizoaffective disorder by ICD-10. Of these 703 patients, 149 were newly treated with aripiprazole, 67 with blonanserin, 95 with olanzapine, 36 with quetiapine, 74 with perospirone, and 120 with risperidone. Table 1 lists the characteristics of patients treated with second-generation antipsychotics. These data indicate that the average daily antipsychotic doses in Japanese patients are somewhat lower than those in Western patients. One of the possible reasons is that Japanese patients have a smaller build and a lower body weight in comparison with Western patients.
Table 1.
Information on patients treated with second-generation antipsychotics
| Aripiprazole (n = 149) | Blonanserin (n = 67) | Olanzapine (n = 95) | Perospirone (n = 36) | Quetiapine (n = 74) | Risperidone (n = 120) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female, n (%) | 91 (61.1) | 35 (52.2) | 55 (57.9) | 23 (63.9) | 44 (59.5) | 71 (59.2) |
| Male, n (%) | 58 (38.9) | 32 (47.8) | 40 (42.1) | 13 (36.1) | 30 (40.5) | 49 (40.8) |
| Age (years) | ||||||
| Mean (SD) | 43.5 (14.8) | 45.6 (15.2) | 49.7 (18.7) | 43.9 (14.8) | 50.5 (16.7) | 45.1 (16.1) |
| Median | 41 | 42 | 43 | 40 | 45 | 42 |
| Disease duration (years) | ||||||
| Mean (SD) | 14.7 (10.5) | 14.7 (10.2) | 12.7 (9.8) | 15.5 (9.0) | 17.7 (11.8) | 15.0 (11.7) |
| Median | 12.0 | 11.0 | 11.0 | 13.5 | 13.5 | 12.0 |
| Mean modal dose (mg per day) | ||||||
| Mean (SD) | 15.9 (10.4) | 9.3 (4.6) | 8.5 (6.4) | 15.6 (13.8) | 275.9 (164.7) | 3.3 (4.9) |
| Median | 12 | 8 | 5 | 10 | 250 | 2 |
Abbreviation: SD, standard deviation.
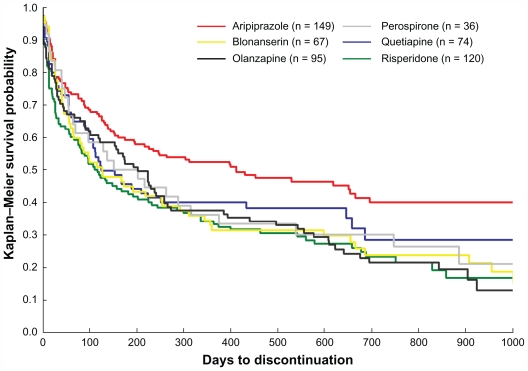
Discontinuation outcomes are presented in Figure 1 and Table 2. Time to discontinuation due to all causes was significantly longer for aripiprazole than for blonanserin (hazard ratio [HR], 0.63; P = 0.008), olanzapine (HR, 0.62; P = 0.003), and risperidone (HR, 0.58; P < 0.001). Although there were no significant differences between aripiprazole and quetiapine or between aripiprazole and perospirone, aripiprazole tended to be continued for longer than quetiapine (HR, 0.74; P = 0.092) or perospirone (HR, 0.68; P = 0.086, Figure 1). There were no significant differences between any of the second-generation antipsychotics except in comparison with aripiprazole (Table 2).
Figure 1.
Kaplan–Meier analysis of time to discontinuation of second-generation antipsychotics.
Table 2.
Summary of comparisons between treatment groups
| Aripiprazole (n = 149) | Blonanserin (n = 67) | Olanzapine (n = 95) | Perospirone (n = 36) | Quetiapine (n = 74) | Risperidone (n = 120) | |
|---|---|---|---|---|---|---|
| Discontinuation n (%) | 83 (55.7) | 53 (79.1) | 74 (77.9) | 27 (75.0) | 48 (64.9) | 92 (76.7) |
| Time to discontinuation (day) by Kaplan–Meier analysis | 543 | 364 | 370 | 407 | 402 | 336 |
| Treatment comparisons by Cox’s proportional hazards regression model | ||||||
| Aripiprazole | ||||||
| Hazard ratio (95% CI) | 0.63 (0.44–0.89) | 0.62 (0.45–0.85) | 0.68 (0.44–1.06) | 0.74 (0.51–1.05) | 0.58 (0.43–0.78) | |
| P value | 0.008** | 0.003** | 0.086 | 0.092 | <0.001** | |
| Blonanserin | ||||||
| Hazard ratio (95% CI) | 0.98 (0.69–1.38) | 1.1 (0.70–1.76) | 1.16 (0.78–1.71) | 0.91 (0.65–1.28) | ||
| P value | 0.9 | 0.69 | 0.48 | 0.59 | ||
| Olanzapine | ||||||
| Hazard ratio (95% CI) | 1.12 (0.71–1.74) | 1.16 (0.80–1.67) | 0.93 (0.69–1.27) | |||
| P value | 0.63 | 0.43 | 0.65 | |||
| Perospirone | ||||||
| Hazard ratio (95% CI) | 1.06 (0.66–1.71) | 0.84 (0.55–1.29) | ||||
| P value | 0.8 | 0.43 | ||||
| Quetiapine | ||||||
| Hazard ratio (95% CI) | 0.80 (0.56–1.13) | |||||
| P value | 0.2 | |||||
Note: P value is statistically significant.
Abbreviation: CI, confidence interval.
Discussion
The primary outcome measure in this study was all causes of treatment discontinuation, which is a global measure of the effectiveness of a drug in the clinical setting.4–7 Treatment continuation is affected by multiple factors, such as the efficacy, safety, and tolerability of the drug, as well as the preference of the patient and caregiver. In this study, aripiprazole had a significantly longer time to discontinuation than blonanserin, olanzapine, or risperidone, and showed a tendency to be continued for longer than quetiapine or perospirone. Therefore, aripiprazole might be more effective than the other five second-generation antipsychotics.
A possible reason for the superiority of aripiprazole in treatment continuation may be related to its unique pharmacological profile.2 Aripiprazole acts as a partial agonist at dopaminergic D28 and serotoninergic 5-HT1A 9 receptors and as an antagonist at 5-HT2A receptors.10 It also shows low affinity for histaminergic H1 and muscarinic cholinergic receptors.2,10 Because of this pharmacological profile, it has a low propensity to cause extrapyramidal symptoms, weight gain, metabolic disruption, hyperprolactinemia, sedation, or prolongation of the QT interval,10,11 and has little effect on psychosocial functioning.12 Aripiprazole is well tolerated13,14 and is more often preferred by patients and caregivers.15 Therefore, aripiprazole may be appropriate for long-term treatment of patients with schizophrenia or schizoaffective disorder.
Our study had several limitations. First, it was retrospective in nature and undertaken in a single outpatient office setting. Generalizing these results to inpatient or outpatient settings in hospitals may not be appropriate. A prospective, multicenter, and naturalistic study needs to be conducted in order to confirm our findings. Second, we were unable to evaluate several cofactors that influence both the course of psychosis and medication adherence, such as years of treatment with other antipsychotics, age at onset of psychosis, duration of possible untreated psychosis, and neuropsychological performance. Third, the analysis in this study included not only monotherapy, but also adjunctive treatment. Strictly speaking, the time to discontinuation obtained in our study may not necessarily indicate effectiveness of second-generation antipsychotic drugs. Fourth, detailed reasons for treatment discontinuation were not evaluated. In a future study, the reasons for discontinuation should be classified according to lack of efficacy, intolerable adverse effects, or a decision by the patient to understand better the differences in effectiveness among second-generation antipsychotics. Fifth, in this study, continuation/discontinuation of treatment and adherence/ nonadherence were assessed by patient response and not by objective data such as antipsychotic blood levels. Recall bias and validity of information from patients may influence the evaluation of antipsychotic effectiveness.
Despite these limitations, our finding of differences in effectiveness among second-generation antipsychotics provides important information, helping clinicians to choose treatments that are appropriate for patients with schizophrenia or schizoaffective disorder.
Conclusion
We retrospectively evaluated the effectiveness of second-generation antipsychotics by comparing their time to discontinuation. Aripiprazole might be considered the best of the available options for long-term treatment of patients with schizophrenia or schizoaffective disorder.
Acknowledgment
The authors thank the staff at Shioiri Mental Clinic for their assistance with this project.
Footnotes
Disclosure
TA has received speaking fees from Astellas, Dainippon-Sumitomo, Eisai, Eli Lilly, Janssen, Meiji, Mochida, Pfizer, Otsuka, and Takeda. The other authors report no conflicts of interest in this work.
References
- 1.Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 2.Gentile S. A systematic review of quality of life and weight gain-related issues in patients treated for severe and persistent mental disorders: focus on aripiprazole. Neuropsychiatr Dis Treat. 2009;5:117–125. doi: 10.2147/ndt.s4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogarty GE, Schooler NR, Baker RW. Efficacy versus effectiveness. Psychiatr Serv. 1997;48:1107. doi: 10.1176/ps.48.9.1107. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 5.Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: A CATIE study. Am J Psychiatry. 2007;164:415–427. doi: 10.1176/ajp.2007.164.3.415. [DOI] [PubMed] [Google Scholar]
- 6.Coley KC, Fabian TJ, Kim E, et al. Predictors of aripiprazole treatment continuation in hospitalized patients. J Clin Psychiatry. 2008;69:1393–1397. doi: 10.4088/jcp.v69n0906. [DOI] [PubMed] [Google Scholar]
- 7.Gorwood P. Meeting everyday challenges: antipsychotic therapy in the real world. Eur Neuropsychopharmacol. 2006;16:5156–5162. doi: 10.1016/j.euroneuro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 9.Jordan S, Koprivica V, Chen R, et al. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 10.Hirose T, Uwahodo Y, Yamada S, et al. Mechanism of action of ariprazole predicts clinical efficacy and a favorable side-effect profile. J Psychopharmacol. 2004;18:375–383. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- 11.Chung AK, Chua SE. Effects on prolongation of Bazett’s corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011;25:646–666. doi: 10.1177/0269881110376685. [DOI] [PubMed] [Google Scholar]
- 12.Bergman F, Zacher A, Nass A, et al. Psychosocial functioning in patients with schizophrenia treated with aripiprazole – an office-based real-world setting. Results from the German post-marketing surveillance study. Pharmacopsychiatry. 2009;42:101–108. doi: 10.1055/s-0028-1112129. [DOI] [PubMed] [Google Scholar]
- 13.Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term placebo-controlled trials. Schizophr Res. 2003;61:123–136. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63:763–771. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- 15.Tandon R, Marcus RN, Stock EG, et al. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patient with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA) Schizophr Res. 2006;84:77–89. doi: 10.1016/j.schres.2005.12.857. [DOI] [PubMed] [Google Scholar]