Abstract
Background
Healing after acute myocardial infarction (AMI) is characterized by an intense inflammatory response and increased Interleukin-1 (IL-1) tissue activity. Genetically engineered mice lacking the IL-1 receptor (IL-1R1-/-, not responsive to IL-1) or the IL-1 receptor antagonist (IL-1Ra, enhanced response to IL-1) have an altered IL-1/IL-1Ra balance that we hypothesize modulates infarct healing and cardiac remodeling after AMI.
Methods
IL-1R1-/- and IL-1Ra-/- male mice and their correspondent wild-types (WT) were subjected to permanent coronary artery ligation or sham surgery. Infarct size (trichrome scar size), apoptotic cell death (TUNEL) and left ventricular (LV) dimensions and function (echocardiography) were measured prior to and 7 days after surgery.
Results
When compared with the corresponding WT, IL-1R1-/- mice had significantly smaller infarcts (−25%), less cardiomyocyte apoptosis (−50%), and reduced LV enlargement (LV end-diastolic diameter increase [LVEDD], −20%) and dysfunction (LV ejection fraction [LVEF] decrease, −50%), whereas IL-1Ra-/- mice had significantly larger infarcts (+75%), more apoptosis (5-fold increase), and more severe LV enlargement (LVEDD increase,+30%) and dysfunction (LVEF decrease, +70%)(all P values <0.05).
Conclusions
An imbalance in IL-1/IL-1Ra signaling at the IL-1R1 level modulates the severity of cardiac remodeling after AMI in the mouse, with reduced IL-1R1 signaling providing protection and unopposed IL-1R1 signaling providing harm.
Introduction
Healing and cardiac remodeling after acute myocardial infarction (AMI) are characterized by an intense inflammatory response within the myocardium. Interleukin-1β (IL-1β) is a potent pro-inflammatory mediator with local and systemic effects mediated by the IL-1 type I receptor (IL-1R1), the only signaling membrane receptor for IL-1β [1]. Genetically engineered mice lacking the gene encoding for the IL-1 type I receptor (IL-1R1-/- mice) are not responsive to IL-1β or IL-1α, but have an otherwise normal phenotype and remain responsive to other pro-inflammatory stimuli such as lipopolysaccharide (LPS) and Tumour Necrosis Factor-α [1]–[2].
Interleukin-1 receptor antagonist is a natural occurring protein that competitively inhibits IL-1β (and IL-1α) signalling, but fails to recruit the IL-1 receptor associated protein and therefore does not transduce signal [1]–[3]. Mice with genetic deletion of the gene encoding for IL-1Ra (IL-1Ra-/-mice) have enhanced response to IL-1, are more susceptible to Listeria monocytogenis infections, and may develop spontaneously occurring inflammatory arthritis and arteritis [4]–[6]. However, no gross cardiac abnormalities have been reported in these mice.
In many conditions, the balance between IL-1β and IL-1Ra at the receptor level determines the inflammatory activity and severity of the disease [3], [7]. IL-1 is known to influence ischemia, heart failure, and cardiac remodeling after acute myocardial infarction in the mouse [8]–[10]. In order to demonstrate the pivotal role of IL-1 signaling on cardiac remodeling after AMI, we studied two independent strains of mice with genetic modifications that alternatively enhanced IL-1 signaling (IL-1Ra deletion, IL-1Ra-/-) or suppressed IL-1 signaling (IL-1R1-/-) in a model of severe ischemic cardiomyopathy due to permanent coronary artery ligation.
Methods
Experimental design
IL-1R1-/- male mice (strain B6.129S1-Il1r1tm1Roml/J) and the corresponding wild-type adult male mice (strain B6.129SF2J) were purchased from Jackson Laboratory (Maine, USA)[11].
IL-1Ra-/- male mice (strain B6.129S-IL-1RNtm1Dih/J) were also purchased from Jackson Laboratory [12] and wild-type adult male mice (strain C56BL/6J) were used as recommended by the supplier in consideration of the backcrossing of the B6-129 generated mouse into the C56BL line [12].
All animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996). The study was approved by the Institutional Animal Care and Utilization Committee (IACUCU) of the Virginia Commonwealth University (AM20114). Mice were received at variable ages. Upon their arrival, all animals were allowed to readjust to the housing environment for at least 7 days before any experiment, with freely accessible standard rodent food and water. All mice were between 14 and 16 weeks at time of surgery.
AMI by permanent left coronary artery ligation was induced in 4 groups of mice (IL-1R1-/- and B6.129 WT mice; IL-1Ra-/- and C57Bl WT mice)(N = 10–12 per group), while 4 additional groups of mice (N = 5) underwent a sham operation including every step except the coronary artery ligation. The mice were then allowed to recover for 7 days.
Surgical procedure
The animals under anesthesia (pentobarbital 50–70 mg/Kg) were orally intubated, and then underwent opening of the chest and ligation of the left coronary artery (or sham surgery, in which the needle was passed around the artery but it was not ligated), as previously described [8].
Histology
For immunofixation, animals were sacrificed at 7 days and the heart was then mounted on a Langendorff apparatus. The coronary arteries were perfused with 10% formalin and subsequently the whole heart was submersed in the same solution. After fixation, the mid-ventricular section of each heart was cut (approx. 3 mm thickness) and then the samples were dehydrated and included in paraffin.
The infarct scar size was measured as scar area in sections stained with Masson's trichrome (Sigma-Aldrich, St Louis, Mo) by computer morphometry using a BIOQUANT imaging software and expressed as a percentage of the whole left ventricle mid-ventricular section.
Nuclear DNA fragmentation (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling–ApopTag, Chemicon) was used as a marker for apoptosis. Only cardiomyocytes (cardiac actin+) expressing TUNEL staining in the nucleus were counted in the peri-infarct area, defined as the zone bordering the infarct where viable myocardium was prevalent and reparative fibrosis was only marginal, and in the remote unaffected myocardium. The apoptotic rate (AR) is expressed as the number of apoptotic cardiomyocytes on all cardiomyocytes per field, analysing 5 of more fields covering the entire infarct and border zones in the mid-ventricular section. The pathologist performing the count was unaware of the mouse genotype or surgical procedure performed.
Echocardiography
Transthoracic echocardiography was performed before surgery and 7 days later, using the Vevo770™ imaging system (VisualSonics Inc., Toronto, Canada), equipped with a 30 MHz probe, as previously described [8]. Briefly, the heart was imaged in the short-axis view of the LV. The M-mode cursor was positioned perpendicular to the anterior wall in order to measure left ventricular end-diastolic and end-systolic diameters (LVEDD and LVESD, respectively) at the level of the papillary muscles below the mitral valve tip. LV ejection fraction (LVEF) and mass were calculated. The investigator performing and reading the echocardiogram was blinded to the mouse genotype and surgical procedure performed.
Statistical analysis
Data are presented as mean and standard error of the mean. Comparisons between the genetically modified mice (IL-1R1-/- or IL-1Ra-/-) and the corresponding WT at a single time point was performed using the Student T test for unpaired data. In order to compare changes over time between 2 groups, ANOVA for repeated measures was using analyzing the effect of time_x_interaction. SPSS 16.0 package was used. Two-tail significance was set at 0.05.
Direct comparison between the IL-1R1-/- and IL-1Ra-/- mice was not possible considering that 2 strains had different genetic background (B6.129 and C57Bl, respectively), therefore in order to visually compare the IL-1R1-/- and IL-1Ra-/- mice, the values of LVEDD, LVESD, LVEF, infarct size and apoptosis was normalized to the corresponding WT, and expressed as% of WT.
Results
Cardiac morphology
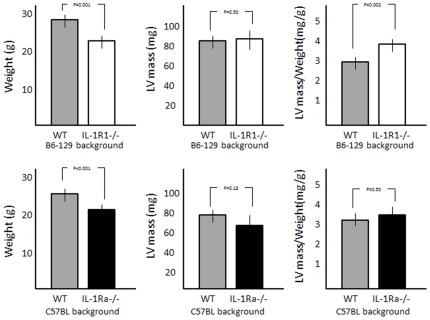
IL-1R1-/- mice had significantly smaller body weight but similar LV mass reflecting a significantly increased LV mass to body weight ratio (Figure 1). IL-1Ra-/- mice also had significantly smaller body weight, but similar LV mass and similar LV mass to body weight ratio (Figure 1).
Figure 1. Body weight and left ventricular mass in IL-1R1-/- and IL-1Ra-/- mice.
Genetically modified mice (both IL-1R1-/- and IL-1Ra-/- ) have significantly smaller body weights compared with the respective wild-type animals (WT). Left ventricular (LV) mass calculated at echocardiography was not statistically different comparing IL-1R1-/- and IL-1Ra-/- and respectively WT, but the IL-1R1-/- had significantly greater LV mass/weight ratio. N = 8–10 per group.
Survival after surgery
Two of 12 (17%) of IL-1R1-/- mice and 5 of 14 (36%) of the corresponding WT mice died after AMI surgery. Eight of 15 (54%) IL-1Ra-/- mice died after coronary artery ligation surgery compared to 4 of 12 (33%) of the corresponding WT mice. Free wall cardiac rupture was found in 2 IL-1Ra-/- mice (13%), none of the IL-1R1-/- mice, and none of the WT mice. None of the sham operated animals died. None of the differences reached the statistical significance set at a P of 0.050.
Infarct scar size
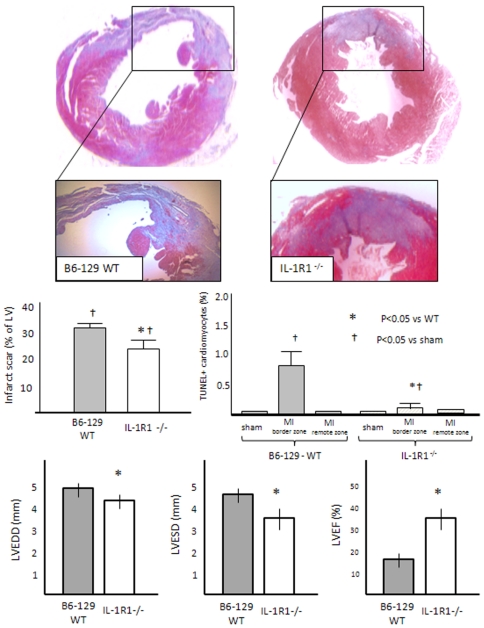
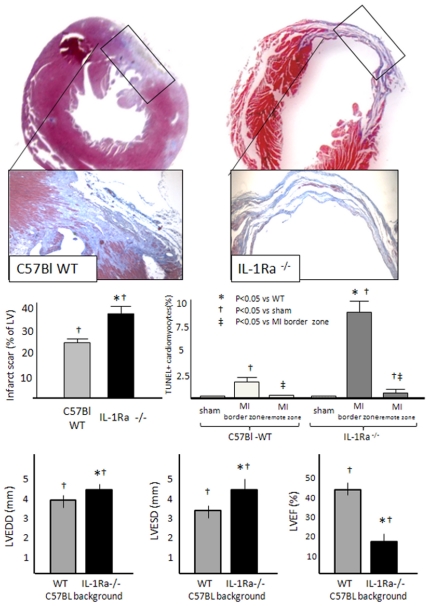
Infarct scar size was measured 7 days after AMI surgery. Figures 2 and 3 show representative images from the AMI groups for the IL-1R1-/- and IL-1Ra-/-, respectively. When compared with the corresponding WT mice, IL-1R1-/- mice had a significantly smaller infarct size, whereas IL-1Ra-/- had a significantly larger infarct size. Sham operated mice had no measurable infarct (data not shown).
Figure 2. Infarct size, apoptosis and left ventricular remodeling in wild-type and IL-1R1-/- mice.
When compared with the corresponding wild-type mice, infarct scar size (Masson's trichrome stain) and cardiomyocyte apoptotic rate (in-situ end labelling of DNA fragmentation–TUNEL) were significantly smaller in IL-1R1-/- mice. Moreover, IL-1R1-/- mice had less left ventricular enlargement and systolic dysfunction 1 week after AMI. N = 6–8 per group.
Figure 3. Infarct size, apoptosis and left ventricular remodeling in wild-type and IL-1Ra-/- mice.
When compared with the corresponding wild-type mice, infarct scar size (Masson's trichrome stain) and cardiomyocyte apoptotic rate (in-situ end labelling of DNA fragmentation–TUNEL) were significantly greater in IL-1Ra-/- mice. Moreover, IL-1Ra-/- mice had less left ventricular enlargement and systolic dysfunction 1 week after AMI. N = 6–8 per group.
Cardiomyocyte apoptosis
Cardiomyocyte apoptosis was significantly greater in the peri-infarct myocardium versus the remote myocardium and versus the anterior wall in sham operated animals in all 4 groups. When compared with the corresponding WT mice, IL-1R1-/- mice had significantly less cardiomyocyte apoptosis in the peri-infarct myocardium (Figure 2), whereas IL-1Ra-/- mice had significantly more cardiomyocyte apoptosis both in the peri-infarct and remote myocardium (Figure 3).
Left ventricular enlargement and systolic dysfunction
AMI lead to LV enlargement and systolic dysfunction versus sham operated mice (LVEDD, LVESD and LVEF–P<0.001). When compared with the corresponding WT mice, IL-1R1-/- mice had significantly less LV enlargement (measured as LVEDD and LVESD) and less LV systolic dysfunction (measured as LVEF)(Figure 2), whereas IL-1Ra-/- mice had worse LV enlargement and dysfunction (Figure 3).
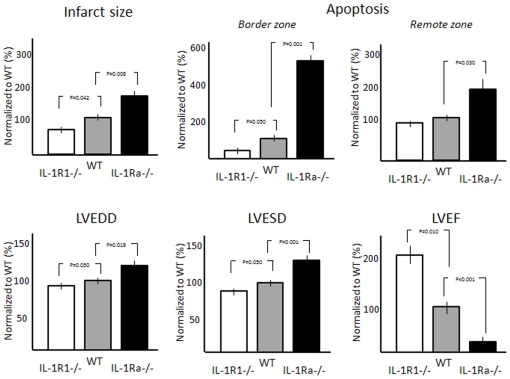
Indirect comparison between IL-1R1-/- and IL-1Ra-/- mice
When data from IL-1R1-/- and IL-1Ra-/- mice were normalized to the WT, the WT mice displayed an intermediate phenotype between the IL-1R1-/- mice–which were protected–and the IL-1Ra-/- mice in which heart damage was exacerbated (Figure 4).
Figure 4. Indirect comparison between the IL-1R1-/- and the IL-1Ra-/-.
Considering that IL-1R1-/- and the IL-1Ra-/- mice were generated in different backgrounds, a direct comparison between the 2 strains cannot be made. The Figure shows an indirect comparison between the 2 strains in which the value of each strain is normalized to the corresponding wild-type mouse. In the comparison, the IL-1R1-/- mouse shows to have smaller infarcts, less apoptosis and more favorable remodeling, wheras the IL-1Ra-/- mouse shows large infarcts, more apoptosis, and more unfavorable remodeling.
Discussion
IL-1 is considered a key inflammatory mediator in the response to sterile inflammation such as acute myocardial infarction. By using 2 genetically modified mouse strains, the current study shows that the lack of IL-1R1 signaling or the unopposed IL-1R1 signaling (due to lack of the IL-1Ra) is sufficient to modulate the natural course of post-AMI cardiac remodeling.
In this mouse model of severe ischemic cardiomyopathy, the lack of signaling through the IL-1R1 signaling post-AMI is associated with a more favorable remodeling. This is an agreement with a prior study showing more favorable remodeling following myocardial ischemia-reperfusion injury due to transient coronary occlusion [13].
The results of these experiments also show that endogenous IL-1Ra plays a protective role in the myocardium during AMI by preventing cell death and ensuing adverse cardiac remodeling. While the data presented are in accordance with the proposed cardioprotective effects of IL-1Ra in myocardial ischemia and infarction [8], [14], the current study shows for the first time that the lack of endogenous IL-1Ra leads to more adverse remodeling. Previous experiments showed that IL-1Ra transgenic mice, in which IL-1Ra is overexpressed in fibroblasts, subjected to global cardiac ischemia-reperfusion surgery simulating heart transplantation had a smaller extent of subendocardial infarct and reduced apoptosis in cardiomyocytes [14]. Moreover, supplementation of recombinant human IL-1Ra to the mouse lead to improved cardiac remodeling after AMI [8].
These findings reinforce the concept that modulation of IL-1 is a viable target for interventions aimed at preventing heart failure after AMI, and, within the challenges and limitations of preclinical and translational studies [15], they provide the frame to interpret studies using pharmacologic IL-1 blockers in patients with AMI [16]. With the exception of one study using a hamster anti-mouse IL-1β antibody (not commercially available) which showed adverse remodeling and increased cardiac rupture [17], all other studies using either a recombinant human IL-1Ra [8], a recombinant fusion protein binding circulation IL-1β and IL-1α (IL-1Trap)[9], or a mouse engineered anti-IL-1β antibody (XMA052 MG1K)[10], have shown a more favorable remodeling associated with IL-1 blockade and no increase in the incidence of cardiac rupture. All together, the data deriving from the genetically manipulated mice and the more recent studies using IL-1 blockers argue against the concept that IL-1β is necessary for infarct healing.
During AMI, tissue injury leads to an intense sterile inflammation that is likely to be initially induced by toll-like receptor (TLR) signaling by the many danger-associated molecular patterns generated during cell death. TLR agonists induce a powerful activation of nuclear factor κ-B and induction of numerous inflammatory mediators that recruit and activate leukocytes within the injured myocardium. IL-1 is certainly among these soluble mediators that greatly amplifies the inflammatory response in a paracrine and autocrine fashion (“IL-1 induces IL-1”)[18]. Pharmacologic IL-1 blockade (or genetic deletion of the IL-1R1) does not impede TLR signaling allowing for the initial inflammatory response to injury, but may limit the occurrence of additional tissue injury induced by IL-1. Cardiomyocytes in the border zones of the AMI synthesize IL-1Ra to protect themselves from IL-1 mediated injury. Mice lacking IL-1Ra lack this endogenous protective mechanism and hence their cardiomyocytes (and other cell types) are at higher risk of death. A clinical syndrome in children characterized by life-threatening systemic inflammation (with particular involvement of skin and bone involvement) has been linked to the genetic deficiency of the IL-1Ra (DIRA), and has been found to be responsive to exogenous IL-1Ra [19]. The DIRA syndrome, in which endogenous IL-1Ra is lacking, is similar to syndromes of enhanced IL-1β production due to genetic alterations of the cryopyrin gene (Cryopyrin-associated periodic syndromes [CAPS]), which are also responsive to exogenous IL-1Ra [20], thus confirming the key role of IL-1/IL-1Ra balance in disease activity.
One major limitation of the current study is that the IL-1R1-/- and IL-1Ra-/- strains were generated in 2 different mouse lines and hence a direct comparison between the 2 mutants cannot be performed. Indeed, mouse strain may determine significant differences in the outcome of wound healing after myocardial infarction [21]. With the use of indirect comparison after normalization to the respective wild-type mouse, we could show that a gradient of severity was present going from the IL-1R1-/- mice through the WT and to the IL-1Ra-/- mice. Moreover, the WT used in each study were not littermates because not commercially available.
The relatively small number of mice studies makes it unable to have reliable data on survival and on cardiac rupture. We noticed a small increase in cardiac rupture in IL-1Ra-/- mice but it did not reach statistical significance. Moreover, the lack of data regarding leukocyte density and activation and MMP activation does not allow us to determine whether enhanced MMP activation in IL-1Ra-/- is the cause of cardiac rupture.
Finally, no data on right ventricular dimensions, function or pathology was recorded. Defining whether IL-1 is involved in biventricular remodeling [22]–[23] could have further improved our understanding of heart failure.
The mechanisms by which IL-1 signaling at the IL-1R1 level drives adverse cardiac remodeling is not completely clear and have not been explored in the current study. In vitro, IL-1 signaling modulates cardiomyocyte survival independent of the function of leukocytes [8], [24]. IL-1 upregulates caspase-1 activity which has been shown to promote cell death in cardiomyocytes [24]–[27]. In vitro, IL-1Ra has been shown to promote cell survival and to inhibit caspase-1 and caspase-9 [8]. Whether IL-1Ra promotes cell survival in ways other than competing with IL-1β at the IL-1R1 levels is unknown, and requires further studies.
We chose the model of non-reperfused AMI because although most of the patients with AMI receive some form of intervention aimed at obtaining reperfusion, presently many patients achieve incomplete tissue level reperfusion (no reflow), which negates the benefit of reperfusion and is associated with an even greater risk of subsequent heart failure [28]–[29].
In conclusion, modification of IL-1 signaling determining an imbalance between IL-1 and IL-1Ra at the IL-1R1 level determines the severity of cardiac remodeling after AMI in the mouse, with reduced IL-1R1 signaling providing protection and unopposed IL-1R1 signaling providing harm.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by an American Heart Association Beginning Grant-in-Aid (Mid-Atlantic Affiliate) to Dr. Abbate. Dr. Salloum is supported by an American Heart Association Scientist Development Grant. Dr. Van Tassell is supported by an institutional KL2RR031989-01. The funding sources had no role in determining the study design nor did they participate in data analysis or reporting.
References
- 1.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory disease. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 3.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71–77. doi: 10.1111/j.1365-2249.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 9.Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, et al. Interleukin-1 trap attenuates cardiac remodeling following experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]
- 10.Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, et al. Interleukin-1β modulation using a genetically engineered antibody prevents adverse cardiac remodeling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 11.The Jackson Laboratory website. Available: http://jaxmice.jax.org/strain/003018.html. Accessed 2011 May 31.
- 12.The Jackson Laboratory website. Available: http://jaxmice.jax.org/strain/004754.html. Accessed 2011 May 31.
- 13.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;3:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, et al. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104(12 Suppl 1):I308–I313. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Baxter G, Bell R, Bøtker HE, Davidson SM, et al. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 17.Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, et al. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol. 2001;38:1546–1553. doi: 10.1016/s0735-1097(01)01591-1. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 19.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep. 2010;10:229–235. doi: 10.1007/s11882-010-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, et al. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 22.Abbate A, Bussani R, Sinagra G, Barresi E, Pivetta A, et al. Right ventricular cardiomyocyte apoptosis in patients with acute myocardial infarction of the left ventricular wall. Am J Cardiol. 2008;102:658–662. doi: 10.1016/j.amjcard.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toldo S, Bogaard HJ, Van Tassell BW, Mezzaroma E, Seropian IM, et al. Right ventricular dysfunction following acute myocardial infarction in the absence of pulmonary hypertension in the mouse. PLoS One. 2011;6:e18102. doi: 10.1371/journal.pone.0018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- 25.Syed FM, Hahn HS, Odley A, Guo Y, Vallejo JG, et al. Pro-apoptotic effects of caspase-1/interleukin-converting enzyme dominate in myocardial ischemia. Circ Res. 2005;96:1103–1109. doi: 10.1161/01.RES.0000166925.45995.ed. [DOI] [PubMed] [Google Scholar]
- 26.Merkle S, Frantz S, Schön MP, Bauersachs J, Buitrago M, et al. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 27.Frantz S, Ducharme A, Sawyer D, Rohde LE, Kobzik L, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 28.Abbate A, Kontos MC, Biondi-Zoccai GG. No-reflow: the next challenge in treatment of ST-elevation acute myocardial infarction. Eur Heart J. 2008;29:1795–7. doi: 10.1093/eurheartj/ehn281. [DOI] [PubMed] [Google Scholar]
- 29.Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]