Abstract
Spinal cord injury increases inhibitory factors that may restrict neurite outgrowth after trauma. The expression of repulsive molecules in reactive astrocytes and the formation of the glial scar at the injury site produce the non-permissive environment for axonal regeneration. However, the mechanism that triggers this astrogliotic response is unknown. The release of nucleotides has been linked to this hypertrophic state.
Our goal is to investigate the temporal profile of P2Y2 nucleotide receptor after spinal cord injury in adult female Sprague–Dawley rats. Molecular biology, immunofluorescence studies, and Western Blots were used to evaluate the temporal profile (2, 4, 7, 14, and 28 days post-injury) of this receptor in rats injured at the T-10 level using the NYU impactor device.
Real time RT-PCR showed a significant increase of P2Y2 mRNA after 2 days post-injury that continues throughout 28 days post-injury. Double labeling studies localized P2Y2 immunoreactivity in neuronal cell bodies, axons, macrophages, oligodendrocytes and reactive astrocytes. Immunofluorescence studies also demonstrated a low level of P2Y2 receptor in sham samples, which increased after injury in glial fibrillary acidic protein positive cells. Western Blot performed with contused spinal cord protein samples revealed an upregulation in the P2Y2 42 kDa protein band expression after 4 days post-injury that continues until 28 days post-injury. However, a downregulation of the 62 kDa receptor protein band after 2 days post-injury that continues up to 28 days post-injury was observed.
Therefore, the spatio-temporal pattern of P2Y2 gene expression after spinal cord injury suggests a role in the pathophysiology response generated after trauma.
Keywords: Astrogliosis, Nucleotide receptor, Regeneration, Glial scar, Trauma, Purinergic
1. Introduction
Spinal cord injury (SCI) leads to a complex series of cellular and molecular events including axotomy, demyelination of surviving axons, nucleotides release, free-radical production, and release of excitatory amino acids (Franke et al., 2006; Tanhoffer et al., 2007). These events are followed by neural tissue loss due to necrosis, apoptosis, macrophage infiltration, and inflammation (Dijkstra et al., 2001; Hulsebosch, 2002; Iannotti et al., 2006; Popovich et al., 2003). In addition, the downregulation of growth factors (Nakamura and Bregman, 2001; Widenfalk et al., 2001), and the upregulation of repulsive factors (Fournier and Strittmatter, 2001) are among the causes that contribute to the production of a non-permissive environment for axonal regeneration and functional locomotor recovery. However, the adult central nervous system (CNS) has the plasticity to promote axonal elongation after trauma, when a supportive substrate and a permissive environment are provided. Inhibitory molecular signals of myelin origin, MAG, NOGO, and Omgp are the neurite outgrowth inhibitors most recognized and studied (McKerracher et al., 1994; Schnell and Schwab, 1990; Wang et al., 2002). Other repulsive factors that block axonal regeneration after SCI are proteoglycans, semaphorin-3, Slit proteins and Eph/ephrins (Silver and Miller, 2004; Willson et al., 2002). Most of the repulsive factors are expressed in glial cells, in particular, oligodendrocytes and reactive astrocytes.
The astrocytes are involved in neural development, synaptic activity and homeostasis of the extracellular environment in the CNS (Fields and Stevens-Graham, 2002; Laird et al., 2008). During injury or trauma to the CNS, the astrocytes go through reactive gliosis or astrogliosis. This cellular process is distinguished for the upregulation of glial fibrillary acidic protein (GFAP), hypertrophy, and proliferation of astrocytes at the lesion site. Chondroitin sulfate proteoglycans (Lemons et al., 1999), tenascin-R (Becker et al., 2000), keratin (Canning et al., 1996) and Eph receptors (Cruz-Orengo et al., 2006; Figueroa et al., 2006; Irizarry-Ramirez et al., 2005; Miranda et al., 1999) are among the repellent proteins expressed by astrocytes after been reactive or activated. In addition, the reactive astrocytes form a glial scar that constitutes a physical and chemical barrier for axonal regeneration across the lesion site. On the other hand, this wall separates healthy from injured cells and limits the extension of the cyst at the lesion epicenter (Busch and Silver, 2007). The molecular events that trigger this gliotic response after injury are unknown but nucleotides release (like ATP) has been linked to this hypertrophic state (Neary et al., 2006).
Several nucleotide or purinergic receptors have been cloned, characterized and their molecular structures determined. The P1 adenosine receptors are G-protein coupled receptors. They are classified into four types: A1, A2A, A2B and A3, and act through adenylate cyclase. On the other hand, nucleotides like ATP and UTP activate two different types of receptors called P2X (ionotropic) and P2Y (metabotropic) receptors. The P2X are ion gated channels which lead to a fast calcium influx and P2Y are G-protein coupled receptors which act through an inositol trisphosphate pathway that increases intracellular calcium by release from internal stores (Burnstock, 1997).
Astrocytes express several P2 receptor subtypes (Lenz et al., 2000), including P2Y2 receptors whose activation stimulates cell proliferation, migration, and communication (Weisman et al., 2005). This receptor has been implicated in the astrocytic response to brain trauma (Wang et al., 2005). In addition, studies in human 1321N1 astrocytoma cells expressing a recombinant P2Y2 receptor suggest an important role in survival and neuroprotective mechanisms under pathological conditions (Chorna et al., 2004). Previous reports confirmed an upregulation in the expression of P2Y2 on cortical and nucleus accumbens astrocytes in vivo after stab wound injury, associating astrogliosis with ATP release and receptor activation (Franke et al., 2004). Moreover, a non-selective nucleotide antagonist, like suramin, disrupts the gliotic response after injury suggesting a role of nucleotide receptors in the reactive gliosis initiated by trauma to the CNS (Di Prospero et al., 1998). All this evidence supports a role for nucleotide receptor expression and activation in the process of astrogliosis after CNS trauma.
The expression profile of P2Y2 receptor and the role that these molecules may play in the non-permissive environment generated by reactive astrocytes after SCI is unknown. Regenerating axons may recognize those inhibitory molecular signals expressed in gliotic astrocytes after their induction by nucleotide receptors, thus blocking axonal elongation at the injury site. Therefore, the focus of this project is to analyze the spatio-temporal expression of P2Y2 receptors in cells of the spinal cord after trauma. Moreover, P2Y2 receptors present novel targets to control reactive astrogliosis because blockade of these receptors may diminish the gliotic response; reducing the repulsive mechanisms from protein expressed by glial cells.
2. Materials and methods
2.1. Spinal cord injury
Adult female Sprague–Dawley rats (~235 g) from Hilltop Lab (Scottdale, PA) were anesthetized with a cocktail of 87.7 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA), 4.2 mg/kg xylazine (Boehringer Ingelheim, Ridgefield, CT), and 0.85 mg/kg acepromazine (Vetus Animal Health, Rockville Center, NY) via intraperitoneal injection. Rats under anesthesia were kept at 37 °C on a heating blanket to prevent hypothermia. To monitor appropriate sedation, the degree of responsiveness to rat’s tail and toe pinch was observed. Each rat received a T-10 laminectomy after a dorsal incision to expose the vertebrae. Rats in the injured group had their spinal cord compressed using the NYU Impactor device by adjusting the impactor (10 g) to a height of 12.5 mm as previously described (Cruz-Orengo et al., 2006; Figueroa et al., 2006). Sham control rats remained uncontused and were sutured after laminectomy. Rats received two daily doses of the antibiotic cefazolin (25 mg/kg, Bristol Myers Squibb, NY) subcutaneously for 7 consecutive days, including the day of surgery. In addition, two daily doses of Buprenex (buprenorphine; Reckett & Colman Pharmaceuticals, Inc., Richmond, VA; 0.05 mg/kg) for 3 days were administered for analgesia. Animals were hydrated with 0.9% NaCl solution, administered subcutaneously after surgery and during postoperative care when needed (≈1 cm3 per 5 g weight loss). Bladders of injured rats were expressed at least three times daily until complete recovery, usually after 7 days. Animals were housed individually on cages with absorbent bedding. Both sham and contused groups were allowed to survive for 2, 4, 7, 14 and 28 days post-injury (DPI). These procedures were approved by the University of Puerto Rico IACUC and followed NIH guidelines for the safe use and care of laboratory animals.
2.2. RNA studies
Sham and injured rats (n = 3 for each time point studied) were anesthetized by intraperitoneal administration of Pentobarbital (40–50 mg/kg) and transcardially perfused with ice-cold 0.01 M phosphate-buffered saline (PBS), pH 7.4 (Sigma–Aldrich, St. Louis, MO) as described by Irizarry-Ramirez et al. (2005). The epicenters (5 mm) of each spinal cord were dissected from the lesion site and total RNA extracted using Trizol (Sigma–Aldrich, Inc., St. Louis, MO). The extracted RNA was treated with DNAse I (Ambion DNA-free kit; Ambion Inc., Austin, TX) to avoid genomic contamination. Integrity of each sample was electrophoretically verified in a 1% agarose–formaldehyde gel and quantification of total RNA was performed using the Eppendorf BioPhotometer system (Eppendorf, AG). Reverse transcription reaction of 1 μg of RNA was performed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Mock cDNA was prepared and used as negative control to assess the possibility of genomic contamination.
2.3. Real time RT-PCR
Real time RT-PCR assay was performed as previously described by Silva et al. (2005) with some modifications to determine P2Y2 mRNA expression. P2Y2 and GAPDH primer sequences (Table 1) were designed using Beacon Designer 6 software (Premier Biosoft International, Palo Alto, CA) and manufactured by Integrated DNA Technologies, Inc. (Coralville, IA). The reactions were performed in an iCycler (Bio-Rad Laboratories, Hercules, CA) using the iQ SYBR Green Supermix (Bio-Rad, CA) as a fluorescent dye. After optimization of RT-PCR conditions, reactions were conducted with SYBR Green master mix, 10 μM forward/reverse primers and 100 ng of each cDNA sample. P2Y2 primers’ amplification curve was done using the following parameters: a hot-start at 95 °C for 3 min and 40 cycles: 95 °C denaturing step for 30 s, 1 min annealing at 55.3 °C and an extension at 72 °C for 1 min. GAPDH was used as a housekeeping gene to demonstrate specificity of the changes taking place in the spinal cord after injury and the annealing temperature used was 62.1 °C. Products generated were confirmed by melt curves and migration to the expected position on a 1.5% agarose gel electrophoresis stained with ethidium bromide. The PCR products were purified with the QIA quick PCR purification kit (QIAGEN Inc., CA) and sequenced to confirm the identity of the products.
Table 1.
P2Y2 and GAPDH’s real time RT-PCR primer sequences.
2.4. Immunofluorescence
Anesthetized rats (n = 3) were perfused intracardially with ice-cold 0.01 M PBS (pH 7.4; Sigma–Aldrich, St. Louis, MO) followed by 4% paraformaldehyde (PFA) at 4 °C. Spinal cords were removed and post-fixed in 4% PFA at 4 °C for 2 h and finally equilibrated in 30% sucrose at 4 °C overnight (ON). The spinal cords were mounted in tissue blocks with tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) and sectioned (20 μm) using a Leica cryostat cryocut 1800 (Nussloch, Germany); then stored at −20 °C. To begin with the immunofluorescence (IMF), the tissue was dried for at least 10 min at room temperature (RT) and delineated with a PAP PEN (Ted Pella INC., Redding, CA). The sections were placed in a humid chamber and washed with 0.01 M PBS for 5 min at RT. Then, they were post-fixed in 4% PFA at 4 °C for 10 min followed by three washes of 0.01 M PBS for 10 min each at RT. Next, the samples were washed with 100 mM NH4Cl/PBS at RT for 10 min followed by three washes (10 min each) with 0.01 MPBS. Subsequently, 0.1% Triton X-100/PBS was added to the sections for 20 min. After blocking for 2 h with 2% BSA/PBS at RT, the sections were incubated ON at 4 °C with rabbit polyclonal IgG anti-P2Y2 antibody (1:50; (H-70) Santa Cruz Biotechnology) or a mixture of anti-P2Y2 with mouse anti-GFAP (1:100; BD Pharmingen), mouse anti-NeuN (1:100; Chemicon, CA), mouse anti-CD68:Biotin (1:500; Serotec, Raleigh, NC), mouse anti-MAB 328 (1:500; Chemicon, CA), or mouse anti-NF-H (1:1000; Chemicon, CA); cell-specific markers for astrocytes, neurons, macrophages, oligodendrocyte, and axons, respectively. Then, the sections were washed three times with 0.01 M PBS for 10 min each and incubated with 1:100 Rhodamine Red Donkey anti-rabbit (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) and 1:250 Alexa 488 goat anti-mouse (Invitrogen) for 2 h at RT in a covered area. Sections were rinsed three times (10 min each) in PBS and coverslipped with Slow Fade Antifade Kit (Molecular Probes, Invitrogen, Oregon). Slices treated without primary antibody were used as controls. Images were visualized with a Zeiss LSM 5 PASCAL Confocal Microscope System (Carl Zeiss MicroImaging Inc., Thornwood, NY) equipped with an argon laser emitting at 488 nm and a helium/neon laser emitting at 543 nm.
2.5. Protein extraction
Sham and injured rats (n = 4–5 for each time point studied) were anesthetized and perfused with ice-cold 0.01 M PBS as described previously. Then 5 mm of lesion epicenter was removed and homogenized in 300 μl of ice-cold lysis buffer I (pH 8) containing 20 mM Tris, 1 mM EDTA, 150 mM NaCl, 1 mM EGTA, 5 mM NaF with the following proteases and phosphatase inhibitors: antipain (2 μg/ml), aprotinin (10 μg/ml), benzamidine (5 mM), DTT (1 mM), leupeptin (10 μg/ml), Na3VO4 (1 mM), PMSF (1 mM), trypsin inhibitors (10 μg/ml). An additional, 700 μl of lysis buffer I was added and mixed for 30 s. The mixture was centrifuged (Eppendorf Centrifuge 5417R, Brinkmann Instruments Inc., NY) at 20,817 × g for 90 min at 4 °C. The supernatant was kept at 4 °C until protein concentration determination.
2.6. Determination of protein concentration
Protein sample (2 μl) was diluted in 798 μl of distilled water. 200 μl of Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories Inc., CA) was added to each test tube and vortex. After incubation at RT for at least 5 min, protein concentrations were measured at 595 nm in a DU-70 Spectrophotometer (Beckman Instruments Inc., CA) in duplicate. A standard curve (0–10 mg/ml) using Albumin Standard (Thermo Scientific, Rockford, IL) was generated.
2.7. Western Blot analysis
The extracted proteins (12.5 μg) were separated electrophoretically on 7.5% resolving acrylamide gel using a Mini PROTEAN 3 Cell electrophoresis unit (Bio-Rad, CA) for approximately 50 min at 200 V. Before the transfer, the Immun-Blot PVDF membrane (Bio-Rad, CA) was wetted in 100% methanol (Fisher Scientific, NJ), then equilibrated for 20 min at RT in ice-cold transfer buffer (20% of methanol, 2.5 mM Tris, 19.2 mM glycine, and 0.01% SDS, pH 8.3). The gel with the separated proteins was also equilibrated in the same transfer buffer. The proteins were transferred to the PVDF membrane ON at 4 °C using the Bio-Rad transfer unit at 15 V. The membrane was washed three times (10 min each) with a solution containing 0.4% Tween-20 (Bio-Rad, CA) in PBS (Bio-Rad, CA). After blocking the PVDF membrane with 3% Blotto (3% Instant Nonfat Dry Milk, 10 mM Tris, 100 mM NaCl, 0.5% Tween-20, pH 7.5) for 1 h, it was exposed to primary polyclonal rabbit anti-P2Y2 antibody (1:200; Alomone Laboratories, Israel), rabbit anti-GAPDH (1:5000; Sigma, MO) or a mixture of polyclonal rabbit anti-P2Y2 antibody and P2Y2 peptide residues 227–244 for preabsorption (Alomone, Israel; accession number P41232) for 2 h at 37 °C. Subsequently, the membrane was washed three times (10 min each) with blocking solution and then incubated with secondary anti-rabbit IgG Peroxidase Conjugate antibody (1:5000; Sigma, MO) for 1 h at RT. After rinsing two times with blocking solution (10 min each) and four times (10 min each) with a solution containing 10 mM Tris, 100 mM NaCl and 0.1% Tween-20, pH 7.5; the membrane was developed with Super Signal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions to enhanced chemiluminescence. The Versa Doc Imaging System and Quantity One Software (Bio-Rad, CA) was used for detection and analysis of the developed membranes. Levels of P2Y2 protein were standardized with GAPDH at each time point.
2.8. Statistical analysis
Data are presented as mean±standard error. ANOVA, followed by Student–Newman–Keuls multiple comparisons post hoc test analysis was used for evaluation of control (sham) and experimental groups (animals from 2, 4, 7, 14, and 28 DPI). A probability <0.05 between control and experimental groups was considered to be statistically significant with three to five animals per time point.
3. Results
3.1. Upregulation of P2Y2 mRNA expression after SCI
Expression of P2Y2 mRNA in the adult spinal cord of rats was assessed by real time RT-PCR using specific primers (Fig. 1). The amplified fragment migrated to the expected position of 119 bps confirming the presence of P2Y2 in the adult spinal cord. The specificity of the primer and the identity of the amplified fragments were determined by sequencing of the PCR products (data not shown). Time course studies were performed in sham and contused spinal cords at 2, 4, 7, 14 and 28 DPI. The expression profile of P2Y2 mRNA was analyzed and standardized to the expression of GAPDH at each time point. One-way ANOVA analysis demonstrated a significant sixfold increase after 2 DPI (603%±166%, p < 0.01) that remain elevated until 28 DPI (495%±36%, p < 0.01) when compared with sham sample (n = 3). At 4, 7,±and 14 DPI, the changes in P2Y2 mRNA expression were four-, three-, and fivefold, respectively (p < 0.05).
Fig. 1.
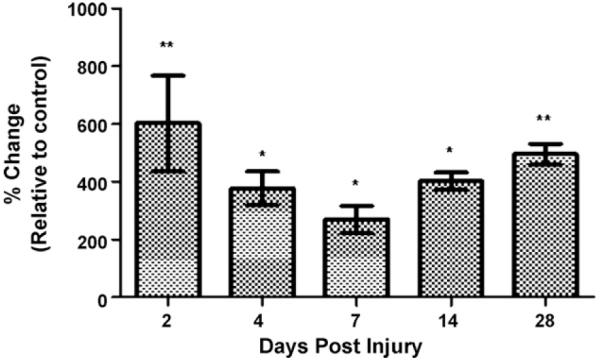
Increase of P2Y2 mRNA expression after spinal cord injury. Real time RT-PCR studies were performed in sham and contused spinal cord at 2, 4, 7, 14 and 28 days post-injury (DPI) and standardized to the expression of GAPDH at each time point. P2Y2 mRNA levels increased significantly across all the time points studied. Data represent the mean±SEM, n = 3; *p < 0.05, and **p < 0.01 significant differences versus control group (ANOVA followed by Student–Newman–Keuls multiple comparisons post hoc test; F = 7.388; df = 5, 12).
3.2. P2Y2 receptors are expressed in cells of the gray and white matter
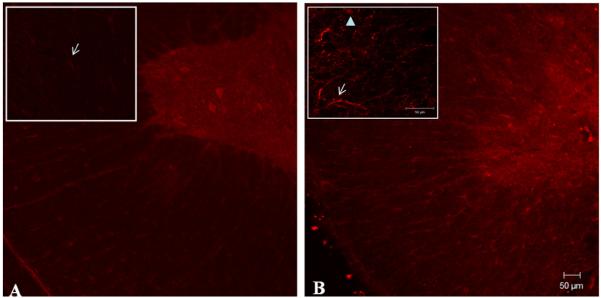
Antibodies against the P2Y2 receptor were used in fixed adult spinal cord to determine the regions of protein expression (n = 3). P2Y2 immunoreactivity was evident in motoneuron-like cells of the ventral horn in sham animals with reduced immunostaining in cells of the white matter (Fig. 2A). However, injured tissue (14 DPI) presented an apparent increase in the amount of immunoreactive cells located in the white matter of the spinal cord close to the lesion epicenter (Fig. 2B) and regions rostral or caudal to it (data not shown). Insert of the white matter shows the increase immunoreactivity of P2Y2 in astrocytes-like cells and axon-like structures.
Fig. 2.
Immunohistochemical analysis of P2Y2 receptors in normal and contused spinal cord. Rabbit anti-P2Y2 receptor antibody was used in fixed adult spinal cord to determine the regions of protein expression in control (A) and injured (B) spinal cord samples (n = 3). Insert indicates the pattern of P2Y2 expression in regions of the white matter visualized at higher magnification. Arrows indicate astrocytes-like cells positively labeled in regions of the white and the arrow-head point out an axon-like structure (gamma = 0.7). Scale bar = 50 μm.
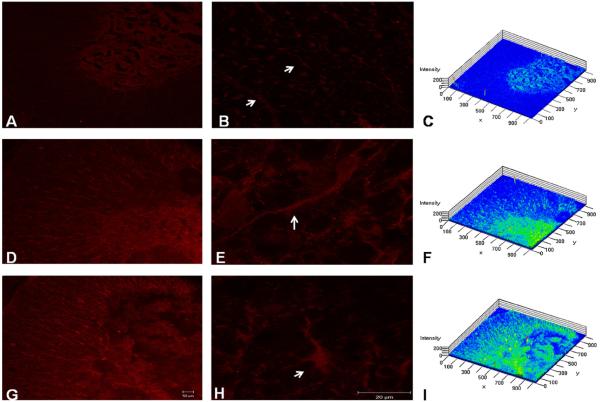
Differences in P2Y2 immunoreactivity were observed between sham samples (Fig. 3A–C) and injury samples at 7 (Fig. 3D–F) and 14 DPI (Fig. 3G–I). Low magnification images demonstrated that trauma to the spinal cord triggers a visible increase in the immunofluorescence of P2Y2 protein receptor in cells of the gray and white matter at 7 and 14 DPI. An enlarged view of the white matter shows the increase immunoreactivity of P2Y2 in astrocytes-like cells. Densitometry analysis of confocal images (Fig. 3D and G) showed an increase in P2Y2 immunoreactivity in samples (n = 3) from injured cords (Fig. 3F and I), relative to sham sections (Fig. 3A and C).
Fig. 3.
Spatial profile of P2Y2 expression. Photo micrographs densitometric analysis demonstrated that contused rats at 7 DPI (D–F) and 14 DPI (G–I) presented significantly more immunoreactivity than sham animals (A–C) in astrocyte-like cells of the white matter (n = 3). Control sections treated without primary antibody were used as controls (gamma = 0.7). Scale bar = 50 μm (A, D, and G) and 20 μm (B, E, and H).
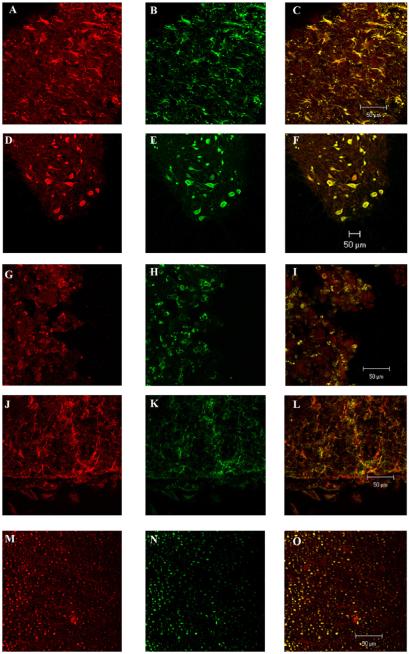
3.3. Identification of the cells expressing the P2Y2 receptor
Double labeling studies were performed with antibodies against the P2Y2 receptor (Fig. 4A, D, G, J, and M) and the antibodies that recognize reactive astrocytes (GFAP) (Fig. 4B), neurons (NeuN) (Fig. 4E), macrophages (MAB 328) (Fig. 4H), oligodendrocytes (CD68: Biotin) (Fig. 4K), and axons (NF-H) (Fig. 4N). Astrocytes, neurons, macrophages, oligodendrocytes, and axons (Fig. 4C, F, I, L, and O) showed expression of P2Y2 receptor in the injured cord (n = 3).
Fig. 4.
Identification of the cell phenotype expressing the P2Y2 receptor in the adult spinal cord. Double labeling studies were performed in samples of rat spinal cord with antibodies against the P2Y2 receptor and antibodies that recognize reactive astrocytes (GFAP), neurons (NeuN), axons (NF-H), macrophages (MAB 328), and oligodendrocytes (CD68: Biotin); (n = 3). The images were visualized with a Zeiss LSM 5 PASCAL Confocal Microscope System. Scale bar = 50 μm.
3.4. Temporal profile of the mature and newly synthesized P2Y2 protein after SCI
Expression of the P2Y2 receptor at the protein level in the adult spinal cord was determined by Western Blot analysis. Immunoreactive bands in the 62 kDa and 42 kDa region were observed; corresponding to previously reported molecular weights of this receptor (Nylund et al., 2007). Specificity of the antibody against both P2Y2 receptor bands was demonstrated by preabsorbing the antibody with the peptide used to generate it (Fig. 5). This 62 kDa protein represents the glycosylated protein because when protein extracts were treated with glycosidase to remove the carbohydrates moieties, the level of the immunoreactive band diminished (data not shown). Moreover, treatment of the protein extracts with 8 M urea did not affect the migration of the 62 kDa immunoreactive band, suggesting that the P2Y2 receptor is not associated with other proteins (data not shown) and is indeed the mature glycosylated P2Y2 receptor that is basally expressed in the adult spinal cord. These results confirm the expression of the mature (glycosylated) and the un-mature (un-glycosylated) forms of the P2Y2 receptor in the adult spinal cord.
Fig. 5.
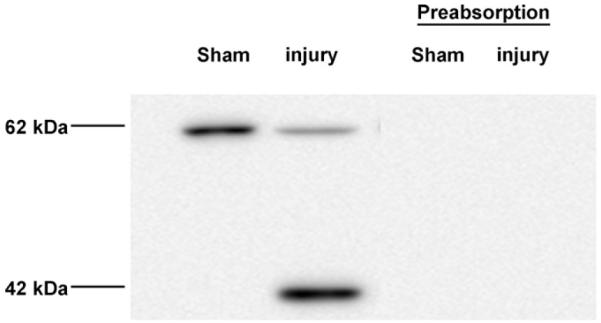
Expression of the P2Y2 receptor in the adult spinal cord. Western Blot analysis of spinal cord samples demonstrated the presence of the P2Y2 42 kDa and the 62 kDa protein bands. Preabsorption of the anti-P2Y2 antibody with control peptide antigen showed the specificity of the antibody for the P2Y2 receptor.
Injury produced an upregulation in the 42 kDa P2Y2 protein band expression (Fig. 6) at 4 DPI that reached significance at 7 DPI (997 ± 286 arbitrary units (AU), p < 0.05, n = 4) and 14 DPI (822±255 AU, p < 0.05, n = 5). At 2 (n = 5), 4 (n = 5), and 28 (n = 4) DPI, the changes in P2Y2 expression were twofold, sixfold, and fivefold, respectively but these changes were not significant (p > 0.05). Opposing results were observed after trauma to the spinal cord in the 62 kDa band. SCI significantly reduced the levels of the P2Y2 62 kDa glycosylated protein (Fig. 7) at 2 DPI (−59±16 AU, p < 0.001, n = 5) and remained downregulated until 28 DPI (−66±12 AU, p < 0.001, n = 5) relative to sham animals (n = 5).
Fig. 6.
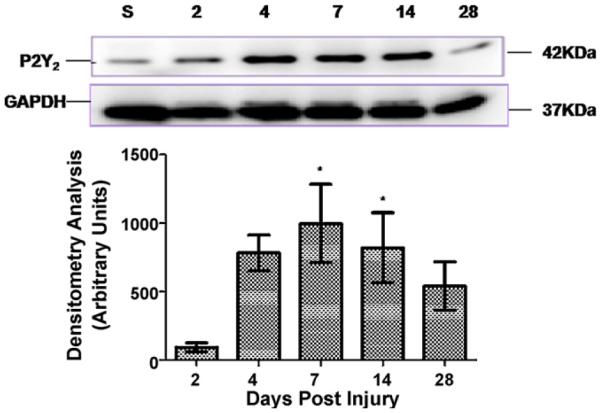
Spinal cord injury triggers the expression of the 42 kDa protein. Analysis of the 42 kDa P2Y2 receptor band by densitometry confirmed an upregulation after SCI. Significant changes were observed at 7 (p < 0.05, n = 4) and 14 DPI (p < 0.05, n = 5) relative to sham animals. Levels of P2Y2 protein were analyzed and compared to the expression of GAPDH at each time point by densitometry analysis. Data represent the mean±SEM; *p < 0.05 significant difference versus control group (ANOVA followed by Student–Newman–Keuls multiple comparisons post hoc test; F = 4.157; df = 5, 22).
Fig. 7.
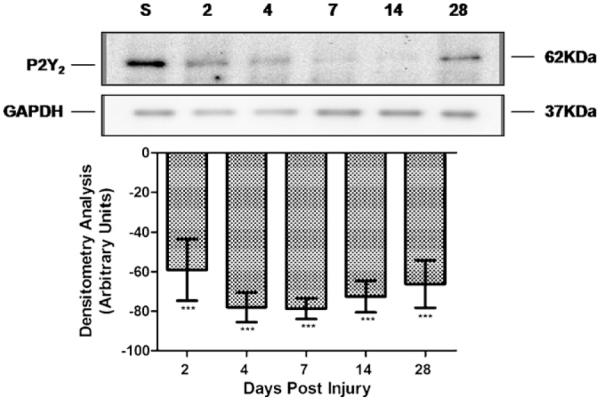
Trauma to the spinal cord decreased the levels of the 62 kDa protein. Densitometric analysis of the immunoreactive 62 kDa band showed a significant decrease in the level of this protein at 2 DPI that remained reduced until 28 DPI (p < 0.001) relative to sham animals. Levels of P2Y2 protein were analyzed and compared to the expression of GAPDH at each time point by densitometry analysis. Data represent the mean±SEM, n = 5; ***p < 0.001 significant difference versus control group (ANOVA followed by Student–Newman–Keuls multiple comparisons post hoc test; F = 9.938; df = 5, 24).
4. Discussion
Any injury or event that causes a rupture in the plasma membrane will cause release of many molecules and nucleotides into the extracellular environment that activate many receptors including the nucleotide receptors (Franke et al., 2001; Inoue et al., 2004; Neary et al., 2003) in neurons, glia (Benarroch, 2010; Franke et al., 1999), and cells of the immune system (Di Virgilio et al., 2001). Release of ATP into extracellular space has been identified following ischemia, high-frequency neuronal stimulation, and mechanical forces (Chorna et al., 2004; Feng et al., 2008). The uncontrolled discharge of ATP can act as an excitotoxin in certain pathological conditions (Wang et al., 2004). Moreover, the inflammatory reactions and oxidative stress inhibit the enzymes that hydrolyze and regulate the extracellular concentration of ATP in normal conditions, resulting in the accumulation of this nucleotide in the extracellular milieu which has serious neurotoxic effect (Franke et al., 2006; Ryu et al., 2002).
In vitro studies with rat brain astrocytes demonstrated that ATP or UTP application significantly increased the levels of P2Y2 receptor mRNA, as well as by submitting the cells to glucose-oxygen deprivation (Ballerini et al., 2006). Similar findings were reported in trauma conditions like the duct-ligated rat sub-mandibular gland, during congestive heart failure or rat aorta lesions and during activation thymocytes (Ahn et al., 2000; Hou et al., 1999; Koshiba et al., 1997; Seye et al., 1997). Our study extends the characterization of the P2Y2 receptor in the spinal cord and evaluates the receptor expression during the experimental SCI of female rats since they are been implicated in proliferative and inflammatory pathways in astrocytes. Therefore, this receptor represents a new target to manage reactive astrogliosis in SCI.
As a first approach, the expression of the mRNA for the P2Y2 receptor in spinal cord injured samples was evaluated. The mRNA levels of P2Y2 were increased during the acute and chronic stages of SCI suggesting a pronounced change in gene expression and regulation of P2Y2 receptor expression at the transcriptional level (synthesis) or post-transcriptional (stability) stage. Similar results were obtained in studies with brain astrocytes in culture when treated with UTP or guanosine. An RNA polymerase inhibitor, Actinomycin D, ended both UTP and guanosine-mediated P2Y2 mRNA upregulation, thus indicating that de novo transcription was required (Ballerini et al., 2006). Moreover, pathological conditions like focal brain ischemia, nucleus accumbens mechanical injury or brain trauma increases P2Y2 mRNA, suggesting the possibility of new P2Y2 receptor protein synthesis in response to the altered condition.
Analysis of the immature 42 kDa revealed a statistically significant upregulation in the P2Y2 protein expression at 7 and 14 DPI. This result may suggest that trauma triggers the re-expression of this receptor and probably the regulation is at the transcriptional level because mRNA and protein change occur in parallel. However, SCI may produce some cellular changes that reduce the processing or maturation of the newly synthesized P2Y2 receptor via degradation.
The 62 kDa P2Y2 protein band was downregulated after SCI, suggesting that the cellular events like necrosis, apoptosis or receptor internalization and further degradation take place. Previous studies in 1321N1 human astrocytoma cells by Sromek and Harden (1998) demonstrated that the P2Y2 receptors originating at the cell surface undergo agonist-promoted movement to an intracellular compartment in response to different concentrations and time of exposure to the agonist. They also demonstrate that P2Y2 receptor desensitize and internalize as a result of agonist incubation, suggesting that desensitization precedes the loss of surface receptors. This internalization can be part of the steps implicated in delivery of the receptor to lysosomes for degradation (Lohse, 1993; Tulapurkar et al., 2005). In our work, a significant decrease in the mature P2Y2 protein expression was observed after 2 DPI.
The IMF studies performed with antibodies against the P2Y2 receptor and antibodies that recognize reactive astrocytes (GFAP), neurons (NeuN), axons (NF-H), macrophages (MAB 328), and oligodendrocytes (CD68: Biotin) show co-localization with the P2Y2 receptor in cells or structures in our spinal cord samples. All physiological effects initiated by nucleotides in these cells may be amplified or altered by increased extracellular concentrations of ATP or UTP. In trauma, extracellular ATP or UTP originate from damaged cells, activated astrocytes, neurons, and microglia (Dubyak and el-Moatassim, 1993; Ferrari et al., 1997; James and Butt, 2002; Neary et al., 1999). This abundance of nucleotides after an injury can exert a hyperactivation effect on glial cells and exacerbate neuronal damage. Published reports demonstrate that mechanical damage to the rat nucleus accumbens up-regulated nucleotide receptors in GFAP positive astrocytes, suggesting that astrogliosis in vivo may be associated with ATP release and receptor activation (Franke et al., 2001). In vitro studies also demonstrated that ATP released extracellularly by mechanical injury of cultured astrocytes, triggers calcium-dependent extracellular signal-regulated kinase (ERK) signaling, suggesting a possible role of P2 nucleotide receptors in the astrocytic response to brain trauma (Neary et al., 2003). Furthermore, there is evidence for roles for P2Y2 receptors in pain hypersensitivity and nociception (Donnelly-Roberts et al., 2008; Liu and Salter, 2005).
Studies with primary cultures of rat cortical astrocytes suggested that signal transducer and activator of transcription 3 (STAT3) have been implicated in astrocytes proliferation and reactive astrogliosis. ATP stimulated the phosphorylation of STAT3 at Ser-727 through P2 receptor activation since suramin, a non-selective nucleotide antagonist, diminished this response. Further characterization of the phosphorylation response after P2Y receptor activation supported a role for P2Y2 receptor (Washburn and Neary, 2006). In addition, other studies with suramin suggests that blockade of nucleotide receptor activation may disrupt the gliotic response following brain injury (Di Prospero et al., 1998). As well, astrogliosis due to in vivo tissue injury caused by microinjection procedure into the rat nucleus accumbens decreased after pyridoxal-phosphate-6-azophenyl-2,4-disulfonic acid (PPADS, non-competitive and non-selective inhibitor of most P2 receptors) was microinfused in situ; sustaining the hypothesis that nucleotides are implicated in these processes via stimulation of P2 receptors in vivo (Franke et al., 1999).
These studies support our hypothesis, that P2Y2 activation could mediate the astrogliotic response; the formation of the glial scar and the production of cytokines and other proinflammatory agents that have detrimental effects on the regeneration process. Moreover, since in both astrocytes and oligodendrocytes we found presence of P2Y2 receptor, it is likely that some of the neuron–glial interaction is through this receptor. In addition, during pathological conditions, nucleotides are released from damaged cells. If the white matter is injured, the increase in calcium, mediated by nucleotide receptor (Kirischuk et al., 1995), could possibly trigger a larger cascade of events involving oligodendrocytes. Given that it has been demonstrated that elevated calcium levels stimulate proteolysis of myelin via calcium-activated neutral protease, it is expected demyelination after injury (Banik et al., 1985).
However, we could not discard the possibility that activation of P2Y2 receptors may regulate important neuroprotective mechanisms under pathological conditions. Chorna et al. (2004) reported that P2Y2 receptors activation in human astrocytoma cells stimulate the expression of genes implicated in nervous system development, neuronal migration, differentiation and survival, and the formation and function of synapses. Other studies demonstrated that activation of P2Y2 receptors, using an in vitro brain trauma technique, mediates survival responses to glial cells experiencing cellular death provoked by trauma (Burgos et al., 2007). In SCI, the formation of the glial scar may be important for the isolation of the lesion, maintenance of healthy cells alienated from injured cells, restraint of edema, and control the extension of the non-permissive environment created after the injury (Faulkner et al., 2004). Future studies should target P2Y2 expression or activation after SCI, monitor the gliotic response and extension of cavity formation, and determine functional locomotor recovery.
Acknowledgements
The authors thank Luz C. Arocho, Johnny D. Figueroa, Laurivette Mosquera, Odrick R. Rosas, and Jose M. Santiago for the excellent technical assistance during surgeries and post-operatory procedures. Also, special thanks to the personnel of the Animal Resources Center (University of Puerto Rico, Medical Science Campus), the Experimental Surgery facilities, and Dr. Silva’s laboratory team for their valuable technical support and advice. Finally, our gratitude to Nildris Cruz, Jose O. Garcia, Kandy Velazquez, and Dr. Alan Preston for their critiques in the manuscript. This work was supported by NIH-MRISP (2 R24MH48190-14), NIH-SNRP (NS39405), MBRS-SCORE (S06-GM008224), MBRS-RISE (GM-68138) and the Associated Deanship of Biomedical Sciences and Graduate Studies of the UPR School of Medicine.
Footnotes
Supported by NIH-MRISP (2 R24 MH 48190-14), NIH-SNRP (NS39405), MBRS-SCORE (S06-GM008224), MBRS-RISE (GM-68138) and the Associated Deanship of Biomedical Sciences and Graduate Studies of the UPR School of Medicine.
References
- Ahn JS, Camden JM, Schrader AM, Redman RS, Turner JT. Reversible regulation of P2Y(2) nucleotide receptor expression in the duct-ligated rat sub-mandibular gland. Am. J. Physiol. Cell Physiol. 2000;279:C286–C294. doi: 10.1152/ajpcell.2000.279.2.C286. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Di IP, Caciagli F, Rathbone MP, Jiang S, Nargi E, Buccella S, Giuliani P, D’Alimonte I, Fischione G, Masciulli A, Romano S, Ciccarelli R. P2Y2 receptor up-regulation induced by guanosine or UTP in rat brain cultured astrocytes. Int. J. Immunopathol. Pharmacol. 2006;19:293–308. doi: 10.1177/039463200601900207. [DOI] [PubMed] [Google Scholar]
- Banik NL, McAlhaney WW, Hogan EL. Calcium-stimulated proteolysis in myelin: evidence for a Ca2+-activated neutral proteinase associated with purified myelin of rat CNS. J. Neurochem. 1985;45:581–588. doi: 10.1111/j.1471-4159.1985.tb04026.x. [DOI] [PubMed] [Google Scholar]
- Becker T, Anliker B, Becker CG, Taylor J, Schachner M, Meyer RL, Bartsch U. Tenascin-R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia. 2000;29:330–346. doi: 10.1002/(sici)1098-1136(20000215)29:4<330::aid-glia4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Adenosine triphosphate: a multifaceted chemical signal in the nervous system. Neurology. 2010;74:601–607. doi: 10.1212/WNL.0b013e3181d03762. [DOI] [PubMed] [Google Scholar]
- Burgos M, Neary JT, Gonzalez FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J. Neurochem. 2007;103:1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr. Opin. Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Canning DR, Hoke A, Malemud CJ, Silver J. A potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. Int. J. Dev. Neurosci. 1996;14:153–175. doi: 10.1016/0736-5748(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J. Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Figueroa JD, Velazquez I, Torrado A, Ortiz C, Hernandez C, Puig A, Segarra AC, Whittemore SR, Miranda JD. Blocking EphA4 upregulation after spinal cord injury results in enhanced chronic pain. Exp. Neurol. 2006;202:421–433. doi: 10.1016/j.expneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Di Prospero NA, Zhou XR, Meiners S, McAuliffe WG, Ho SY, Geller HM. Suramin disrupts the gliotic response following a stab wound injury to the adult rat brain. J. Neurocytol. 1998;27:491–506. doi: 10.1023/a:1006995624754. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Borea PA, Illes P. P2 receptors meet the immune system. Trends Pharmacol. Sci. 2001;22:5–7. doi: 10.1016/s0165-6147(00)01574-1. [DOI] [PubMed] [Google Scholar]
- Dijkstra S, Geisert EE, Jr., Dijkstra CD, Bar PR, Joosten EA. CD81 and microglial activation in vitro: proliferation, phagocytosis and nitric oxide production. J. Neuroimmunol. 2001;114:151–159. doi: 10.1016/s0165-5728(01)00240-5. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J. Pharmacol. Exp. Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HL, Yan L, Cui LY. Effects of repetitive transcranial magnetic stimulation on adenosine triphosphate content and microtubule associated protein-2 expression after cerebral ischemia-reperfusion injury in rat brain. Chin. Med. J. (Engl.) 2008;121:1307–1312. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal SM, Collo G, Buell G, Di VF. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JD, Benton RL, Velazquez I, Torrado AI, Ortiz CM, Hernandez CM, Diaz JJ, Magnuson DS, Whittemore SR, Miranda JD. Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J. Neurosci. Res. 2006;84:1438–1451. doi: 10.1002/jnr.21048. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Schmidt R, Grosche J, Reichenbach A, Illes P. P2 receptor-types involved in astrogliosis in vivo. Br. J. Pharmacol. 2001;134:1180–1189. doi: 10.1038/sj.bjp.0704353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Malmsjo M, Moller S, Pantev E, Bergdahl A, Zhao XH, Sun XY, Hedner T, Edvinsson L, Erlinge D. Increase in cardiac P2X1- and P2Y2-receptor mRNA levels in congestive heart failure. Life Sci. 1999;65:1195–1206. doi: 10.1016/s0024-3205(99)00353-7. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Iannotti C, Zhang YP, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma. 2006;23:853–865. doi: 10.1089/neu.2006.23.853. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M, Koizumi S. Chronic pain and microglia: the role of ATP. Novartis Found. Symp. 2004;261:55–64. [PubMed] [Google Scholar]
- Irizarry-Ramirez M, Willson CA, Cruz-Orengo L, Figueroa J, Velazquez I, Jones H, Foster RD, Whittemore SR, Miranda JD. Upregulation of EphA3 receptor after spinal cord injury. J. Neurotrauma. 2005;22:929–935. doi: 10.1089/neu.2005.22.929. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J. Physiol. 1995;483(Pt 1):41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp. Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- Lenz G, Gottfried C, Luo Z, Avruch J, Rodnight R, Nie WJ, Kang Y, Neary JT. P(2Y) purinoceptor subtypes recruit different MEK activators in astrocytes. Br. J. Pharmacol. 2000;129:927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Salter MW. Purines and pain mechanisms: recent developments. Curr. Opin. Investig. Drugs. 2005;6:65–75. [PubMed] [Google Scholar]
- Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim. Biophys. Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR. Induction of Eph B3 after spinal cord injury. Exp. Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp. Neurol. 2001;169:407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Shi YF, Tran MD, Wanner IB. P2 receptor signalling, proliferation of astrocytes, and expression of molecules involved in cell-cell interactions. Novartis Found. Symp. 2006;276:131–143. [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund G, Hultman L, Nordgren S, Delbro DS. P2Y2 and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton. Autacoid. Pharmacol. 2007;27:79–84. doi: 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, van RN, Hickey WF, Preidis G, McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp. Neurol. 2003;182:275–287. doi: 10.1016/s0014-4886(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Kim J, Choi SH, Oh YJ, Lee YB, Kim SU, Jin BK. ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport. 2002;13:1611–1615. doi: 10.1097/00001756-200209160-00008. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler. Thromb. Vasc. Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- Silva WI, Maldonado HM, Velazquez G, Rubio-Davila M, Miranda JD, Aquino E, Mayol N, Cruz-Torres A, Jardon J, Salgado-Villanueva IK. Caveolin isoform expression during differentiation of C6 glioma cells. Int. J. Dev. Neurosci. 2005;23:599–612. doi: 10.1016/j.ijdevneu.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sromek SM, Harden TK. Agonist-induced internalization of the P2Y2 receptor. Mol. Pharmacol. 1998;54:485–494. doi: 10.1124/mol.54.3.485. [DOI] [PubMed] [Google Scholar]
- Tanhoffer RA, Yamazaki RK, Nunes EA, Pchevozniki AI, Pchevozniki AM, Nogata C, Aikawa J, Bonatto SJ, Brito G, Lissa MD, Fernandes LC. Glutamine concentration and immune response of spinal cord-injured rats. J. Spinal Cord Med. 2007;30:140–146. doi: 10.1080/10790268.2007.11753925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulapurkar ME, Schafer R, Hanck T, Flores RV, Weisman GA, Gonzalez FA, Reiser G. Endocytosis mechanism of P2Y2 nucleotide receptor tagged with green fluorescent protein: clathrin and actin cytoskeleton dependence. Cell Mol. Life Sci. 2005;62:1388–1399. doi: 10.1007/s00018-005-5052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with alpha integrin mediates astrocyte migration. J. Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience. 2006;142:411–423. doi: 10.1016/j.neuroscience.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol. Neurobiol. 2005;31:169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson CA, Irizarry-Ramirez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11:229–239. [PubMed] [Google Scholar]