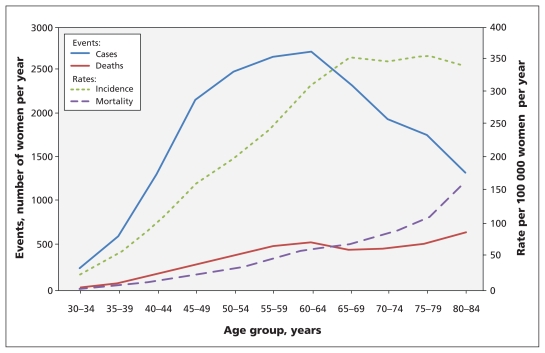
Of the newly diagnosed cases of breast cancer in Canada, 80% were in women over the age of 50 years, and about 28% were in women aged 70 years or older (Figure 1),1 with little variation by province. Regular screening for breast cancer with mammography, breast self-examinations and clinical breast examinations are widely recommended to reduce mortality due to breast cancer. Although controversy remains over precisely which screening services should be provided and to whom, these methods are frequently used in contemporary practice.2–4
Figure 1:
Incidence of breast cancer and associated mortality among Canadian women in 2007. Sources: Canadian Cancer Registry and Canadian Vital Statistics — Death Databases, Statistics Canada.
Outcomes of screening for breast cancer such as tumour detection and mortality must be put into context of the harms and costs of false-positive results, overdiagnosis and overtreatment. Consideration of benefits, harms and costs is complicated by variations in risk factors and in the types and stages of cancer.
Any positive result from screening has emotional costs such as anxiety and worry for patients and their families, and financial costs to both the patient and the health care system as a result of additional and potentially unnecessary diagnostic tests. For women with positive results on screening tests, additional diagnostic tests will usually be recommended, such as further mammography, ultrasound and/or tissue sampling with core needle biopsy.
This document updates the previous guidelines issued by the Canadian Task Force on Preventive Health Care (2001).5,6 The absence of current Canadian recommendations, the recent controversy over the best way to screen for breast cancer among women at average risk of the disease,7,8 the availability of new technologies such as magnetic resonance imaging (MRI) and a recent review of the evidence9 were the basis for selecting this topic for an update by the revitalized Canadian Task Force on Preventive Health Care.
Recommendations are presented for the use of mammography, MRI, breast self-examination and clinical breast examination to screen for breast cancer among women at average risk of disease (defined as those with no previous breast cancer, no history of breast cancer in a first-degree relative, no known mutations in the BRCA1/BRCA2 genes or no previous exposure of the chest wall to radiation). Recommendations are provided separately for women aged 40–49, 50–69 and 70–74 years and are aimed at clinicians and policy-makers. The recommendations are intended to inform both organized and opportunistic screening.
Methods
The Canadian Task Force on Preventive Health Care is an independent panel of clinicians and methodologists with expertise in prevention, primary care, literature synthesis, critical appraisal and the application of evidence to practice and policy. The task force makes recommendations about clinical manoeuvres aimed at primary and secondary prevention. (Please see www.canadiantaskforce.ca/members_eng.html for a list of current members of the task force.)
Work on each recommendation is led by a workgroup of two to five members of the task force; a list of members of the workgroup for the current guidelines is available at the end of this article. Each workgroup establishes the research questions and analytical framework for the guideline.
The Evidence Review and Synthesis Centre (the details of which are available at www.canadiantaskforce.ca/about_eng.html) assembles a team of methodologists who will perform the systematic review, usually in conjunction with one or more clinical experts. The team provides input on the analytical framework, then summarizes the evidence using a systematic review and quantitative summary of the relevant available evidence; narrative summaries are developed when quantitative synthesis is not feasible. A list of members of the evidence review team for the current guidelines is available at the end of this article.
Once the systematic review is available from the evidence review team, the task force workgroup independently develops the recommendation statements by consensus, based on a detailed review of the evidence. In formulating recommendations, workgroups consider both the benefits and harms associated with a screening test, patient values and preferences, the quality of the evidence and, in some cases, the costs of the intervention (Box 1). The strength of evidence is determined using the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system.10 The draft recommendations as developed by the workgroup are revised and approved by the entire task force.
Box 1: Grading of recommendations.
Recommendations are graded as either strong or weak according to the Grades of Recommendation Assessment, Development and Evaluation system (GRADE).10 GRADE offers two strengths of recommendation: strong and weak. The strength of recommendations is based on the quality of supporting evidence, the degree of uncertainty about the balance between desirable and undesirable effects, the degree of uncertainty or variability in values and preferences, and the degree of uncertainty about whether the intervention represents a wise use of resources.
Strong recommendations are those for which the task force is confident that the desirable effects of an intervention outweigh its undesirable effects (strong recommendation for an intervention) or that the undesirable effects of an intervention outweigh its desirable effects (strong recommendation against an intervention). A strong recommendation implies that most people will be best served by the recommended course of action.
Weak recommendations are those for which the desirable effects probably outweigh the undesirable effects (weak recommendation for an intervention) or undesirable effects probably outweigh the desirable effects (weak recommendation against an intervention) but appreciable uncertainty exists. A weak recommendation implies that most women would want the recommended course of action, but many would not. For clinicians, this means they must recognize that different choices will be appropriate for individual women, and they must help each woman arrive at a management decision consistent with her own values and preferences. Policy-making will require substantial debate and involvement of various stakeholders. Weak recommendations result when the balance between desirable and undesirable effects is small, the quality of evidence is lower, and there is more variability in the values and preferences of patients.
Evidence is graded as high, moderate, low or very low based on how likely further research is to change our confidence in the estimate of effect.
Although members of a task force workgroup are not necessarily content experts in the clinical area of the guideline, a content expert is part of the evidence review team, and the research questions, systematic review and recommendations undergo internal and external peer review by experts in the field and by stakeholders and partners, such as the Canadian Breast Cancer Screening Initiative for these guidelines. Details about the task force’s methods can be found elsewhere11 and in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110334/-/DC1).
Key questions and analytic framework for systematic review
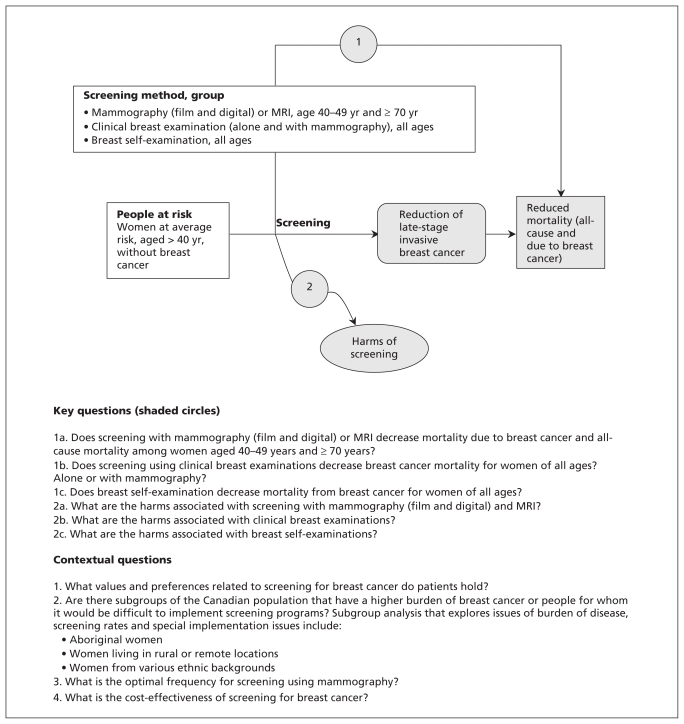
The Breast Cancer Workgroup established key questions and an analytic framework for the systematic review on screening for breast cancer (Figure 2). Key questions were addressed by systematic review. Contextual questions (issues judged not to require a systematic review, but that were addressed by targeted literature searches) are also shown in Figure 2.
Figure 2:
Analytical framework and key questions posed by the workgroup. MRI = magnetic resonance imaging.
Because previous guidelines have emphasized the benefit of mammography for screening women aged 50–69 years, the focus of the review was women aged 40–49 years and women aged 70 years and older; data were collected for women aged 40 years and older. Randomized and quasi-randomized controlled trials were used to determine the effectiveness of screening interventions (Figure 2, key questions 1a, 1b and 1c). Observational studies and mathematical models were not used to assess efficacy owing to their potential for bias and because there was sufficient trial data to answer the key research questions.
Randomized trials often fail to capture detailed information on clinically relevant harms and generally do not study patient values and preferences. We used observational data to assess these issues (Figure 2, key questions 2a, 2b and 2c, and contextual questions).
Because a high-quality systematic review from the United States Preventive Services Task Force10 (a national organization with a mandate similar to that of the Canadian Task Force on Preventive Health Care) had recently been published, we chose to update their search to avoid duplication of effort. The previous summary of evidence was used for evidence up to the end of 2008, and an updated search for new evidence published after that time was done by the evidence review team. Because our initial search did not identify sufficient age-specific data to assess the harms of mammography, we did an additional search of data from Canadian organizations including the Public Health Agency of Canada and the Canadian Institute for Health Information. The systematic review upon which the current guidelines are based and an update as of October 2011 are published on the task force’s website.12
Recommendations
A summary of the recommendations for clinicians and policy-makers is shown in Box 2. More detailed explanations of the recommendations are available in Appendices 2 and 3 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110334/-/DC1).
Box 2: Summary of recommendations for clinicians and policy-makers.
Recommendations are presented for the use of mammography, magnetic resonance imaging (MRI), breast self-examination and clinical breast examination to screen for breast cancer (see Box 1). These recommendations apply only to women at average risk of breast cancer aged 40 –74 years. They do not apply to women at higher risk because of personal history of breast cancer, history of breast cancer in first-degree relatives, known mutations of the BRCA1/BRCA2 genes or previous exposure of the chest wall to radiation. No recommendations are made for women aged 75 years and older, given the lack of data available for this group.
Mammography
For women aged 40–49 years, we recommend not routinely screening with mammography. (Weak recommendation; moderate-quality evidence)
For women aged 50–69 years, we recommend routinely screening with mammography every two to three years. (Weak recommendation; moderate-quality evidence)
For women aged 70–74 years, we recommend routinely screening with mammography every two to three years. (Weak recommendation; low-quality evidence)
Magnetic resonance imaging
We recommend not routinely screening with MRI scans. (Weak recommendation; no evidence)
Clinical breast examination
We recommend not routinely performing clinical breast examinations alone or in conjunction with mammography to screen for breast cancer. (Weak recommendation; low-quality evidence)
Breast self-examination
We recommend not advising women to routinely practice breast self-examination. (Weak recommendation; moderate-quality evidence)
Mammography
Women aged 40–49 years
For women 40–49 years of age, we recommend not routinely screening for breast cancer with mammography. (Weak recommendation; moderate-quality evidence.)
Mammography is associated with significant reductions in the relative risk of death from breast cancer among women aged 40–49 years (Table 1).13 However, the absolute benefit is lower for this age group than for older women because of the younger group’s lower risk of cancer. We calculated the number needed to screen (NNS), defined here as the number of women who would need to be screened about once every 2 years over a median of about 11 years to prevent a single death from breast cancer. Because not all women in the randomized trials who were invited to attend screening actually had mammography, these NNS may underestimate the absolute benefit of screening.
Table 1:
Summary of evidence of the benefits associated with using mammography to screen for breast cancer
| No. of studies | Quality assessment | Summary of findings | GRADE quality of evidence | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths from breast cancer | Effect | |||||||||||
| Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Screening, no. (%) | Control, no. (%) | RR (95% CI) | Absolute (95% CI) | |||
| Breast cancer mortality for ages 40–49 yr* | ||||||||||||
| 8 | Randomized trials† | Serious‡ | No serious inconsistency§ | No serious indirectness¶ | No serious imprecision** | None†† | n = 152 300 448 (0.29) | n = 195 919 625 (0.32) | 0.85 (0.75–0.96) | 474 fewer per 1 000 000 (from 115 to 792 fewer), NNS 2108 | Moderate | Critical |
| Breast cancer mortality for ages 50–69 yr | ||||||||||||
| 7 | Randomized trials | Serious‡‡ | No serious inconsistency§§ | No serious indirectness¶ | No serious imprecision** | None†† | n = 135 068 639 (0.47) | n = 115 206 743 (0.64) | 0.79 (0.68–0.90) | 1 387 fewer per 1 000 000 (from 622 to 2 050 fewer), NNS 721 | Moderate | Critical |
| Breast cancer mortality for ages 70–74 yr | ||||||||||||
| 2 | Randomized trials¶¶ | Serious*** | No serious inconsistency††† | No serious indirectness¶ | Serious‡‡‡ | None†† | n = 10 339 49 (0.47) | n = 7 307 50 (0.68) | 0.68 (0.45–1.01) | 2 218 fewer per 1 000 000 (from 3 734 fewer to 39 more), NNS 451 | Low | Critical |
Note: Estimates of relative risk are based on a random-effects meta-analysis. GRADE allows evidence to be rated as very low, low, moderate or high quality.10 Randomized controlled trials begin as high-quality evidence, whereas observational data begin as low. Evidence can be downgraded as a result of study limitations (e.g., lack of blinding or allocation concealment), inconsistency of results, indirectness of evidence, imprecision of estimates (wide confidence intervals) or publication bias. Evidence can be upgraded if there is a large magnitude of effect, low likelihood of plausible confounding and a dose–response gradient.13 CI = confidence interval, GRADE = Grades of Recommendation Assessment, Development and Evaluation, NNS = number needed to screen (patients who need to be screened about once every 2 yr over a median of about 11 yr to prevent 1 death from breast cancer), RR = relative risk.
The available data were based on women aged 39–49 yr, although the focus of the review was women aged 40–49 yr.
Five quasi-randomized and three truly randomized trials.
Blinding and concealment were not clear for five studies; only three of them are considered truly randomized.
No heterogeneity exists; p = 0.48, I2 = 0%.
The question addressed is the same for the evidence regarding the population, intervention, comparator and outcome.
Total sample size is large and the total number of events is greater than 300 (a threshold rule-of-thumb value).
Insufficient number of studies to assess publication bias.
Blinding and concealment were not clear for five studies; only two of them are considered truly randomized.
No heterogeneity exists; p = 0.12, I2 = 41%.
Quasi-randomized.
Blinding and concealment were not clear.
No heterogeneity exists, p = 0.75, I2 = 0%.
Total sample size is large, but the total number of events is less than 300 (a threshold rule-of-thumb value).
The NNS to prevent one death from breast cancer for women aged 40–49 years is 2108, as compared with 721 for women aged 50–69 years. In addition, the risk of a false-positive result from mammography is higher for women younger than 50 years. Thus, screening about 2100 women aged 40–49 years once every 2–3 years for about 11 years would prevent a single death from breast cancer, but it would also result in about 690 women having a false-positive result on a mammogram, leading to unnecessary follow-up testing, and 75 women having an unnecessary biopsy of their breast.12
No primary studies looked at the risk of overdiagnosis (diagnosis of breast cancer that will not affect life expectancy or quality of life) specifically among women aged 40–49 years, but studies involving older women have estimated that the frequency of overdiagnosis ranges from 30% to 52%.12 Data from our systematic review show that for every 1000 women aged 39 years and older who are screened using mammography, 5 will have an unnecessary lumpectomy or mastectomy as a result of overdiagnosis.12 In addition to unnecessary intervention, false-positive results can lead to fear, anxiety and distress.14 In the judgment of the task force, this ratio of potential benefit to harm does not justify routine screening in women aged 40–49 years.
This recommendation places a relatively low value on a very small absolute decrease in mortality and reflects concerns with false-positive results, the incidence of unnecessary biopsies and overdiagnosis of breast cancer. The implications of a weak recommendation are that most women would follow the recommended course of action, but many would not. Clinicians should discuss the benefits and harms with their patients and must help each woman to make a decision that is consistent with her values and preferences. Women who place a higher value on a small reduction in mortality and are less concerned about undesirable consequences are likely to choose screening. Because it is likely that the benefit of screening increases in a continuous fashion with increasing age (rather than a sharp increase at 50 years of age), women aged 40–49 years may be more inclined to receive screening as they grow older, even if their preferences do not change.
Women aged 50–69 years
For women aged 50–69 years, we recommend routinely screening for breast cancer with mammography every two to three years. (Weak recommendation; moderate-quality evidence.)
Mammography is associated with significant reductions in the relative risk of death from breast cancer in this age group, and the absolute benefits are greater than those seen among women aged 40–49 years (Table 1). The benefits of mammography for women aged 60–69 years (NNS 432) are greater than for women aged 50–59 years (NNS 910). Screening about 720 women aged 50–69 years once every 2–3 years for about 11 years would prevent 1 death from breast cancer, but it would also result in about 204 women having a false-positive result on a mammogram and 26 women having an unnecessary biopsy of their breast (Table 2).12 In the judgment of the task force, the larger absolute benefits for women aged 50–69 years justify a weak recommendation for screening, in contrast to the recommendation for women aged 40–49 years.
Table 2:
Estimated number of women with adverse outcomes following screening mammography12
| Adverse outcome | Women affected by age range, no. | ||
|---|---|---|---|
| 40–49 yr | 50–69 yr | 70–74 yr | |
| Per 1000 women screened | |||
| False-positive result on mammogram | 327 | 282 | 212 |
| Unnecessary biopsy | 36 | 37 | 26 |
| Per single death prevented | |||
| Number needed to screen | 2108 | 721 | 451 |
| False-positive result on mammogram | 690 | 204 | 96 |
| Unnecessary biopsy* | 75 | 26 | 11 |
Note: Results are expressed per thousand women screened for a median of 11 yr (estimated as a total of 4 screening mammograms per woman assuming a screening interval of 2–3 yr). The period of 11 yr was chosen because it was the approximate median duration of follow-up during the randomized trials included in the systematic review. Data assume that rescreening rates stay constant over time.
Percutaneous or surgical biopsies of the breast that were subsequently found not to have cancer.
The absolute benefits of screening remain small among women aged 50–69 years, and a substantial proportion of women will have false-positive results on mammography leading to unnecessary and invasive investigation. Again, the potential benefits and harms of screening should be discussed with each patient in the context of her preferences. Women aged 50–69 years who do not place a high value on a small reduction in mortality and who are concerned about false-positive results, unnecessary diagnostic testing and potential overdiagnosis of breast cancer are likely to decline screening.
Women aged 70–74 years
For women aged 70–74 years, we recommend routinely screening for breast cancer with mammography every two to three years. (Weak recommendation; low-quality evidence.)
The reduction in relative risk of death from breast cancer associated with mammography for women aged 70–74 years is statistically non-significant (Table 1). However, the point estimate for relative risk is similar to that seen for younger women, and the 95% confidence intervals extend only marginally above unity. Given the higher absolute risk in this age group, these considerations suggest that the absolute benefits of mammography are likely to be similar to those seen among women aged 50–69 years. However, patient preferences remain important for determining who should and should not be screened.
Screening about 450 women aged 70–74 years once every 2–3 years for about 11 years would prevent 1 death from breast cancer, but it would also result in about 96 women having a false-positive result on a mammogram and 11 women having an unnecessary biopsy of their breast (Table 2).12 Women aged 70–74 years who do not place a high value on a small reduction in mortality and who are concerned about false-positive results, unnecessary diagnostic testing and potential overdiagnosis of breast cancer are likely to decline screening.
Clinical considerations for women aged 50–74 years
The trials included in our review screened women at intervals ranging from 12 to 33 months (median 22 mo).15–21 The optimal frequency of screening cannot be determined at present, but data from the sole randomized trial comparing different screening intervals suggest no significant difference between screening intervals of one year and three years. However, that trial was not adequately powered to detect a small benefit of more frequent screening.22 Pooled analyses suggest that the effect of screening on mortality is similar in trials with a screening interval of 24 months or more and those with a screening interval of less than 24 months (Table 3). Further stratified analyses suggested that the benefit of screening appeared similar in trials with screening intervals of 33 months (two trials involving 98 431 women; relative risk [RR] 0.70, 95% confidence interval [CI] 0.45–1.09),16,18 24 months or longer (three trials involving 193 905 women (RR 0.77, 95% CI 0.58–1.03)16,18 and annually (four trials involving 311 165 women; RR 0.87, 95% CI 0.77–0.99).15,17,20,21 The small number of women screened at intervals of 33 months did not permit further stratification by age. Therefore, for women aged 50–74 years, we suggest a screening interval of two to three years, which appears to preserve the benefit of annual screening but reduces adverse effects, inconvenience to women and cost.
Table 3:
Effect of screening with mammography on relative risk of death from breast cancer, stratified by age and screening interval
| Age range, yr | Screening interval < 24 mo | Screening interval ≥ 24 mo | ||||
|---|---|---|---|---|---|---|
| No. of trials | RR (95% CI) | GRADE quality of evidence | No. of trials | RR (95% CI) | GRADE quality of evidence | |
| 40–49* | 515,17–20 | 0.82 (0.72–0.94) | High | 316,18 | 1.04 (0.72–1.50) | Low |
| 50–69 | 415,18,19,21 | 0.86 (0.75–0.98) | High | 316,18 | 0.67 (0.51–0.88) | Moderate |
| ≥ 70 | NA† | — | — | 216,18 | 0.68 (0.45–1.01) | Low |
| All ages | 615,17–21 | 0.83 (0.76–0.92) | High | 316,18 | 0.77 (0.58–1.03) | Low |
Note: CI = confidence interval, GRADE = Grades of Recommendation Assessment, Development and Evaluation,10 NA = not available, RR = relative risk.
The evidence used to support this recommendation is based on data for women aged 39–49 yr.
No trials performed in women aged ≥ 70 yr.
Since no studies show that the type of mammography influences the anticipated reduction in mortality associated with screening, either digital or film mammography is acceptable.
Appropriate clinical actions following mammography are outside the scope of this document, but they are summarized elsewhere.23
Magnetic resonance imaging
We recommend not routinely screening for breast cancer using MRI scans. (Weak recommendation; no evidence.)
There are no data evaluating whether screening women at average risk of breast cancer using MRI scans reduces mortality as compared with mammography or no screening. Thus, screening women at average risk using MRI scans is not recommended.
Breast examinations
We recommend not routinely performing clinical breast examinations alone or in conjunction with mammography to screen for breast cancer. (Weak recommendation; low-quality evidence.)
We recommend not advising women to routinely practice breast self-examination. (Weak recommendation; moderate-quality evidence.)
No evidence was found to show that clinical breast examination or breast self-examination reduced mortality due to breast cancer or all-cause mortality.24 Two large trials identified no reduction in breast cancer mortality associated with teaching breast self-examination to women aged 31–64 years, but evidence of increased harm was seen (RR 1.5, 95% CI 1.1–1.9 for benign finding on breast biopsy).25,26
Screening women aged 75 years and older
We did not identify data addressing the benefits of screening mammography for women more than 74 years of age. It is possible that screening might reduce breast cancer mortality in this group. Given the small absolute reduction in mortality associated with screening, benefit is unlikely among people with limited life expectancy. Practitioners should communicate this information to patients so that it can be considered during joint decision-making about whether to proceed with screening.
Considerations for implementation
Screening with mammography leads to relatively small reductions in mortality, together with increased harm associated with false-positive results and unnecessary interventions. Although the absolute benefit of screening may increase with longer follow-up, it remains relatively small. There was no evidence that screening with mammography reduces all-cause mortality. Although screening might permit surgery for breast cancer at an earlier stage than diagnosis of clinically evident cancer (thus permitting the use of less-invasive procedures for some women), available trial data suggest that the overall risk of mastectomy is significantly increased among recipients of screening compared with women who have not undergone screening.27
Although available data suggest that some women would prefer to undergo screening despite its potential harms, many would not.28–33 These data show that determining the preferences of individual women about the relative importance of potential benefits and harms is critical in determining who should undergo screening. Sources of information for women should accurately portray the value of mammography and the potential for harm rather than simply provide encouragement.7,34 For example, the Public Health Agency of Canada has created a leaflet to assist women with deciding whether or not to undergo screening.35 In addition, one-page information sheets are available for both clinicians and patients to help with shared decision-making (Appendix 3; additional knowledge translation tools are available at http://canadiantaskforce.ca/patient-resources.html and at http://canadiantaskforce.ca/GRADE.html).
Introducing organized screening programs appears to increase the proportion of women who undergo mammography; such programs should be structured to encourage women to make an informed decision about whether to participate. In some provinces, women may self-refer to organized screening programs; our recommendations are relevant to physicians advising their patients about the potential merits of mammography within or outside of such programs. Reminders linked to an electronic medical record might be helpful for increasing the proportion of women with whom the risks and benefits of mammography are discussed, but this would require further study.
Certain ethnic groups may have higher (e.g., Ashkenazi Jews)36 or lower (East Asians)33 risk of death from breast cancer, which may alter the absolute benefit of screening. Rates of screening are low among Aboriginal populations,37,38 women with low incomes and recent immigrants;39 further work is needed to explain these findings and determine their potential impact.
Access to high-quality facilities with the necessary equipment and expertise in mammography is required for screening. Provincial and regional decision-makers should consider whether access is adequate for people in their jurisdictions who reside outside of major centres. Mobile screening units may help to increase access to screening among women who live in rural or remote communities.
Suggested performance measures
An ideal performance measure for preventive services would allow clinicians to assess the quality of care that they are delivering to patients, and allow the writers of guidelines to assess whether their recommendations have influenced clinical practice. The objective of these guidelines is to improve health among women aged 40–74 years, which requires balancing the potential benefits and harms of using mammography to screen for breast cancer. Although uptake rates of screening are often used as performance measures, women aged 50–74 years who are well-informed might reasonably choose not to undergo mammography. Therefore, performance measures based solely on the number or proportion of women in each age group who undergo mammography may not be suitable.
For health care providers, the proportion of women aged 40–74 years with whom the benefits and harms of mammography are discussed is an appropriate measure of performance. The proportion of women aged 50–74 years who undergo screening mammography at least every three years could be used as a proxy for access to screening services. However, the optimal proportion of women who should undergo screening is dependent on preferences and thus may vary between populations. Measures of quality assurance for facilities providing mammography should be required routinely, including the evaluation of the percentage of women screened who are referred for further testing because of abnormal results found with a program screen (i.e., abnormal call rate) and the number of women detected as having invasive cancer during a routine screening episode per 1000 women screened (i.e., invasive cancer detection rate).40
Economic implications of screening
Available data suggest that screening with mammography every two years is associated with costs per quality-adjusted life year that are generally considered to represent good value for money in developed countries.41,42 However, many such analyses are based on observational data, which may overestimate the potential benefit of screening compared with trial data. Longer screening intervals will be more economically attractive than shorter screening intervals, assuming that the benefit in terms of reducing breast cancer mortality is retained, as the available evidence suggests.
Other guidelines
The current guideline differs from previous recommendations made by the Canadian Task Force on Preventive Health Care by lengthening the screening interval from one year to two to three years. Several other organizations have developed recommendations for screening for breast cancer (Table 4).5,6,43–45 The US Preventive Services Task Force and the National Health Service in the United Kingdom recommend routine screening for women aged 50–74 years, but not for women aged 40–49 years. The US Preventive Services Task Force recommends screening for women aged 50–74 years every two years, whereas the National Health Service recommends screening for women aged 50–70 years every three years. The explanation for these differences may be varying judgments about the quality of available evidence.
Table 4:
Comparison of recommendations for screening for breast cancer
| Organization | Recommendation | ||||
|---|---|---|---|---|---|
| Mammography, age range, yr | Breast self-examination | Clinical breast examination | |||
| 40–49 | 50–74 | ≥75 | |||
| Canadian Task Force on Preventive Health Care (current), Canada | Recommend against routine screening | Every 2–3 yr | No recommendation | Recommend against | Recommend against |
| Canadian Task Force on Preventive Health Care (1994; 2001),5,6 Canada | No recommendation (2001) | Every year for women 50–69 yr (1994) | No recommendation (1994) | Not recommended (2001) | Every year for women 50–69 yr (1994) |
| US Preventive Services Task Force,43 USA | Recommend against routine screening. Individual decision and patient context | Every 2 yr | Insufficient evidence | Recommend against teaching to women | Insufficient evidence |
| BreastScreen Australia Program, Australia44 | No active recruitment | Every 2 yr for women 50–69 yr | No active recruitment | NA | NA |
| National Health Service breast cancer screening program,45 UK | No active recruitment* | Recruited for screening every 3 yr until age 70 yr | Women > 70 yr not routinely recruited for screening* | Not recommended | Not recommended |
Note: NA = not available.
Program is expanding to extend screening mammography every 3 years to women aged 47–73 yr.
The Canadian Task Force on Preventive Health Care will update this guideline within five years of publication.
Gaps in knowledge
Little is known about the benefit and harms of screening using mammography for women older than 74 years or younger than 40 years. New technologies such as MRI scans have not been adequately studied in the screening of women at average risk. Digital mammography is a new technology that is widely used in contemporary clinical practice. The overall diagnostic accuracy of digital versus film mammography as a means of screening for breast cancer is similar, but digital mammography is more sensitive and has similar specificity for women younger than 50 years of age, women with radiographically dense breasts and premenopausal or perimenopausal women.46
Although improvements in technology suggest that screening may be more effective now than in the past, mortality among women with breast cancer continues to decline (perhaps due to better adjuvant treatment). It is possible, though speculative, that the absolute benefit attributable to screening might have declined in parallel. Because all of the trials identified by our review used film mammography, determining whether digital mammography or MRI scan of the breast improve the benefit associated with screening (especially for younger women) would require further randomized trials, which would be of great interest to clinicians, patients and policy-makers.
We have recommended a screening interval of every two to three years for women 50–74 years of age using available evidence from randomized controlled trials. The concept of individualizing the interval for screening with mammography based on breast density or other risk factors is appealing but requires further study.47
Finally, given the importance of patients’ preferences for appropriate decision-making, further studies are needed to determine the best way to communicate information about the potential benefits and harms of mammography.
Conclusion
Although screening mammography reduces mortality from breast cancer among women aged 40–74 years, the absolute benefit is small — especially for younger women — and is partially offset by harms caused by unnecessary intervention. Despite its potential to reduce mortality, appropriate use of mammography will require thoughtful discussion between clinicians and patients about the balance between benefits and harms. Finally, available evidence does not support the use of MRI scans, clinical breast examination or breast self-examination to screen for breast cancer among women at average risk.
Key points
The reduction in mortality associated with screening mammography is relatively small for women aged 40–74 years at average risk of breast cancer.
A greater reduction in mortality is seen with mammography for women at average risk aged 50–74 years than among similar women aged 40–49 years; however, harms of overdiagnosis and unnecessary biopsy may be greater for younger women than for older women.
When deciding whether to recommend mammography to a specific patient, providers should first discuss the tradeoff between benefits and harms, as well as the patient’s values and preferences.
For women at average risk who choose to have screening mammography, an interval of every two to three years appears appropriate.
There is no evidence that screening women at average risk of breast cancer using magnetic resonance imaging, clinical breast examination or breast self-examination reduces the risk of mortality or other clinically relevant adverse outcomes.
Supplementary Material
Acknowledgements
The authors thank members of the research team from the Evidence Review and Synthesis Centre who conducted the systematic review upon which these recommendations were based, the staff at the Task Force Office of the Public Health Agency of Canada, and the peer reviewers whose thoughtful comments helped to improve the quality of this manuscript.
See related commentary by Gøtzsche on page 1957 and at www.cmaj.ca/lookup/doi/10.1503/cmaj.111721
Footnotes
Competing interests: Marcello Tonelli, Michel Joffres, James Dickinson, Harminder Singh, Gabriela Lewin and Richard Birtwhistle have received support for travel to meetings from the Public Health Agency of Canada. Gabriela Lewin is an employee of Kemptville District Hospital. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors made substantial contributions to the conception and design of the article, the acquisition, analysis and interpretation of data, drafted the article and revised it critically for important intellectual content and approved the final version submitted for publication.
Funding: Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada and the Canadian Institutes of Health Research. The views of the funding bodies have not influenced the content of the guideline; competing interests have been recorded and addressed. The views expressed in this article are those of the authors and do not represent those of the Public Health Agency of Canada.
Guidelines writing group: Marcello Tonelli, Sarah Connor Gorber, Michel Joffres, James Dickinson, Harminder Singh, Gabriela Lewin, Richard Birtwhistle.
Systematic review writing group: Donna Fitzpatrick-Lewis, Nicole Hodgson, Donna Ciliska, Marcello Tonelli, Mary Gauld, Yan Yun Liu.
References
- 1.Canadian Cancer Society/National Cancer Institute of Canada Canadian Cancer Statistics 2007. Toronto (ON): The Society; 2007 [Google Scholar]
- 2.Shields M, Wilkins K. An update on mammography use in Canada. Health Rep 2009;20:7–19 [PubMed] [Google Scholar]
- 3.Del Giudice E, Tannenbaum D, Goodwin PJ. Breast self-examination: resistance to change. Can Fam Physician 2005;51: 698–9 [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey V, Glazier R. Explanations for the preventive care checklist form. Can Fam Physician 2006;52:48–55 [PMC free article] [PubMed] [Google Scholar]
- 5.Ringash J; Canadian Task Force on Preventive Health Care Preventive health care, 2001 update: screening mammography among women aged 40–49 years at average risk of breast cancer. CMAJ 2001;164:469–76 [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison BJ. Screening for breast cancer. In: The Canadian guide to clinical preventive health care. Ottawa (ON): Health Canada; 1994 [Google Scholar]
- 7.Welch HG. Screening mammography — a long run for a short slide? N Engl J Med 2010;363:1276–8 [DOI] [PubMed] [Google Scholar]
- 8.Quanstrum KH, Hayward R. Lessons from the mammography wars. N Engl J Med 2010;363:1076–9 [DOI] [PubMed] [Google Scholar]
- 9.Nelson HD, Tyne K, Nalk A, et al. Screening for breast cancer: systematic evidence review update for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality; 2009. Report No.: 10-05142-EF-1 [PubMed] [Google Scholar]
- 10.Schünemann H, Brozek J, Oxman A, editors. GRADE handbook for grading the quality of evidence and the strength of recommendations. The GRADE Working Group; 2009 [Google Scholar]
- 11.Canadian Task Force on Preventive Health Care Procedure manual. Available: http://canadiantaskforce.ca/methods-manual-2011.html (accessed 2011 Oct. 17).
- 12.Fitzpatrick-Lewis D, Hodgson N, Ciliska D, et al. Breast cancer screening. Available: http://canadiantaskforce.ca/recommendations/2011_01_eng.html (accessed 2011 Oct. 17).
- 13.Salz T, Richman AR, Brewer NT. Meta-analyses of the effect of false-positive mammograms on generic and specific psychosocial outcomes. Psychooncology 2010;19:1026–34 [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Kunz R. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habbema JD, van Oortmarssen GJ, van Putten DJ, et al. Age-specific reduction in breast cancer mortality by screening: an analysis of the results of the Health Insurance Plan of Greater New York study. J Natl Cancer Inst 1986;77:317–20 [PubMed] [Google Scholar]
- 16.Tabár L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age. New results from the Swedish two-county trial. Cancer 1995;75:2507–17 [DOI] [PubMed] [Google Scholar]
- 17.Miller AB, To T, Baines CJ, Wall C. Canadian national breast screening study: 1. Breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40s. Ann Intern Med 2002;137:305–12 [DOI] [PubMed] [Google Scholar]
- 18.Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909–19 [DOI] [PubMed] [Google Scholar]
- 19.Bjurstam N, Björneld L, Warwick J, et al. The Gothenburg breast screening trial. Cancer 2003;97:2387–96 [DOI] [PubMed] [Google Scholar]
- 20.Moss S, Cuckle H, Evans A, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006;368:2053–60 [DOI] [PubMed] [Google Scholar]
- 21.Miller AB, To T, Bained CJ, Wall C. Canadian national breast screening study: 2. 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst 2000;92:1490–9 [DOI] [PubMed] [Google Scholar]
- 22.The Breast Screening Frequency Trial Group The frequency of breast cancer screening: results from the UKCCCR randomized trial. Eur J Cancer 2002;38:1458–64 [DOI] [PubMed] [Google Scholar]
- 23.Canadian Cancer Society Treatment for breast cancer. Toronto (ON): The Society; 2011. Available: www.cancer.ca/sitecore/content/Home/Canada-wide/About%20cancer/Types%20of%20cancer/Treatment%20for%20breast%20cancer.aspx (accessed 2011 Oct. 13). [Google Scholar]
- 24.Humphrey LL, Helfland M, Chan BKS, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347–60 [DOI] [PubMed] [Google Scholar]
- 25.Baxter N. with the Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837–46 [PMC free article] [PubMed] [Google Scholar]
- 26.Kösters JP, Gøtzsche PC. Regular self-examination or clinical examination for early detection of breast cancer [review]. Cochrane Database Syst Rev 2003;(2):CD003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gøtzsche PC, Neilsen M. Screening for breast cancer with mammography [review]. Cochrane Database Syst Rev 2011;CD001877. [DOI] [PubMed] [Google Scholar]
- 28.Phillips KA, Van Bebber S, Marshall D, et al. A review of studies examining stated preferences for cancer screening. Prev Chronic Dis 2006;3:A75. [PMC free article] [PubMed] [Google Scholar]
- 29.Gyrd-Hansen D. Cost-benefit analysis of mammography screening in Denmark based on discrete ranking data. Int J Technol Assess Health Care 2000;16:811–21 [DOI] [PubMed] [Google Scholar]
- 30.Grann VR, Patel P, Bharthuar A, et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat 2010;119:177–84 [DOI] [PubMed] [Google Scholar]
- 31.Ganott MA, Sumkin JH, King JL, et al. Screening mammography: Do women prefer a higher recall rate given the possibility of earlier detection of cancer? Radiology 2006;238:793–800 [DOI] [PubMed] [Google Scholar]
- 32.Rozenberg S, Carly B, Liebens F, et al. Effect of screening programme on mortality from breast cancer. Women might not accept mammography if benefit is lower than is currently thought. BMJ 2000;321:1527–8 [PubMed] [Google Scholar]
- 33.McDermott S, Desmeules M, Lewis R, et al. Cancer incidence among Canadian immigrants, 1980–1998: results from a national cohort study. J Immigr Minor Health 2011;13:15–26 [DOI] [PubMed] [Google Scholar]
- 34.Gøtzsche PC, Hartling OJ, Nielson M, et al. Breast screening: the facts — or maybe not. BMJ 2009;338:b86. [DOI] [PubMed] [Google Scholar]
- 35.Public Health Agency of Canada Information on mammography for women aged 40 and older: a decision aid for breast cancer screening in Canada. Ottawa (ON): The Agency; 2009. Available: www.phac-aspc.gc.ca/cd-mc/mammography-mammographie-eng.php (accessed 2011 Oct. 17). [Google Scholar]
- 36.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336:1401–8 [DOI] [PubMed] [Google Scholar]
- 37.Access to cancer screening and First Nations. Ottawa (ON): Assembly of First Nations; 2009. Available: http://64.26.129.156/cmslib/general/AFN%20Cancer%20Screening%20Review-final-ENG.pdf (accessed 2011 Jan. 6). [Google Scholar]
- 38.Sheppard AJ, Chiarel AM, Marrett LD, et al. Detection of later stage breast cancer in First Nations women in Ontario, Canada. Can J Public Health 2010;101:101–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shields M, Wilkins K. An update on mammography use in Canada. Health Rep 2009;20:7–19 [PubMed] [Google Scholar]
- 40.Quality Determinants of organized breast cancer screening programs, 2003. Ottawa (ON): Public Health Agency of Canada; 2003 [Google Scholar]
- 41.Lee SY, Jeong SH, Kim YN, et al. Cost-effective mammography screening in Korea: high incidence of breast cancer in young women. Cancer Sci 2009;100:1105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahern CH, Shen Y. Cost-effectiveness analysis of mammography and clinical breast examination strategies: a comparison with current guidelines. Cancer Epidemiol Biomarkers Prev 2009;18:718–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Preventive Services Task Force Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:716–26 [DOI] [PubMed] [Google Scholar]
- 44.BreastScreen Australia Program. Canberra (Australia): Australian Government, Department of Health and Ageing; 2010. Available: www.health.gov.au/internet/screening/publishing.nsf/Content/breastscreen-about (accessed 2010 Dec. 27) [Google Scholar]
- 45.NHS Breast Screening Programme. London (UK): National Health Service; Available: www.cancerscreening.nhs.uk/breastscreen/ (accessed 2011 Oct. 13). [Google Scholar]
- 46.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773–83 [DOI] [PubMed] [Google Scholar]
- 47.Schousboe JT, Kerlikowske K, Loh A, et al. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med 2011;155:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.