Abstract
Background
The prevalence of gestational diabetes (GDM) is increasing all over the world. Hence, the impact of GDM on maternal and infant health is an important topic of research. No study has been conducted in Qatar to evaluate the outcome of pregnancies complicated by diabetes mellitus.
Objective
The aim of the study was to determine the prevalence of GDM, compare the maternal–neonatal complications among women with GDM and non-GDM pregnant women, and investigate the risk factors and potential outcomes associated with GDM.
Design
This is a prospective cohort study.
Setting
The survey was carried out at the antenatal clinics of the Women’s Hospital, Qatar.
Subjects and methods
A representative sample of 2056 pregnant women who attended the antenatal clinics of the Women’s Hospital were surveyed during the period from the first week of January 2010 to April 2011. From this sample, 1608 women (78.2%) expressed their consent to participate in the study. Questionnaires were administered to pregnant women who were seeking antenatal care at this urban hospital. The questionnaire covered variables related to sociodemographic factors, family history, medical history, maternal complications, and neonatal outcome.
Results
The prevalence of GDM in Qatar was 16.3%. Women with GDM were significantly higher in the age group of 35–45 years (45%; P = 0.001). Family history of diabetes (31.7%; P < 0.001), increased parity (55.3%; P = 0.004), and obesity (59.2%; P < 0.001) were determinants of GDM in pregnant women. Maternal complications like pregnancy-induced hypertension (19.1% vs 10.3%; P < 0.001), pre-eclampsia (7.3% vs 3.8%; P = 0.012), antepartum hemorrhage (19.2% vs 14.6%; P = 0.05), and cesarean (27.9% vs 12.4%; P < 0.001) were significantly higher in GDM women. Neonates were at increased risk of preterm birth (12.6% vs 8.3%; P = 0.03), macrosomia (10.3% vs 5.9%; P = 0.01), and birth trauma (8% vs 3%; P < 0.001).
Conclusion
The study findings revealed that GDM was higher in women in Qatar and that they were at increased risk of developing maternal and neonatal complications. Obesity emerged as an essential risk factor for subsequent GDM. The advanced maternal age, low monthly income, family history of diabetes, and obesity were the main significant risk factors for GDM.
Keywords: gestational diabetes, obstetric risks, macrosomic, Qatar
Introduction
Gestational diabetes (GDM) is a glucose tolerance disorder that occurs or is diagnosed for the first time during pregnancy.1 GDM is a public health problem that currently affects a large part of the female population and has short- and long-term consequences for the fetus and the mother. It has been reported that GDM affects 1%–14% of all pregnancies, and that its incidence has been steadily rising.2 GDM is a major cause of perinatal morbidity and mortality, as well as maternal morbidity.3 It is therefore highly important that these mothers are diagnosed during pregnancy and that they have a regular postpartum follow-up for identification and treatment of any complications.
Although the risks associated with GDM are well recognized, the impact on maternal and neonatal health outcomes is less clear. The factors that have been postulated to influence the risk of GDM among mothers include obesity, a positive family history of diabetes, treatment for infertility, recurrent urinary tract infections, macrosomic infant, unexplained neonatal death, prematurity, pre-eclampsia, diabetes in previous pregnancy, and advancing maternal age.4 Women with GDM have increased risk for potential morbidity and for impaired glucose tolerance, and it identifies a population of women who are at high risk of developing type 2 diabetes in the years following the pregnancy.5 In addition to higher risk of perinatal morbidity, the offspring of mothers with GDM face increased risk of childhood obesity and early onset of type 2 diabetes mellitus.6 GDM is a condition that can be effectively controlled, thereby decreasing the associated risks and eventually leading to the delivery of healthy infants. Thus, appropriate management of GDM will improve both maternal and perinatal outcomes.
The data on the prevalence of GDM and its complications in Asian populations are very few.7 Previous studies of Bener et al8–11 have reported that the prevalence of diabetes mellitus and its complications was high in Qatar. It was documented in the literature that GDM women and their offspring are more likely to develop metabolic syndrome or type 2 diabetes in later life.12,13 Because Qatar has a high prevalence rate of diabetes mellitus,8 it is important to determine the prevalence of GDM in women. Early diagnosis of GDM is necessary to reduce maternal and fetal morbidity and to help to prevent or delay the onset of type 2 diabetes. Therefore, this study was conducted to analyze the population characteristics of women with GDM and identify the risk factors associated with GDM.
Subjects and methods
This is a prospective cohort study that was conducted among Qatari and other Arab pregnant women over a period from January 2010 to April 2011. The study surveyed all pregnant women who attended the antenatal clinics of the Women’s Hospital. The hospital is an urban hospital, and patients were referred from private clinics and primary health care centers. A representative sample of 2056 pregnant women were approached and 1608 women (78.2%) expressed their consent to participate in the study. Women with diabetes before pregnancy were not included in the study. A total of 448 women were excluded from the study due to incomplete questionnaires or if they did not want to respond to the questionnaire due to lack of time. A series of pregnant women were taken consecutively from the register and included in the study sample. Research assistants screened medical files of the subjects for any queries about the pregnancy and neonatal complications.
In 2010, there were a total of 16,188 deliveries in the Women’s Hospital. Our study sample included 1608 pregnant women, which is 9.9% of the mothers delivered. The study was approved by both institutional review boards at the Weill Cornell Medical College and Hamad Medical Corporation prior to commencing data collection. Each participant was provided with brief information about the study and was assured of strict confidentiality.
Sociodemographic data of the pregnant women were obtained during the face-to-face interview. During the study period, women attending the antenatal clinics were screened for GDM. If any woman was found to have a risk factor for diabetes during her antenatal visits, she was screened during the same visit. If her plasma glucose on screening was ≥140 mg/dL, she underwent a 75 g 2-hour oral glucose tolerance test to confirm the diagnosis of GDM. Women who did not have GDM were followed up until the third trimester of pregnancy, then every fortnight in the third trimester of pregnancy. There was a follow-up for both the groups until their delivery at the clinic for identifying maternal and neonatal complications. Body mass index (BMI) was calculated by dividing the weight in kilograms by the height in meters squared.
A well-designed and pilot-tested questionnaire was used to collect data. Face-to-face interviews were conducted by qualified nurses using a validated self-administered questionnaire in the local language. The questionnaire covered sociodemographic characteristics of the pregnant women, family and medical history, type of maternal complication, and neonatal outcome. A translated Arabic version of the questionnaire was revised by a bilingual consultant. The survey instrument was then tested on 100 randomly selected pregnant women from the list for the validity of the questionnaire. The investigators had made the necessary corrections and modifications after considering the minor differences and discrepancies that had been found during the pilot study.
Statistical analyses were performed using SPSS software (v 18.0; SPSS Inc, Chicago, IL). Fisher’s exact test and χ2 analysis were performed to test for differences in the proportions of categorical variables between two or more groups. Student’s t-test (two-tailed) was used to determine the significance of difference between two continuous variables and confirmed by nonparametric Mann–Whitney test. Multiple logistic regression analysis using the forward inclusion and backward deletion method was used to assess the relationship between dependent and independent variables and to adjust for potential confounders and orders of importance of risk factors (determinant) for the GDM. The level P < 0.05 was taken as the cut-off value for significance.
Results
Table 1 shows the prevalence and sociodemographic characteristics of pregnant women with GDM and without GDM. The prevalence of GDM was 16.3%, with 8.8% among Qatari women and 7.5% among other Arab women. Women who had GDM were significantly older (33.4 ± 6.5 years) than women who had no GDM (31.9 ± 6.3 years) (P = 0.001). Women with GDM were more likely to be in the age group 35–45 years (45%; P = 0.001). There was no significant difference in the level of education and occupation between the two groups, but most of the GDM women were more likely to be of a lower economic status (Qatari Riyal 5000–9999 [44.7%]).
Table 1.
Sociodemographic risk factors for gestational diabetes mellitus (GDM) in Qatar (N = 1608)
| Variables | GDM (n = 262) | Non-GDM (n = 1346) | P value* |
|---|---|---|---|
| Mean age (years) | 33.4 (6.5) | 31.9 (6.3) | 0.001 |
| Nationality | |||
| Qatari | 142 (54.2) | 604 (44.9) | 0.008 |
| Non-Qatari | 120 (45.8) | 742 (55.1) | |
| Age group (years) | |||
| <25 | 35 (13.4) | 224 (16.6) | |
| 25–34 | 109 (41.6) | 679 (50.4) | 0.001 |
| 35–45 | 118 (45) | 443 (32.9) | |
| Education level | |||
| Illiterate | 14 (5.3) | 89 (6.6) | 0.167 |
| Primary | 33 (12.6) | 118 (8.8) | |
| Intermediate | 26 (9.9) | 126 (9.4) | |
| Secondary | 73 (27.9) | 451 (33.5) | |
| University | 116 (44.3) | 562 (41.8) | |
| Occupation | |||
| Housewife | 165 (63) | 810 (60.2) | 0.519 |
| Sedentary/professional | 64 (24.4) | 379 (28.2) | |
| Manual | 22 (8.4) | 89 (6.6) | |
| Business | 8 (3.1) | 41 (3.0) | |
| Police/army | 3 (1.1) | 27 (2.0) | |
| Housing condition | |||
| Villa | 193 (73.3) | 860 (63.9) | 0.005 |
| Traditional house | 48 (18.3) | 377 (28) | |
| Apartment | 21 (8.0) | 109 (8.1) | |
| Monthly income (Qatari Riyal) | |||
| <5000 | 17 (6.5) | 124 (9.2) | |
| 5000–9999 | 117 (44.7) | 497 (36.9) | 0.075 |
| 10,000–14,999 | 57 (21.8) | 278 (20.7) | |
| 15,000–20,000 | 51 (19.5) | 298 (22.1) | |
| >20,000 | 20 (7.6) | 149 (11.1) | |
Note: Based on χ2 test.
Table 2 reveals the determinants of GDM according to family and medical history. A total of 31.7% of women with GDM had a positive family history of diabetes, compared with 12.8% in normal women (P < 0.001). Consanguinity was higher in women with GDM (52.7%) than in normal women (48.9%). Women who had GDM were significantly more parous (>four children 55.3%; P = 0.004) and obese (59.2%; P < 0.001). Past history of abortion (24% vs 16.9%; P = 0.006) and stillbirth (11.8% vs 7.9%; P = 0.04) were significant past obstetric risks in GDM women compared with the normal group.
Table 2.
Determinants of gestational diabetes mellitus (GDM) according to family and medical history (N = 1608)
| Predictors | GDM (n = 262) | Non-GDM (n = 1346) | P value* |
|---|---|---|---|
| Consanguinity | 138 (52.7) | 658 (48.9) | 0.262 |
| Family history of diabetes | 83 (31.7) | 172 (12.8) | <0.001 |
| Parity number | |||
| <2 | 53 (20.2) | 379 (28.2) | |
| 2–3 | 64 (24.4) | 364 (27) | 0.004 |
| >4 | 145 (55.3) | 603 (44.8) | |
| Appropriate antenatal care | 206 (78.6) | 1042 (77.4) | 0.667 |
| Body mass index | |||
| <25 | 35 (13.4) | 478 (35.5) | <0.001 |
| 25–30 | 72 (27.5) | 529 (39.3) | |
| >30 | 155 (59.2) | 339 (25.2) | |
| Obstetric risks in the past | |||
| Abortion | 63 (24) | 228 (16.9) | 0.006 |
| Stillbirth | 31 (11.8) | 107 (7.9) | 0.040 |
Note: Based on χ2 test.
Table 3 compares the maternal and neonatal complications between women with GDM and normal women. Women with GDM were more likely to develop pregnancy- induced hypertension (19.1% vs 10.3%; P < 0.001), pre-eclampsia (7.3% vs 3.8%; P = 0.012), antepartum hemorrhage (19.2% vs 14.6%; P = 0.05), preterm labor (19.8% vs 8.5%; P < 0.001), and cesarean delivery (27.9% vs 12.4%; P < 0.001) than those without GDM. Infants born to women with GDM were at increased risk of being born preterm (12.6% vs 8.3%; P = 0.03) and were also significantly more likely to be macrosomic (10.3% vs 5.9%; P = 0.012). Birth trauma was significantly higher in offspring of GDM mothers (8% vs 3%; P < 0.001).
Table 3.
Comparison of maternal and neonatal complications between women with gestational diabetes mellitus (GDM) and women without GDM (non-GDM)
| Variables | GDM (n = 262) | Non-GDM (n = 1346) | P value* |
|---|---|---|---|
| Maternal complications | |||
| Pregnancy-induced hypertension | 50 (19.1) | 138 (10.3) | <0.001 |
| Pre-eclampsia | 19 (7.3) | 51 (3.8) | 0.012 |
| Urinary tract infections | 64 (24.4) | 353 (26.2) | 0.543 |
| Antepartum hemorrhage | 50 (19.2) | 196 (14.6) | 0.050 |
| Preterm labor | 52 (19.8) | 114 (8.5) | <0.001 |
| Premature rupture of membrane | 40 (15.3) | 53 (3.9) | <0.001 |
| Labor | |||
| Spontaneous | 195 (74.4) | 943 (70.1) | 0.050 |
| Induced | 40 (15.3) | 187 (13.9) | |
| Augmented | 27 (10.3) | 216 (16.0) | |
| Cesarean | |||
| Elective | 50 (19.1) | 127 (9.4) | <0.001 |
| Emergency | 23 (8.8) | 41 (3.0) | <0.001 |
| Neonatal complications | |||
| Births | |||
| Preterm birth | 33 (12.6) | 112 (8.3) | 0.03 |
| Full-term birth | 229 (87.4) | 1234 (91.7) | |
| Apgar score at 1 minute | |||
| <7 | 56 (21.4) | 342 (25.4) | 0.166 |
| ≥7 | 206 (78.6) | 1004 (74.6) | |
| Apgar score at 5 minutes | |||
| <7 | 22 (8.4) | 131 (9.7) | 0.500 |
| ≥7 | 240 (91.6) | 1215 (90.3) | |
| Birth weight (g) | |||
| Low birth weight (<2500) | 12 (4.6) | 99 (7.4) | 0.012 |
| Normal weight (2500–4000) | 223 (85.1) | 1167 (86.7) | |
| Macrosomia (>4000) | 27 (10.3) | 80 (5.9) | |
| Birth defects | |||
| Birth trauma | 21 (8.0) | 41 (3.0) | <0.001 |
| Jaundice | 33 (12.6) | 83 (6.2) | <0.001 |
| Congenital anomaly | 9 (3.4) | 38 (2.8) | 0.591 |
| Growth retardation | 14 (5.3) | 41 (3.0) | 0.060 |
| Sepsis antibiotics | 21 (8.0) | 49 (3.6) | 0.001 |
Note: Based on χ2 test.
Table 4 shows the predictors for GDM in women and their offspring using stepwise logistic regression analysis. Low monthly income (odds ratio [OR]: 1.9, 95% confidence intervals [CI]: 1.1–3.2; P = 0.05), advanced maternal age (35–45 years) (OR: 1.7, 95% CI: 1.2–2.6; P = 0.001), obesity (OR: 6.6, 95% CI: 4.4–9.9; P < 0.001), family history of diabetes (OR: 3.6, 95% CI: 2.5–5.0; P < 0.001), previous history of abortion (OR: 1.4, 95% CI: 1.1–2.0; P = 0.048), macrosomia (OR: 1.4, 95% CI: 1.1–2.3; P = 0.046), antepartum hemorrhage (OR: 2.2, 95% CI: 1.4–3.6; P = 0.001), and emergency cesarean (OR: 2.7, 95% CI: 1.9–3.7; P < 0.001) were the main factors associated with GDM.
Table 4.
Stepwise logistic regression analysis for gestational diabetes mellitus (GDM) (N = 1608)
| Predictors | OR | 95% CI | P value* |
|---|---|---|---|
| Low monthly income (Qatari riyal) | 1.9 | 1.1–3.2 | 0.050 |
| Age group (years) | |||
| <25 (ref) | 1 | 0.001 | |
| 25–34 | 1.1 | 0.7–1.5 | |
| 35–45 | 1.7 | 1.2–2.6 | |
| Body mass index | |||
| <25 (ref) | 1 | <0.001 | |
| 25–30 | 1.7 | 1.1–2.7 | |
| >30 | 6.6 | 4.4–9.9 | |
| Family history of diabetes | 3.6 | 2.5–5.0 | <0.001 |
| Previous abortion | 1.4 | 1.1–2.0 | 0.048 |
| Neonatal birth weight (g) | |||
| 2500–4000 (ref) | 1 | 0.046 | |
| <2500 | 0.5 | 0.3–0.9 | |
| >4000 | 1.4 | 1.1–2.3 | |
| Antipartum hemorrhage | 2.2 | 1.4–3.6 | 0.001 |
| Cesarean | |||
| Elective (y/n) | 2.3 | 1.6–3.2 | <0.001 |
| Emergency (y/n) | 2.7 | 1.9–3.7 | <0.001 |
Note: Two-sided P value based on −2 log likelihood statistics.
Abbreviations: CI, confidence interval; OR, odds ratio; ref, reference category.
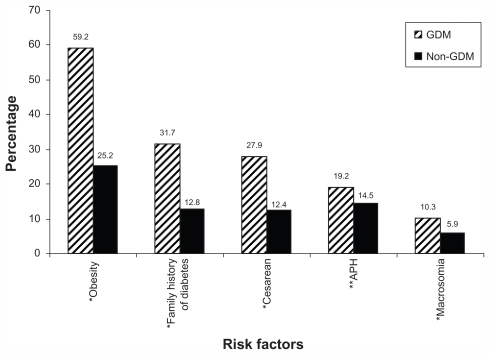
Table 5 presents the global prevalence rate of gestational diabetes mellitus across different countries. Figure 1 compares the main associated factors of GDM between women with GDM and normal women.
Table 5.
Global prevalence rate (%) of gestational diabetes mellitus across different countries
| Country | Reference | Year | Sample size | Diagnostic criteria | Prevalence rate (%) |
|---|---|---|---|---|---|
| Saudi Arabia | Ardawi et al15 | 2000 | 1056 | 1 hour 50 g GCT, cut-off 130 mg/dL, followed by 3 hours fasting 100 g OGTT after 3 days of 200 g CHO per day | 12.5 |
| Bahrain | Al Mahroos et al14 | 2001–2002 | 10,495 | 1 hour 50 g GCT, cut-off 140 mg/dL followed by 3 hours fasting 75 g OGTT | 13.5 |
| Malaysia | Tan et al31 | 2006 | 1600 | 1 hour 50 g GCT, cut-off 140 mg/dL followed by 3 hours fasting 75 g OGTT | 11.4 |
| United Arab Emirates | Agarwal et al16 | 2007 | 1172 | 2 hours fasting 75 g OGTT, cut-off ≥140 mg/dL | 20.6 |
| India | Seshiah et al30 | 2008 | 4151 | 2 hours fasting 75 g OGTT, cut-off ≥140 mg/dL | 17.8 |
| US | Ferrara5 | 1996 | 28,330 | 1 hour 50 g GCT, cut-off 140 mg/dL, followed by 3 hours fasting 100 g OGTT | 4.8 |
| Australia | Moses et al20 | 2010 | 1275 | 1 hour 50 g GCT, 2 hours 75 g OGTT after overnight fast, cut-off 140 mg/dL (ADIPS) criteria | 9.5 |
| Canada | Ryan18 | 2010 | 4150 | Random plasma glucose screening and 2 hours 75 g OGTT | 17.8 |
| France | Schneider et al19 | 2006 | 11,545 | 2 hours fasting 75 g OGTT, cut-off ≥140 mg/dL | 12.1 |
| Qatar | Present study | 2010–2011 | 1608 | 2 hours fasting 75 g OGTT, cut-off ≥140 mg/dL | 16.3 Qatari: 8.8 Other Arab: 7.5 |
Abbreviations: ADIPs, Australasian Diabetes in Pregnancy Society; CHO, carbohydrate; GCT, glucose challenge test; OGTT, oral glucose tolerance test.
Figure 1.
Comparison of the associated factors of gestational diabetes mellitus (GDM) between women with GDM and normal women (non-GDM).
Notes: *P < 0.001; **P = 0.058.
Abbreviation: APH, antepartum hemorrhage.
Discussion
To the best of our knowledge, this study is the first attempt to examine the prevalence of GDM and its associated factors among women in Qatar. GDM is one of the most common medical complications in pregnancy, and there is a general consensus that the incidence of GDM is increasing globally. Through this prospective cohort study we have examined the prevalence of GDM and its associated factors in mothers and their offspring. The prevalence of GDM in our study sample was 16.3%, which is higher than the rates observed in the neighboring Gulf countries Bahrain14 and Saudi Arabia (12.5%),15 but lower than the rate of the United Arab Emirates (20.6%).16 These rates are comparable to the incidence rate reported in the literature that the incidence of GDM ranges 1%–14% of all pregnancies.17 Also, the prevalence rate in Qatar found a similar rate in developed countries like Canada (17.8%)18 and France (12.1%),19 but was higher than the rate observed in Australia (9.5%)20 and the US (4.8%).5 However, the study revealed that GDM was considerably higher among women in Qatar.
This study provides information about the risks of GDM, which could potentially help to incorporate early intervention measures. Our studied women with GDM had a higher risk of adverse health outcomes and were more likely to develop maternal complications. The most common risk factors observed in the studied women with GDM were advanced maternal age, low economic status, increasing maternal BMI, family history of diabetes, and parity. The potential outcomes associated with GDM were maternal complications like pregnancy-induced hypertension, pre-eclampsia, antepartum hemorrhage, cesarean, and neonatal complications like macrosomia, preterm birth, birth trauma, and congenital anomalies. A study conducted in Iran has also confirmed that there is a higher risk of pregnancy complications and adverse fetal outcomes with GDM.21
It was reported that socioeconomic status influences the prevalence of DM in pregnancy.22 On the contrary, there was no significant difference in level of education and job status between both the groups except for their advanced maternal age and low monthly income in the study sample. The proportion of GDM increased with increasing age: 13.4% in women below 25 years, 41.6% in women aged 25–34 years, and 45% in women aged 35–45 years. Although we did not find any significant difference between both the groups in their socioeconomic status, women with GDM were often of lower economic status (44.7%) and housewives (63%). This shows that women with GDM may have had a poor understanding of diabetes and its significance. To improve outcomes in women with GDM, vigorous attempts need to be made to raise their educational awareness.
In terms of family and medical history of women with GDM, the data revealed that GDM was more prevalent in women with a family history of diabetes (31.7%), those who were more parous (55.3%), and those who were obese (59.2%) compared with the normal group. Our study showed that overweight and obese women were more prone to developing GDM, as observed in other studies.23,24 Hence, obese women considering pregnancy should be informed of all the risks of maternal obesity and of how it can complicate their pregnancies. Epidemiologic studies have consistently identified a family history of diabetes and increased parity as primary risk factors for the development of GDM.25–27
In the study sample, the risk of adverse maternal outcome overall was higher in women with GDM compared with normal women. Women with GDM were at increased risk of developing pregnancy-induced hypertension (19.1%), pre- eclampsia (7.3%), antepartum hemorrhage (19.2%), preterm labor (19.8%), premature rupture of membrane (15.3%), and have cesarean section (27.9%) than those with a normal glucose tolerance. Similar results have been reported in previous studies28,29 that pregnancy-induced hypertension (17.9%) and cesarean session (17.1%) were higher in women with GDM than in those with normal glucose. Another study also indicated similar maternal complications among GDM women in China.7
For the neonates, the study findings revealed that they are at increased risk of macrosomia, birth injuries, and hyperbilirubinemia, which is similar to the results found in a study by Langer et al.4 The study identified an increased risk of macrosomia (10.3%), preterm birth (12.6%), jaundice (12.6%), and birth trauma (8%) among offspring of GDM mothers. This may be explained by the higher rate of pregnancy- induced hypertension and cesarean delivery for fetal distress among GDM mothers. Another study by Hong et al28 reported that infants born to GDM mothers were more likely to be born preterm (10.7% vs 6.4%) or have macrosomia (4.3% vs 1.7%). Prevention of macrosomia and perinatal complications are primary goals in the treatment of women with pregnancies complicated by GDM.
It is evident from the aforementioned study findings that the presence of maternal diabetes mellitus during pregnancy has important consequences for both mother and child. Stepwise logistic regression revealed that advanced maternal age, obesity, family history of diabetes, antepartum hemorrhage, cesarean section, and macrosomia were significant associated risk factors for GDM among women in Qatar. The greatest perinatal risk in GDM cases is fetal macrosomia, which has been associated with a higher rate of cesarean delivery. As seen in the results, GDM is seen more frequently in obese women and can be an important confounder for the association with birth weight.
Out of the six risk factors observed in the current study, obesity is a modifiable factor clearly associated with GDM. Hence, there is a need for more research on the effectiveness of various interventions aimed at reducing weight among women of reproductive age and their impact on pregnancy complications. This study demonstrated that the risk of these outcomes can be reduced by obstetricians with standard treatment consisting of individual dietary and lifestyle advice during pregnancy.
Conclusion
The present study revealed that GDM was higher in women in Qatar and that they were at increased risk of developing pregnancy-induced hypertension, pre-eclampsia, antepartum hemorrhage, premature rupture of membrane, and cesarean delivery. The risk of GDM increased steadily with maternal BMI, and obesity emerged as an essential risk factor for subsequent GDM. Infants born to women with GDM were significantly more likely to be macrosomic. Congenital anomalies and birth injuries were significantly higher in the offspring of GDM mothers. Advanced maternal age, low monthly income, family history of diabetes, and obesity were the main associated risk factors for GDM.
Acknowledgments
The project was supported partly by Qatar Diabetes Association, Qatar Foundation, and Qatar National Research Fund Grant No. UREP-08-090-3-018. We would also like to thank Hamad Medical Corporation (HMC Research Protocol #10146/10) and the Weill Cornell Medical College-Qatar Institutional Review Board (IRB# 2010-0021) for their ethical approval of this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30:S45–S46. [Google Scholar]
- 3.Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 2003;102:857–868. doi: 10.1016/j.obstetgynecol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192:989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara A. Increasing prevalence of gestational diabetes mellitus. Diabetes Care. 2007;30:S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 6.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long term effects of the intrauterine environment. Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21:B142–B149. [PubMed] [Google Scholar]
- 7.Yang X, Hsu-Hage B, Zhang H, et al. Gestational diabetes mellitus in women of single gravidity in Tianjin City, China. Diabetes Care. 2002;25:847–851. doi: 10.2337/diacare.25.5.847. [DOI] [PubMed] [Google Scholar]
- 8.Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84:99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Bener A, Zirie M, Al-Rikabi R. Genetics, obesity and environmental risk factors associated with type 2 diabetes. Croat Med J. 2005;46:302–307. [PubMed] [Google Scholar]
- 10.Bener A, Micallef R, Afifi M, Derbala M, Al-Mulla HM, Usmani MA. Association between type 2 diabetes mellitus and helicobacter pylori infection. Turk J Gastroenterol. 2007;18(4):225–229. [PubMed] [Google Scholar]
- 11.Bener A, Al-Saied A, Al-Ali MG, et al. Vitamin D deficiency in the young population with type 1 diabetes: a case-control study. Diabetes Res Clin Pract. 2008;79:S37–S37. [Google Scholar]
- 12.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Jang HC, Park HK, Metzger BE, Cho NH. Prevalence of type 2 diabetes among women with a previous history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2008;81:124–129. doi: 10.1016/j.diabres.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Al Mahroos S, Nagalla DS, Yousif W, Sanad H. A population based screening for gestational diabetes mellitus in non-diabetic women in Bahrain. Ann Saudi Med. 2005;25:129–133. doi: 10.5144/0256-4947.2005.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardawi MSM, Nasrat HA, Jamal HS, Al-Sagaaf HM, Mustafa BE. Screening for gestational diabetes mellitus in pregnant females. Saudi Med J. 2000;21:155–160. [PubMed] [Google Scholar]
- 16.Agarwal MM, Dhatt GS, Zayed R, Bali N. Gestational diabetes: relevance of diagnostic criteria and preventive strategies for type 2 diabetes mellitus. Arch Gynecol Obstet. 2007;276:237–243. doi: 10.1007/s00404-007-0334-4. [DOI] [PubMed] [Google Scholar]
- 17.Bevier WC, Jovanovic-Peterson L, Peterson CM. Diagnosis, management and outcome of gestational diabetes. Endocrinol Metab Clin North Am. 1995;24:103–138. [PubMed] [Google Scholar]
- 18.Ryan EA. Diagnosing gestational diabetes. Diabetologia. 2011;54:480–486. doi: 10.1007/s00125-010-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider S, Hoeft B, Freerksen N, et al. Neonatal complications and risk factors among women with gestational diabetes mellitus. Acta Obstetricia et Gynecologica Scandinavia. 2010;90:231–237. doi: 10.1111/j.1600-0412.2010.01040.x. [DOI] [PubMed] [Google Scholar]
- 20.Moses RG, Morris GJ, Petocz P, San Gil F, Garg D. The impact of potential new diagnostic criteria on the prevalence of gestational diabetes mellitus in Australia. Med J Aust. 2011;194:338–340. doi: 10.5694/j.1326-5377.2011.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 21.Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors, and pregnancy outcome. Diabetes Res Clin Pract. 2005;69:279–286. doi: 10.1016/j.diabres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.El-Hazmi MAF, Warsy AS, Al-Swailem AR, Al-Swailem AM, Sulaimani R. Diabetes mellitus as a health problem in Saudi Arabia. East Mediterr Health J. 1998;4:58–66. [Google Scholar]
- 23.Doherty DA, Mogann EF, Francis J, Morrison JC, Newnham JP. Pre- pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet. 2006;95:242–247. doi: 10.1016/j.ijgo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Hadaegh F, Tohidi M, Harati H, Kheirandish M, Rahimi S. Prevalence of GDM in southern Iran (Bandar Abbas City) Endocr Pract. 2005;11:313–318. doi: 10.4158/EP.11.5.313. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of GDM and its association with type 2 diabetes. Diabet Med. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 26.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guideline. Aust N Z J Obstet Gynaecol. 2011;51:26–30. doi: 10.1111/j.1479-828X.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 27.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 28.Hong JU, Rumbold AR, Wilson KJ, Crowther CA. Borderline gestational diabetes mellitus and pregnancy outcomes. BMC Pregnancy Childbirth. 2008;8:31. doi: 10.1186/1471-2393-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunderson EP, Lewis CE, Tsai AL, et al. A 20 year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception; the Coronary Artery Risk Development In Young Adults (CARDIA) Study. Diabetes. 2007;56:2990–2996. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshiah V, Balaji V, Balaji MS, et al. Prevalence of gestational diabetes mellitus in south India (Tamil Nadu): a community based study. J Assoc Physicians India. 2008;56:329–333. [PubMed] [Google Scholar]
- 31.Tan PC, Ling LP, Omar SZ. Screening for gestational diabetes at antenatal booking in a Malaysian university hospital: the role of risk factors and threshold value for the 50 g glucose challenge test. Aust N Z J Obstet Gynaecol. 2007;47:191–197. doi: 10.1111/j.1479-828X.2007.00717.x. [DOI] [PubMed] [Google Scholar]