Abstract
Objective
To identify C. trachomatis antigens that can be used to differentially diagnose tubal factor infertility in comparison to previously reported Heat Shock Protein 60 (HSP60).
Design
In Vitro Study
Patients
Infertile women with and without tubal pathology diagnosed laparoscopically.
Setting
Academic medical center.
Main Outcome Measures
Antibody responses to C. trachomatis in infertile women with or without tubal pathologies using a C. trachomatis genome-wide proteome array.
Results
Comparison of the antibody profiles revealed 30 C. trachomatis antigens that were preferentially recognized by tubal factor infertility women with a detection sensitivity and specificity of 80.6% and 56.5%, respectively, 10 of which showed 100% specificity. A combination of CT443 and CT381 antigens yielded the highest detection sensitivity (67.7%) while maintaining 100% specificity.
Conclusion
These findings have demonstrated that antibodies to CT443 and CT381, when used in combination, have higher sensitivity and specificity in predicting tubal factor infertility than other indicators for tubal factor infertility such as HSP60 antibodies (35.5%, 100%) or hysterosalpingogram (65%, 83%). Using a panel of C. trachomatis antigens to serologically diagnose tubal factor infertility can save the patients from undertaking expensive and invasive procedures for determining tubal pathology and choosing treatment plans.
Keywords: Tubal factor infertility, Chlamydia trachomatis, Heat Shock Protein 60, Outer Membrane Protein Complex B
Introduction
Twenty-five to 35% of patients presenting for infertility evaluation have tubal disease (1-4). Chlamydia trachomatis is the primary sexually transmitted infection responsible for tubal factor infertility (TFI) (5-7) with C. trachomatis antibodies in approximately 70% of patients (8). C. trachomatis infected cells produce inflammatory cytokines (9, 10) which may contribute to upper genital tract inflammatory damage (11-13). Lunefeld, et al found that among patients undergoing in vitro fertilization, those with C. trachomatis antibodies had decreased pregnancy rates (14).
C. trachomatis infection is often asymptomatic so patient history cannot dictate the presence of tubal disease (15, 16). Searching for biomarkers to predict chlamydial infection-associated tubal infertility is under intensive investigation. Elevated titers of anti-C. trachomatis antibodies are associated with TFI, but detection of overall antibody levels lacks the sensitivity and specificity required for differential diagnosis (17).
Measuring anti-C. trachomatis antibodies at the single antigen level may offer increased sensitivity and specificity for predicting TFI. Elevated anti-chlamydial heat shock protein 60 (HSP60, CT110) antibodies are associated with TFI (18-27). Anti-HSP60 antibodies are associated with decreased pregnancy rates in patients with an ectopic pregnancy history (17). When HSP60 antibodies are in follicular fluid, there are decreased implantation rates (28, 29). Some have postulated that chlamydial HSP60 incites a strong inflammatory response that may cross-react with the highly conserved human HSP60 (25, 30, 31). HSP60 may induce T-cell responses that contribute to the tubal damage (32, 33). Regardless of how HSP60 or anti-HSP60 antibodies can mechanistically contribute to tubal disease, the specificity of anti-HSP60 antibody as a predictor for TFI has demonstrated that it may be used to differentially diagnose TFI.
We have developed a C. trachomatis whole-genome scale protein array that can profile antigen specificities of anti-Chlamydia trachomatis antibodies (34). We hypothesized that C. trachomatis-infected women with TFI or controls (IFC) may develop antibodies recognizing unique sets of antigens.
Materials and Methods
Human patients
31 TFI and 23 IFC patients were enrolled at the University of Texas Health Science Center at San Antonio following Institutional Review Board approval. All women were at least 21 years old and underwent diagnostic laparoscopy with chromotubation as part of their infertility evaluation. Diagnosis of tubal infertility was defined as fallopian pathology consistent with hydrosalpinx, fimbrial phimosis, or peri-tubal adhesions. Exclusion criteria included prior tubal ligation, surgical finding of endometriosis, or a history of pelvic infection or inflammation other than pelvic inflammatory disease such as appendicitis. IFC patients had normal pelvic findings and tubal patency at laparoscopy. After the blood draw, serum samples were stored at −20°C until analyzed.
Cell culture and chlamydial infection
As previously described, HeLa cells (American Type Culture Collection, Manassas VA 20108) were cultured in DMEM (GIBCO PRL, Rockville, MD) with 10% fetal calf serum (FCS; GIBCO BRL) at 37°C with 5% Carbon dioxide (CO2) (34-36). C. trachomatis serovar D or Chlamydia pneumoniae AR39 organisms were grown, purified and titrated as previously described (36-38). For immunofluorescence assay, chlamydial organisms were used to infect HeLa cells grown on glass coverslips in 24-well plates. The sub-confluent HeLa cells were treated with DMEM containing 30 μg/ml of DEAE-Dextran (Sigma, St. Louis, MO) for 10 minutes at 37°C. After removal of DEAE-Dextan solution, chlamydial organisms were added to the wells for 2 hours at 37°C. The infected cells were continuously cultured in DMEM with 10% FCS and 2μg/ml of cycloheximide (Sigma, St. Louis, MO).
Immunofluorescence assay (IFA)
Anti-chlamydial organism antibodies in human sera were titrated using an Immunofluorescence assay (IFA) as previously described (34, 36, 39, 40). Briefly, HeLa cells grown on coverslips were infected with C. trachomatis or C. pneumoniae organisms, fixed 48h post-infection for C. trachomatis and 72h for C. pneumoniae with 2% paraformaldehyde, and permeabilized with 2% saponin at room temperature for 1 hour. After blocking, human sera were added to the Chlamydia-infected cell samples. The primary Ab binding was visualized with a goat anti-human IgG conjugated with Cy3 (red; Jackson ImmunoResearch Laboratories, West Grove, PA), and DNA was labeled with Hoechst dye (blue; Sigma-Aldrich). The highest dilution of a serum that still gave a positive reactivity was defined as the titer of the given serum sample. Serum samples were serially diluted and the appropriate dilutions were repeated multiple times based on the results obtained from prior dilutions in order to obtain a more accurate titer for each serum. Images were acquired with an Olympus AX70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY) as previously described (36, 40).
Chlamydial fusion protein-arrayed microplate Enzyme-linked immunosorbent assay (ELISA)
Glutathione S-transferase (GST) fusion protein enzyme-linked immunosorbent assay (ELISA) for detecting human antibody recognition of chlamydial proteins was carried out as previously described (36). Bacterial lysates containing individual chlamydial GST fusion proteins were added to 96 well microplates pre-coated with glutathione (Pierce, Rockford, IL) at a 1:10 dilution in PBS with a total volume of 200 μl/well. Lysates containing GST alone, as negative, and GST-chlamydial protease-like activity factor (CPAF), as positive controls, were also included on each plate. The plates were incubated overnight at 4°C to allow GST fusion proteins to bind to the plate-immobilized glutathione then blocked with 2.5% milk in PBS and washing with PBST (PBS with 0.05% Tween 20; Sigma-Aldrich).
The human sera was pre-absorbed with a bacterial lysates containing GST at 4°C overnight, then incubated with Glutathione beads (bioWorld, Dublin, OH) for 1 hour at room temperature to reduce background caused by non-specific human antibodies. The human antibody reactivity was detected with a goat anti-human-IgG, IgA & IgM conjugated with horse-radish peroxidase (HRP; Jackson ImmunoResearch Laboratories) plus the substrate 2,2′-azino-bi(2-ethylbenzothiazoline-6-sulforic acid) diammonium salt (ABTS; Sigma). The optical density (OD) was measured at 405nm using a microplate reader (Molecular Devices Corporation, Sunnyvale, CA). To confirm the antibody binding specificity, all sera were further absorbed with lysates made from either HeLa cells alone or C. trachomatis serovar D-infected HeLa cells prior to reacting with the fusion protein-coated plates. The absorption was carried out as following: HeLa cells with or without chlamydial infection were lysed via sonication at 2 × 107 cells per ml of PBS containing a cocktail of protease inhibitors. The pre-diluted serum samples were incubated with cell lysates overnight at 4°C prior to reacting with the plate-immobilized chlamydial fusion proteins. The antibody binding that remained positive after HeLa-alone lysate absorption but significantly reduced by Chlamydia-HeLa lysate absorption was considered true positive.
Data Analyses
Data were analyzed using SPSS v. 15.0 software (IBM, Chicago, IL) as previously described (36, 39). Briefly, titer values were log-transformed to produce a normal distribution and analyses were performed on transformed values. Student’s t-Test was utilized to assess anti-C. trachomatis and anti-C. pneumoniae antibodies to evaluate overall mean differences between the 2 groups of patients. Because the a priori hypothesis was that the TFI group would have higher titers than the IFC group, a one-tailed analysis was used for the C. trachomatis data, but a two-tailed analysis was performed on the C. pneumonia because there was no a priori hypothesis. Because the antibody titers had large variations within a given group, the serum titers were evaluated by ranges of <1:10 (Negative), 1:10 to 1:10,000 (Low), and >1:10,000 (High). Chi-Squared and Fisher’s Exact Test were employed to compare overall antibodies to C. trachomatis and antibodies to C. pneumoniae.
ELISA results were analyzed using Student’s t-Test and Fisher’s Exact Test as appropriate. For the genome-wide ELISA, both Student’s t-Test (for comparing quantitative OD value data) and Fisher’s Exact Test (for comparing the number of sera positively reacted with a given antigen) were preformed. Using both methods allows us to identify C. trachomatis antigens that are both clinically and statistically significant. When Student’s t-Test was utilized, the OD values after subtracting background from the same plate were used. When Fisher’s Exact Test was utilized, a response was determined positive when the OD value was equal to or greater than 2 standard deviations above the mean calculated from the same 96 well plate as described previously (39, 41).
Results
I. Infertile women with laparoscopy-identified tubal pathologies developed significantly higher titers of anti-C. trachomatis antibodies
Sera from TFI or IFC were titrated using HeLa cells infected with either C. trachomatis or C. pneumoniae organisms as antigens in an IFA. TFI Patients developed high titers of antibodies to C. trachomatis (p<0.001) but not C. pneumoniae (p=0.269) (Table 1). When the patients were categorized based on levels of anti-chlamydial antibodies, most TFI patients developed high titers of anti-C. trachomatis antibodies (61.3%) while most IFC patients displayed lower titers (82.6%; p<0.001).
Table 1. Titers of antibodies against C. trachomatis and C. pneumoniae in infertile women with or without tubal pathology.
Serum samples from women with TFI and IFC were 2 fold serially diluted starting with 1:10 and reacted with HeLa cells infected with either C. trachomatis or C. pneumoniae. The antibody reactivity was detected using an immunofluorescence assay as described in the materials and method section. The highest dilution that still gave a positive reactivity was defined as the serum titer. Each serum sample was titrated in triplicate and the average was used as the geometric titer of a given serum sample. Student’s t-Test was used to quantitatively analyze the differences between the two groups of patients. There is a statistically significant difference in titers of antibodies against C. trachomatis (p<0.001) but not C. pneumoniae (p=0.269) organisms. When the serum samples were divided into 3 categories (Negative, Low & High) based on antibody titers, the qualitative analysis with Chi-squared test still revealed a significant difference in the number of sera in different categories between the two groups of patients for antibodies against C. trachomatis (p<0.001) but not C. pneumoniae (p=0.634) organisms. Further pairwise Chi-squared analyses of the anti-C. trachomatis antibodies revealed significant differences between the high vs. low and high vs. negative groups. The number of individuals with high titers of anti-C. trachomatis antibodies in TFI group is significantly higher than that in the IFC group.
| Antibodies to C. trachomatis | Antibodies to C. pneumoniae | ||||
|---|---|---|---|---|---|
| TFI (n=31) | IFC (n=23) | TFI (n=31) | IFC (n=23) | ||
| Quantitative analyses |
Mean | 69928 | 3814 | 41503 | 25861 |
| Standard Deviation |
106709 | 8270 | 65848 | 35847 | |
| Student’s t Test |
p=0.0009 | p=0.269 | |||
| Categorization of serum samples into Negative, Low and High titer groups | |||||
| Qualitative analyses | Negative titers (<1:10) |
1 (3.2%) | 3 (13.0%) | 3 (9.7%) | 4 (17.4%) |
| Low titers (1:10- 1:10000) |
11 (35.5%) | 19 (82.6%) | 11 (34.5%) | 6 (26.1%) | |
| High titers (>1:10000) |
19 (61.3%) | 1 (4.4%) | 17 (54.8%) | 13 (56.5%) | |
| χ2 Test | p < 0.001 | p = 0.634 | |||
| Pairwise χ2 Tests |
High vs. Negative | p = 0.008 | N/A | ||
| High vs. Low | p < 0.001 | ||||
| Low vs. Negative | p = 0.556 | ||||
II. Identification of C. trachomatis antigens preferentially recognized by infertile women with or without tubal pathology
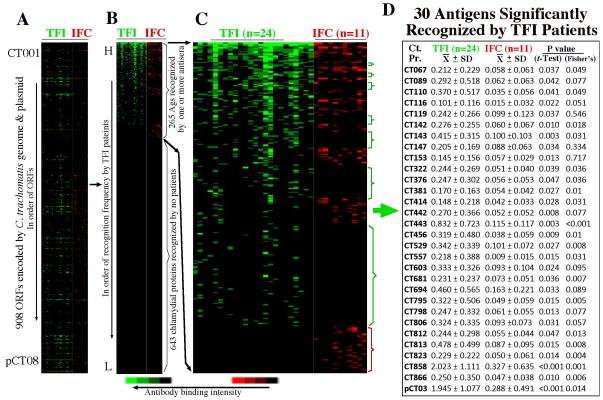
It is difficult to use the quantitative difference in overall anti-C. trachomatis antibodies to diagnose TFI. To identify antigens that are recognized by TFI patients, Anti-C. trachomatis antibodies in 24 TFI and 11 IFC patients were mapped at the genome-wide scale since these patients displayed an overall anti-C. trachomatis antibody titer above 1:1000. As shown in Fig.1, these 35 sera recognized C. trachomatis antigens distributed across the genome (panel A) with 265 antigens recognized by at least one antiserum and 643 antigens not detected by any sera (B). Many C. trachomatis antigens are recognized by both groups of patients, but there are antigens preferentially recognized by either group as highlighted with color-coded brackets (C). Thirty antigens were significantly recognized by TFI based on either mean OD values (Student’s t-test) or recognition frequency (Fisher’s exact test). Reactivity was confirmed using absorption against either HeLa alone or C. trachomatis-infected HeLa lysates as described previously (36).
Figure 1. Reactivity of 54 infertility sera with 933 GST-chlamydial fusion proteins representing 908 unique ORFs of C. trachomatis.
Each of the human Antibodies (displayed along the x-axis on top of each panel) after 1/2000 dilution was reacted with each of the 933 GST fusion proteins (listed along the y-axis) immobilized onto the 96-well microplates. Each colored bar represents a positive reactivity between a given fusion protein and a patient serum with the tubal factor infertility (TFI) patient sera in green and infertile control (IFC) in red. The results are expressed as optical density (OD) readings obtained at the wavelength of 405 nm. Any reaction with an OD ≥ mean + 2 Standard deviations calculated from the same plate is defined positive. The positive OD values are expressed as binding intensity in increasing brightness of fluorescent color. The negative OD values are black. The 933 fusion proteins representing the C. trachomatis genome are first listed in order of the ORFs from CT001 to pCT08 (panel A). Both letter and number extensions were used to distinguish multiple fusion proteins and protein fragments that share the same ORF names. (B) The 933 fusion proteins were then reordered based on binding frequency by the TFI sera from high (top) to low (bottom). As indicated on the right of the panel, a total of 265 ORFs were recognized by one or more sera. (C) By visual inspection of the expanded view of the positive antigens, the fusion proteins preferentially recognized by either TFI (green) or IFC (red) patients were marked with brackets on the right of the panel. (D) A total of 30 antigens were significantly recognized by TFI patients.
III. Identification of C. trachomatis antigens uniquely recognized by TFI patients
To identify antigens that can be used to predict TFI in infertility clinics, we identified antigens that were uniquely recognized by TFI patients. The 30 antigens preferentially recognized by TFI patients (as described above) were reacted with sera from all 54 patients (including 31 TFI and 23 IFC) regardless of their overall anti-C. trachomatis antibody titers. As listed in Figure 2, the HSP60 (CT110) reacted with 22 of the 31 TFI and 4 of 23 IFC sera with a specificity and sensitivity of 82.6% and 71.0% respectively in predicting TFI.
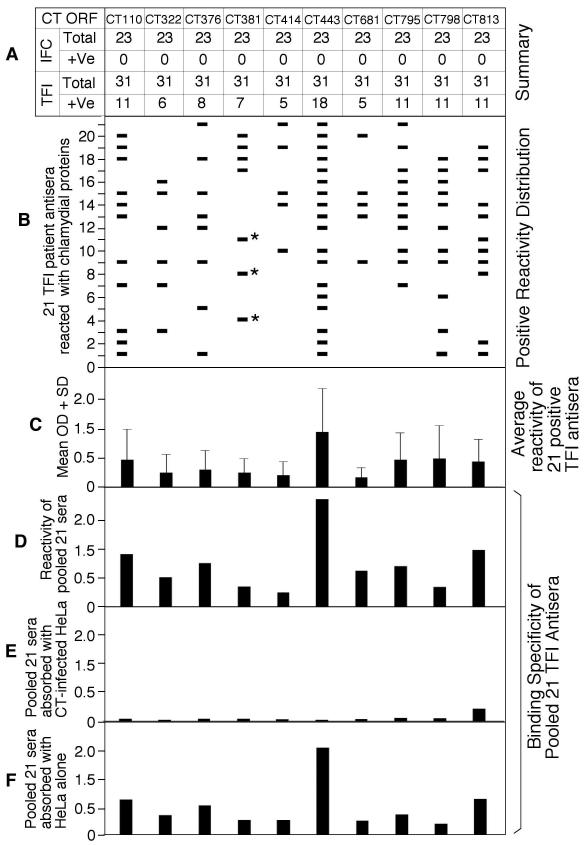
Figure 2. Reactivity of 30 C. trachomatis antigens with 54 patient sera at 1:4000 dilution.
The 30 antigens were reacted with the 54 human sera as described in table Table 2 note except that each serum was diluted 1:4000 (data not shown). (A) Ten of the 30 antigens, failing to react with any of the 23 IFC sera, were thus presented in the figure. Note that HSP60 (CT110) and OmcB (CT443) maintained a detection sensitivity of 35.5% and 58% respectively. (B) The reactivity of each of the 10 antigens was analyzed at individual antiserum level. Note that the combinations of CT443 with CT381 or HSP60 with CT376, CT381 & CT798 can have the highest sensitivity of 67.7% while maintaining 100% specificity. (C) The reactivity intensity between each antigen and the 21 positive sera (measured at individual antiserum level) was expressed as mean OD plus standard deviation. (D) Each of the 10 antigen were reacted with an antiserum sample pooled from the 21 sera at equal ratio without (D) or with absorption with C. trachomatis (CT)-infected HeLa lysate (E) or HeLa alone lysate (F). Note that absorption with CT-HeLa but not HeLa alone lysates removed the reactivity of each antigen with the pooled antiserum.
To further increase specificity, a 5-fold dilution (final dilution of 1:4000) was used to reduce the false-positive rate (Figure 2). At this dilution, 10 of the 30 antigens, including CT110, CT322, CT376, CT381, CT414, CT443, CT681, CT795, CT798 & CT813, failed to react with any sera from the IFC group. Thus, these 10 antigens were uniquely recognized by TFI patients with a detection specificity of 100% (panel A). Dilution of the samples decreased detection sensitivity. HSP60 (CT110) only reacted with 11 out of 31 TFI sera, dropping the detection sensitivity to 35.5% along with three immunodominant antigens (CT795, CT798, CT813). CT443 reacted with 18 of the 31 TFI sera, maintaining a sensitivity of 58.1%. When the reactivity of the 10 antigens was analyzed at each individual antiserum level (Panel B), we found that the 10 antigens all together reacted with 21 independent sera of 31 total TFI sera, with a sensitivity of 67.7%. More importantly, this sensitivity can be maintained using fewer antigens. Combining HSP60 with CT376, CT381 & CT798 (total 4 antigens) or CT443 with CT381 (only 2 antigens) maintains sensitivity of 67.7%. As for other immunodominant antigens with a detection specificity of <100%, their detection sensitivity can be 80% even after serum dilution. Due to their ability to generating false positive results by reacting with IFC samples, it is clinically undesirable to use these antigens for screening for TFI.
Discussion
C. trachomatis organisms cause pathologies in the fallopian tubes, leading to complications such as ectopic pregnancy and infertility. Since infertility can be caused by many different factors, distinguishing tubal infertility from other causes is required for guiding treatment plans. There is an ongoing debate on whether C. trachomatis-specific serology can aid in differential diagnosis of TFI. The goal of the current study is to test whether we can identify C. trachomatis antigens that can improve specificity and sensitivity in detecting TFI.
The finding that anti-C. trachomatis but not anti-C. pneumoniae antibodies are highly associated with TFI is consistent with a well-established concept in the literature (42-44). Efforts have been made to develop individual C. trachomatis antigen-based detection methods. Previous reports demonstrate that anti-HSP60 antibodies are detected in 70-80% of TFI patients (21, 22, 45, 46). Our genome-wide search for additional markers of TFI not only confirmed these findings but also revealed new information for further increasing specificity and sensitivity in detecting TFI.
30 antigens were preferentially recognized by TFI patients. At 1:800 dilution, HSP60 reacted with 22 of the 31 TFI sera (71% sensitivity) and 4 of the 23 IFC sera (82.6% specificity), which is consistent with previous findings and suggests our ELISA is comparable to other assays. When the sera were diluted to 1:4000 in order to further increase specificity, 10 of the 30 antigens achieved 100% specificity. Although the sensitivity decreased, careful examination revealed that a combination of 2 antigens [CT381 and CT443 (Outer membrane complex B, OmcB)], or 4 antigens [CT110 (HSP60), CT376, CT381 & CT798] detected TFI with a specificity and sensitivity of 100% and 67.7% respectively (Fig. 2). We conclude that these combinations of antigens improved the C. trachomatis serology approach for diagnosing tubal infertility over using HSP60 alone (35.5% sensitivity), which represents a clinically significant improvement.
Hysterosalpingogram (HSG) has a detection specificity and sensitivity of 83% and 65% respectively for detecting tubal pathology (47). C. trachomatis antigen-based serology diagnosis has numerous advantages over HSG besides improved detection, including sparing patients from the discomfort, radiation, and potential for infectious sequellae. This conclusion is consistent with previous reports that elevated chlamydial antibody levels are comparable to HSG (48) in diagnosing TFI and that HSG does not add to the medical knowledge on whether C. trachomatis infection contribute to tubal pathology (49).
It is unknown whether these antigens themselves or antigen-specific immune responses contribute to the inflammatory pathologies in the fallopian tubes. The protein CT443, or OmcB, displayed the highest rate of reactivity with TFI patient sera. OmcB is a highly conserved immunodominant antigen, but the precise location of OmcB in the organisms and its role during infection is poorly understood (34, 50-52).
Despite the overwhelming evidence of C. trachomatis infection association with TFI, not every patient is infected with C. trachomatis or developed immune responses to C. trachomatis. Interventions such as early antibiotic therapy may cause a negative or low titers in patients, but it is unlikely that tubal pathology would be attributed to the C. trachomatis infection in these patients. Tubal pathology in TFI patients without positive C. trachomatis titers might be caused by other sources of infection such as Neisseria gonorrhoeae (53, 54) and Mycoplasma genitalium (55, 56). Thus, to further increase the sensitivity in diagnosing TFI, other infection causes should also be taken into account.
The current study has presented a promising approach for globally identifying C. trachomatis-specific serological markers for diagnosis of TFI, Although statistically, the identified antigens or their combinations seem to provide definitive diagnosis of TFI, large sample sizes of infertile women from more diverse sources must be analyzed with a genome-wide scale proteome assay to maximize the potential of the C. trachomatis antigen-specific serology-based diagnosis of TFI. Efforts are underway to expand our studies to China where we hope to enroll several thousand patients.
Capsule.
Chlamydia trachomatis proteome array identified 30 antigens which are preferentially recognized by tubal factor infertility patients compared to controls. Combining CT443 and CT381 has 100% specificity and 67.7% sensitivity.
Table 2.
Reactivity of 30 significant C. trachomatis proteins with 31 TFI & 23 IFC patient antisera (@1:800)
The 30 C. trachomatis antigens significantly recognized by 24 TFI patients as identified in Fig.1 were reacted with 54 patient sera (33 TFI and 21 IFC). All sera were diluted at 1:800 regardless of their over anti-C. trachomatis antibody titers as determined with the immunofluorescence assay. The mean ODs of each antigen were compared between TFI and IFC groups were compared using Student t-Test and the corresponding p values were listed in the last column of the left panel, confirming that all 30 antigens were significantly recognized by TFI patients. The number of positive recognition (determined as described Fig. 1 legend) by either TFI or IFC was used to calculate recognition specificity and sensitivity as well as positive or negative predicting values (PPV or NPV; right panel). Note that HSP60 (CT110) displayed a detection specificity of 82.6% and sensitivity of 71% and many other immundominant antigens such as pCT03 (Pgp3, a plasmid-encoded secreted protein), CT858 (CPAF, a chlamydial protease/proteasome-like activity factor that is secreted into host cell cytosol), CT823 (cHtrA, a secreted stress response serine protease), CT813 (an inclusion membrane protein), CT443 (OmcB, outer membrane complex protein B) & CT143 (a hypothetical protein) behaved similarly. Only the hypothetical protein CT557 had a 100% specificity but its sensitivity was only 29%. Thus, under this assay condition, no single antigen or combinations of antigens can achieve 100% specificity with a sensitivity of >50%.
| CT | TFI (n=31) | IFC (n=23) | t-Test | Specificity | Sensitivity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| ORF | X +/− SD | X +/− SD | |||||
| CT067 | 0.407 ± 0.483 | 0.045 ± 0.115 | <0.001 | 87.0% | 51.6% | 84.2% | 57.1% |
| CT089 | 0.645 ± 0.936 | 0.189 ± 0.407 | 0.020 | 78.3% | 45.2% | 73.7% | 51.4% |
| CT110 | 0.679 ± 0.756 | 0.069 ± 0.112 | <0.001 | 82.6% | 71.0% | 84.6% | 67.9% |
| CT116 | 0.176 ± 0.252 | 0.012 ± 0.059 | 0.001 | 95.7% | 32.3% | 90.9% | 51.2% |
| CT119 | 0.375 ± 0.501 | 0.082 ± 0.196 | 0.005 | 87.0% | 48.4% | 83.3% | 55.6% |
| CT142 | 0.468 ± 0.522 | 0.098 ± 0.138 | 0.001 | 78.3% | 54.8% | 77.3% | 56.3% |
| CT143 | 1.012 ± 0.818 | 0.166 ± 0.220 | <0.001 | 73.9% | 71.0% | 78.6% | 65.4% |
| CT147 | 0.789 ± 0.678 | 0.303 ± 0.185 | 0.001 | 34.8% | 80.6% | 62.5% | 57.1% |
| CT153 | 0.404 ± 0.561 | 0.071 ± 0.110 | 0.003 | 91.3% | 45.2% | 87.5% | 55.3% |
| CT322 | 0.366 ± 0.586 | 0.055 ± 0.112 | 0.007 | 95.7% | 41.9% | 92.9% | 55.0% |
| CT376 | 0.453 ± 0.616 | 0.072 ± 0.097 | 0.002 | 95.7% | 41.9% | 92.9% | 55.0% |
| CT381 | 0.330 ± 0.346 | 0.059 ± 0.074 | <0.001 | 95.7% | 51.6% | 94.1% | 59.5% |
| CT414 | 0.327 ± 0.469 | 0.061 ± 0.082 | 0.004 | 95.7% | 51.6% | 94.1% | 59.5% |
| CT442 | 0.486 ± 0.622 | 0.055 ± 0.070 | 0.001 | 91.3% | 48.4% | 88.2% | 56.8% |
| CT443 | 1.145 ± 1.020 | 0.110 ± 0.173 | <0.001 | 87.0% | 71.0% | 88.0% | 69.0% |
| CT456 | 0.803 ± 0.879 | 0.241 ± 0.558 | 0.006 | 73.9% | 64.5% | 76.9% | 60.7% |
| CT529 | 0.854 ± 0.644 | 0.444 ± 0.310 | 0.003 | 13.0% | 87.1% | 57.4% | 42.9% |
| CT557 | 0.358 ± 0.638 | 0.028 ± 0.057 | 0.007 | 100% | 29.0% | 100% | 51.1% |
| CT603 | 0.579 ± 0.654 | 0.141 ± 0.161 | 0.001 | 78.3% | 61.3% | 79.2% | 60.0% |
| CT681 | 0.363 ± 0.386 | 0.060 ± 0.078 | <0.001 | 87.0% | 51.6% | 84.2% | 57.1% |
| CT694 | 0.698 ± 0.848 | 0.150 ± 0.293 | 0.002 | 78.3% | 54.8% | 77.3% | 56.3% |
| CT795 | 0.647 ± 0.771 | 0.034 ± 0.101 | <0.001 | 95.7% | 61.3% | 95.0% | 64.7% |
| CT798 | 0.622 ± 0.827 | 0.038 ± 0.089 | <0.001 | 91.3% | 51.6% | 88.9% | 58.3% |
| CT806 | 0.673 ± 0.772 | 0.104 ± 0.212 | <0.001 | 82.6% | 54.8% | 81.0% | 57.6% |
| CT812 | 0.555 ± 0.667 | 0.061 ± 0.087 | <0.001 | 91.3% | 54.8% | 89.5% | 60.0% |
| CT813 | 0.673 ± 0.689 | 0.095 ± 0.165 | <0.001 | 78.3% | 67.7% | 80.8% | 64.3% |
| CT823 | 0.649 ± 0.709 | 0.071 ± 0.074 | <0.001 | 91.3% | 71.0% | 91.7% | 70.0% |
| CT858 | 1.947 ± 1.276 | 0.338 ± 0.666 | <0.001 | 78.3% | 74.2% | 82.1% | 69.2% |
| CT866 | 0.574 ± 0.738 | 0.062 ± 0.079 | 0.001 | 91.3% | 48.4% | 88.2% | 56.8% |
| pCT03 | 1.761 ±1.366 | 0.166 ± 0.573 | <0.001 | 82.6% | 74.2% | 85.2% | 70.4% |
Acknowledgement
This work was supported in part by grant R01AI64537 (to G. Zhong) from the National Institutes of Health. Both NB and SG have contributed equally and should be considered second authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healy DL, Trounson AO, Andersen AN. Female infertility: causes and treatment. Lancet. 1994;343:1539–1544. doi: 10.1016/s0140-6736(94)92941-6. [DOI] [PubMed] [Google Scholar]
- 2.Musich JR, Behrman SJ. Surgical management of tubal obstruction at the uterotubal junction. Fertil Steril. 1983;40:423–441. doi: 10.1016/s0015-0282(16)47349-8. [DOI] [PubMed] [Google Scholar]
- 3.Serafini P, Batzofin J. Diagnosis of female infertility. A comprehensive approach. J Reprod Med. 1989;34:29–40. [PubMed] [Google Scholar]
- 4.Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Zbucka M, Wolczynski S, Grygoruk C, et al. Chlamydia trachomatis urogenital infection in women with infertility. Adv Med Sci. 2009;54:82–85. doi: 10.2478/v10039-009-0007-6. [DOI] [PubMed] [Google Scholar]
- 5.Confino E, Tur-Kaspa I, DeCherney A, Corfman R, Coulam C, Robinson E, et al. Transcervical balloon tuboplasty. A multicenter study. JAMA. 1990;264:2079–2082. [PubMed] [Google Scholar]
- 6.Grant A. Infertility surgery of the oviduct. Fertil Steril. 1971;22:496–503. [PubMed] [Google Scholar]
- 7.Malik A, Jain S, Rizvi M, Shukla I, Hakim S. Chlamydia trachomatis infection in women with secondary infertility. Fertil Steril. 2009;91:91–95. doi: 10.1016/j.fertnstert.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 8.Barlow RE, Cooke ID, Odukoya O, Heatley MK, Jenkins J, Narayansingh G, et al. The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in-situ hybridisation. J Med Microbiol. 2001;50:902–908. doi: 10.1099/0022-1317-50-10-902. [DOI] [PubMed] [Google Scholar]
- 9.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 10.Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun. 2008;76:942–951. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajonuma LC, Ng EH, Chan HC. New insights into the mechanisms underlying hydrosalpinx fluid formation and its adverse effect on IVF outcome. Hum Reprod Update. 2002;8:255–264. doi: 10.1093/humupd/8.3.255. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M, Sethi S, Daftari S, Malhotra S. Evidence of chlamydial infection in infertile women with fallopian tube obstruction. Indian J Pathol Microbiol. 2003;46:680–683. [PubMed] [Google Scholar]
- 13.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun. 2008;76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunenfeld E, Shapiro BS, Sarov B, Sarov I, Insler V, Decherney AH. The association between chlamydial-specific IgG and IgA antibodies and pregnancy outcome in an in vitro fertilization program. J In Vitro Fert Embryo Transf. 1989;6:222–227. doi: 10.1007/BF01132869. [DOI] [PubMed] [Google Scholar]
- 15.El Hakim EA, Epee M, Draycott T, Gordon UD, Akande VA. Significance of positive Chlamydia serology in women with normal-looking Fallopian tubes. Reprod Biomed Online. 2009;19:847–851. doi: 10.1016/j.rbmo.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 17.Sziller I, Fedorcsak P, Csapo Z, Szirmai K, Linhares IM, Papp Z, et al. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60-kDa heat shock protein is associated with decreased spontaneous fertility rate in ectopic pregnant women treated by salpingectomy. Am J Reprod Immunol. 2008;59:99–104. doi: 10.1111/j.1600-0897.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 18.Brunham RC, Maclean IW, Binns B, Peeling RW. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985;152:1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- 19.LaVerda D, Kalayoglu MV, Byrne GI. Chlamydial heat shock proteins and disease pathology: new paradigms for old problems? Infect Dis Obstet Gynecol. 1999;7:64–71. doi: 10.1155/S1064744999000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkin SS, Jeremias J, Toth M, Ledger WJ. Cell-mediated immune response to the recombinant 57-kDa heat-shock protein of Chlamydia trachomatis in women with salpingitis. J Infect Dis. 1993;167:1379–1383. doi: 10.1093/infdis/167.6.1379. [DOI] [PubMed] [Google Scholar]
- 21.Toye B, Laferriere C, Claman P, Jessamine P, Peeling R. Association between antibody to the chlamydial heat-shock protein and tubal infertility. J Infect Dis. 1993;168:1236–1240. doi: 10.1093/infdis/168.5.1236. [DOI] [PubMed] [Google Scholar]
- 22.Ault KA, Statland BD, King MM, Dozier DI, Joachims ML, Gunter J. Antibodies to the chlamydial 60 kilodalton heat shock protein in women with tubal factor infertility. Infect Dis Obstet Gynecol. 1998;6:163–167. doi: 10.1002/(SICI)1098-0997(1998)6:4<163::AID-IDOG5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieterle S, Wollenhaupt J. Humoral immune response to the chlamydial heat shock proteins hsp60 and hsp70 in Chlamydia-associated chronic salpingitis with tubal occlusion. Hum Reprod. 1996;11:1352–1356. doi: 10.1093/oxfordjournals.humrep.a019387. [DOI] [PubMed] [Google Scholar]
- 24.Tiitinen A, Surcel HM, Halttunen M, Birkelund S, Bloigu A, Christiansen G, et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Hum Reprod. 2006;21:1533–1538. doi: 10.1093/humrep/del014. [DOI] [PubMed] [Google Scholar]
- 25.Linhares IM, Witkin SS. Immunopathogenic consequences of Chlamydia trachomatis 60 kDa heat shock protein expression in the female reproductive tract. Cell Stress Chaperones. 2010;15:467–473. doi: 10.1007/s12192-010-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karinen L, Pouta A, Hartikainen AL, Bloigu A, Paldanius M, Leinonen M, et al. Antibodies to Chlamydia trachomatis heat shock proteins Hsp60 and Hsp10 and subfertility in general population at age 31. Am J Reprod Immunol. 2004;52:291–297. doi: 10.1111/j.1600-0897.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 27.Karinen L, Pouta A, Hartikainen AL, Bloigu A, Paldanius M, Leinonen M, et al. Association between Chlamydia trachomatis antibodies and subfertility in the Northern Finland Birth Cohort 1966 (NFBC 1966), at the age of 31 years. Epidemiol Infect. 2004;132:977–984. doi: 10.1017/s0950268804002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakus S, Neuer A, Dieterle S, Bongiovanni AM, Witkin SS. Antibody to the Chlamydia trachomatis 60 kDa heat shock protein in follicular fluid and in vitro fertilization outcome. Am J Reprod Immunol. 2008;59:85–89. doi: 10.1111/j.1600-0897.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 29.Equils O, Lu D, Gatter M, Witkin SS, Bertolotto C, Arditi M, et al. Chlamydia heat shock protein 60 induces trophoblast apoptosis through TLR4. J Immunol. 2006;177:1257–1263. doi: 10.4049/jimmunol.177.2.1257. [DOI] [PubMed] [Google Scholar]
- 30.Beatty WL, Byrne GI, Morrison RP. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 31.Campanella C, Marino Gammazza A, Mularoni L, Cappello F, Zummo G, Di Felice V. A comparative analysis of the products of GROEL-1 gene from Chlamydia trachomatis serovar D and the HSP60 var1 transcript from Homo sapiens suggests a possible autoimmune response. International Journal of Immunogenetics. 2009;36:73–78. doi: 10.1111/j.1744-313X.2008.00819.x. [DOI] [PubMed] [Google Scholar]
- 32.Kinnunen A, Molander P, Morrison R, Lehtinen M, Karttunen R, Tiitinen A, et al. Chlamydial heat shock protein 60--specific T cells in inflamed salpingeal tissue. Fertil Steril. 2002;77:162–166. doi: 10.1016/s0015-0282(01)02922-3. [DOI] [PubMed] [Google Scholar]
- 33.Kinnunen A, Surcel HM, Halttunen M, Tiitinen A, Morrison RP, Morrison SG, et al. Chlamydia trachomatis heat shock protein-60 induced interferon-gamma and interleukin-10 production in infertile women. Clin Exp Immunol. 2003;131:299–303. doi: 10.1046/j.1365-2249.2003.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol. 2010;185:1670–1680. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- 35.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193:935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, et al. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. Am J Obstet Gynecol. 2010;203:494–e497. 494–e414. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo J, Liu G, Zhong Y, Jia T, Liu K, Chen D, et al. Characterization of hypothetical proteins Cpn0146, 0147, 0284 & 0285 that are predicted to be in the Chlamydia pneumoniae inclusion membrane. BMC Microbiol. 2007;7:38. doi: 10.1186/1471-2180-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Chen L, Chen F, Zhang X, Zhang Y, Baseman J, et al. A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity in mice against inflammatory pathologies in the upper genital tract. J Immunol revision. 2008 doi: 10.1016/j.vaccine.2009.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun. 2006;74:1490–1499. doi: 10.1128/IAI.74.3.1490-1499.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta R, Jha R, Salhan S, Mittal A. Chlamydia trachomatis-specific heat shock proteins 60 antibodies can serve as prognostic marker in secondary infertile women. Infection. 2008;36:374–378. doi: 10.1007/s15010-008-7129-9. [DOI] [PubMed] [Google Scholar]
- 42.Persson K, Osser S, Birkelund S, Christiansen G, Brade H. Antibodies to Chlamydia trachomatis heat shock proteins in women with tubal factor infertility are associated with prior infection by C. trachomatis but not by C. pneumoniae. Hum Reprod. 1999;14:1969–1973. doi: 10.1093/humrep/14.8.1969. [DOI] [PubMed] [Google Scholar]
- 43.Gijsen AP, Land JA, Goossens VJ, Leffers P, Bruggeman CA, Evers JL. Chlamydia pneumoniae and screening for tubal factor subfertility. Hum Reprod. 2001;16:487–491. doi: 10.1093/humrep/16.3.487. [DOI] [PubMed] [Google Scholar]
- 44.Sarov I, Lunenfeld E, Sarov B, Hanuka N, Rosenzweig R, Potashnik G, et al. Chlamydia specific IgG and IgA antibodies in women with obstructive infertility as determined by immunoblotting and immunoperoxidase assays. Eur J Epidemiol. 1988;4:216–223. doi: 10.1007/BF00144755. [DOI] [PubMed] [Google Scholar]
- 45.Arno JN, Yuan Y, Cleary RE, Morrison RP. Serologic responses of infertile women to the 60-kd chlamydial heat shock protein (hsp60) Fertil Steril. 1995;64:730–735. doi: 10.1016/s0015-0282(16)57847-9. [DOI] [PubMed] [Google Scholar]
- 46.Dadamessi I, Eb F, Betsou F. Combined detection of Chlamydia trachomatis-specific antibodies against the 10 and 60-kDa heat shock proteins as a diagnostic tool for tubal factor infertility: Results from a case-control study in Cameroon. FEMS Immunology & Medical Microbiology. 2005;45:31–35. doi: 10.1016/j.femsim.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Swart P, Mol BW, van der Veen F, van Beurden M, Redekop WK, Bossuyt PM. The accuracy of hysterosalpingography in the diagnosis of tubal pathology: a meta-analysis. Fertility & Sterility. 1995;64:486–491. doi: 10.1016/s0015-0282(16)57781-4. [DOI] [PubMed] [Google Scholar]
- 48.Mol BW, Dijkman B, Wertheim P, Lijmer J, van der Veen F, Bossuyt PM. The accuracy of serum chlamydial antibodies in the diagnosis of tubal pathology: a meta-analysis. Fertility & Sterility. 1997;67:1031–1037. doi: 10.1016/s0015-0282(97)81435-5. [DOI] [PubMed] [Google Scholar]
- 49.den Hartog JE, Lardenoije CM, Severens JL, Land JA, Evers JL, Kessels AG. Screening strategies for tubal factor subfertility. Hum Reprod. 2008;23:1840–1848. doi: 10.1093/humrep/den237. [DOI] [PubMed] [Google Scholar]
- 50.Mygind P, Christiansen G, Persson K, Birkelund S. Analysis of the humoral immune response to Chlamydia outer membrane protein 2. Clin Diagn Lab Immunol. 1998;5:313–318. doi: 10.1128/cdli.5.3.313-318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gervassi AL, Grabstein KH, Probst P, Hess B, Alderson MR, Fling SP. Human CD8+ T cells recognize the 60-kDa cysteine-rich outer membrane protein from Chlamydia trachomatis. J Immunol. 2004;173:6905–6913. doi: 10.4049/jimmunol.173.11.6905. [DOI] [PubMed] [Google Scholar]
- 52.Goodall JC, Yeo G, Huang M, Raggiaschi R, Gaston JS. Identification of Chlamydia trachomatis antigens recognized by human CD4+ T lymphocytes by screening an expression library. Eur J Immunol. 2001;31:1513–1522. doi: 10.1002/1521-4141(200105)31:5<1513::AID-IMMU1513>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 53.Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, Ambrosini G, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140:3–11. doi: 10.1016/j.ejogrb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Morales P, Reyes P, Vargas M, Rios M, Imarai M, Cardenas H, et al. Infection of human fallopian tube epithelial cells with Neisseria gonorrhoeae protects cells from tumor necrosis factor alpha-induced apoptosis. Infect Immun. 2006;74:3643–3650. doi: 10.1128/IAI.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility--a prospective study. Fertil Steril. 2008;90:513–520. doi: 10.1016/j.fertnstert.2006.12.056. 2008. [DOI] [PubMed] [Google Scholar]
- 56.Haggerty CL. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr Opin Infect Dis. 2008;21:65–69. doi: 10.1097/QCO.0b013e3282f3d9ac. [DOI] [PubMed] [Google Scholar]