Abstract
Quantitative trait locus mapping of chemical/inflammatory pain in the mouse identified the Avpr1a gene, encoding the vasopressin-1A receptor (V1AR), as responsible for strain-dependent pain sensitivity to formalin and capsaicin. A genetic association study in humans revealed the influence of a single nucleotide polymorphism (rs10877969) within AVPR1A on capsaicin pain levels, but only in male subjects reporting stress at the time of testing. The analgesic efficacy of the vasopressin analog, desmopressin, revealed a similar interaction between the drug and acute stress, as desmopressin inhibition of capsaicin pain was seen only in non-stressed subjects. Additional experiments in mice confirmed the male-specific interaction of V1AR and stress, leading to the conclusion that vasopressin activates endogenous analgesia mechanisms unless they have already been activated by stress. These findings represent the first explicit demonstration of analgesic efficacy depending on the emotional state of the recipient, and illustrate the heuristic power of a bench-to-bedside-to-bench translational strategy.
Introduction
Arginine vasopressin (AVP) has well-known roles in the regulation of varied biological systems, with effects mediated by one of three receptors: V1AR, V1BR and V2R 1-2. V1AR is believed to predominate in the central nervous system (CNS) 3 and its involvement in affiliation and social communication in microtine rodents 4 and humans 5-6 is known to be male-specific and genetically determined. In humans, two studies using intranasal AVP analogs observed no 7 or modest effects on acute pain 8. In rodents, AVP produces clear but modest inhibition of acute, thermal pain after systemic, supraspinal or spinal injection in rodents, but most of the evidence suggests that this analgesia is mediated by the V1BR 9 or the V2R 10-11.
Pain and analgesic responses in humans and rodents feature robust interindividual variability, and the responsible genes are just starting to be identified 12. An explicit rodent-to-human translational strategy was successfully employed on a few occasions in this domain 13-16. We describe herein the identification of the mouse Avpr1a gene (encoding V1AR) as responsible for variable pain sensitivity to formalin and capsaicin. Translation of this finding to humans revealed that V1AR's role in pain interacts not only with genotype but with sex and acute stress, and this interaction was then confirmed and explained in new experiments in the mouse.
Positional Refinement of a Formalin Test Gene
Standard inbred mouse strains display robust variability on virtually all nociceptive assays 17-18, including on the formalin test, a widely used assay of spontaneous, tonic chemical/inflammatory nociception 19. Using an F2 intercross between the phenotypically divergent A/J (resistant) and C57BL/6J (B6; sensitive) strains, we previously identified a quantitative trait locus (QTL), which we named Nociq2, on distal mouse chromosome 10 (>58 cM), associated with variability in the late/tonic phase (10-60 min post-injection) of the formalin test 20. A recent QTL mapping study of the related acetic acid abdominal constriction test also identified significant linkage in this region 21.
We used a variety of strategies to confirm and refine the position of the QTL, including the testing of recombinant congenic strains 22 and the breeding and testing of marker-assisted congenic and subcongenic strains. A comparison of the formalin test phenotype and chromosome 10 genotype of these strains confirmed that the responsible polymorphism(s) could only lie between DNA markers D10Mit25 (at 121.781 Mb) and D10Mit103 (at 125.176 Mb) (Figs. S1–S3).
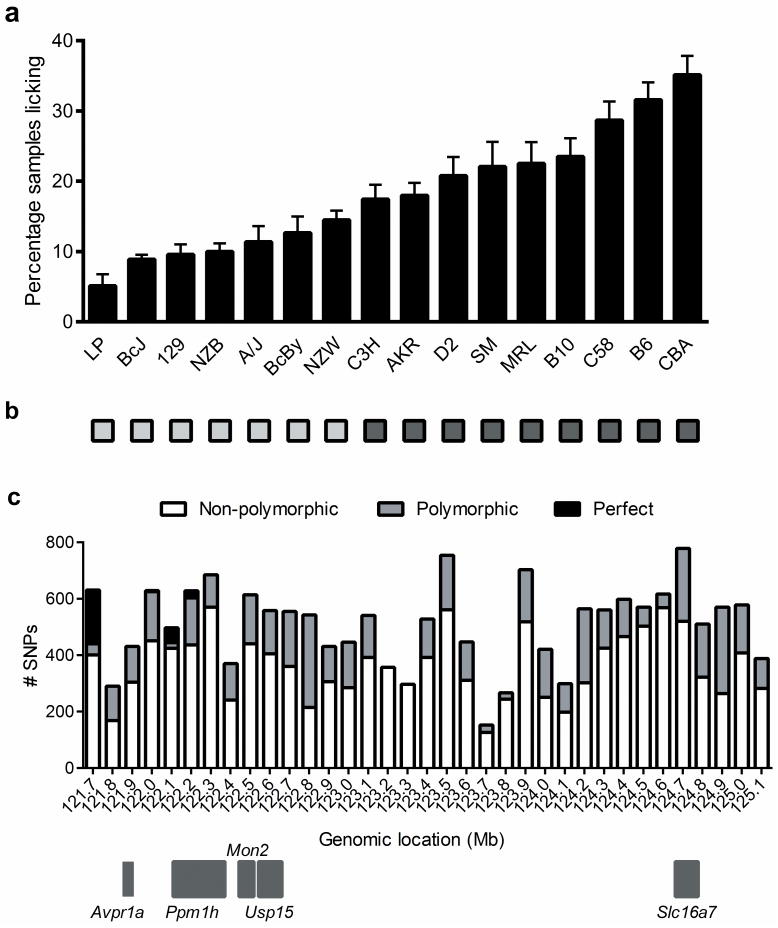
This 3.4-Mb region of chromosome 10 contains only 11 unique transcripts, including five annotated genes (Table S1). Of these, the only obvious candidate was Avpr1a, although no attempt was made to rule out the others functionally. Instead, we conducted a haplotype mapping analysis comparing the late-phase formalin test phenotypes of 16 strains (Fig. 1a) with inheritance of single nucleotide polymorphisms (SNPs) throughout the region. We investigated SNP haplotype patterns in the Mouse Phenome Database (MPD) high-density (≈8 million; CGD1 imputed 74-strain data set) SNP map (http://phenome.jax.org/SNP) in the 3.4-Mb linked region. We noted a large number of SNPs in the proximal part of this region featuring two haplotypes with perfect alignment with formalin sensitivity (Fig. 1b). We then examined individual SNPs in this region, in 100-kb intervals. Of SNPs observed to be polymorphic among the 16 strains tested, a very high percentage showed perfect correspondence in the 121.7–121.8 Mb region (Table S2), with smaller numbers of perfectly corresponding SNPs between 122.1–122.3 Mb (Fig. 1c). Only two genes lie near these regions: Avpr1a (121.886 Mb) and Ppm1h (122.115 Mb). Strains with the B6-like (high phenotypic) haplotype at SNPs within the 121.7–121.8 Mb region (e.g., rs29348576 at 121.75 Mb) exhibited more than double the pain behavior of strains with the A/J-like (low phenotypic) haplotype (24.7±1.0 vs. 11.8±0.7% samples featuring licking; t246 = 9.4, p<0.001).
Fig. 1.
Haplotype mapping localizes Nociq2 to a region of distal chromosome 10 upstream of Avpr1a. Bars in graph a show mean (± SEM) % positive samples in the late-phase formalin test of 16 inbred mouse strains (n=10–26/strain). Two contrasting haplotypes (b; light vs. dark squares) were noted in many SNPs in the 3.4-Mb linked region in the CGD1 SNP data set 32. c) An exhaustive analysis (by 100-kb segments) of SNPs within this region revealed high percentages of non-polymorphic SNPs among the 16 strains tested. Of SNPs showing polymorphisms, those with perfect correspondence (“perfect”) as defined in b were found only from 121.7–121.8 Mb, just upstream from Avpr1a (121.886 Mb), and from 122.1–122.3 Mb, upstream or within Ppm1h (122.115 Mb).
Avpr1a Affects Inflammatory Pain Sensitivity in Mice
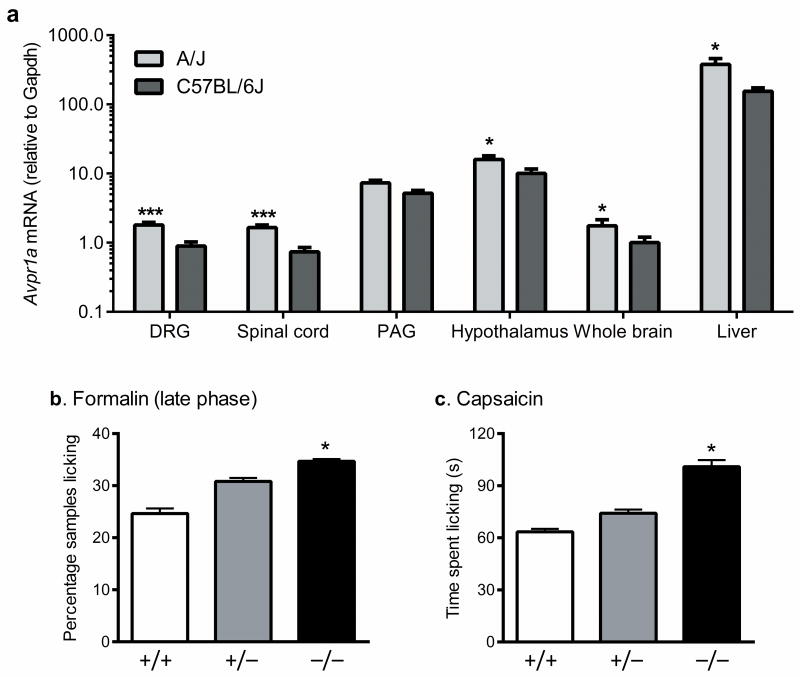
Given that multiple polymorphic SNPs exist upstream of Avpr1a, and that no SNPs are polymorphic between A/J and B6 in the coding region of the gene itself, it is likely that one or more of these SNPs are affecting the expression of Avpr1a. Quantitative real time RT-PCR experiments confirmed differential basal expression of Avpr1a between the strains in multiple regions of the CNS and the liver (Fig. 2a). Since A/J, the pain-resistant strain, displays higher Avpr1a expression than B6, we tentatively concluded that higher basal expression is protective against pain, or analgesic.
Fig. 2.
Functional evidence for Avpr1a's involvement in pain. a) Formalin pain-resistant A/J mice display significantly higher basal expression of Avpr1a than pain-sensitive B6 mice in various loci. A strong trend (p=0.13) towards significance was seen in the periaqueductal gray (PAG). Bars represent mean (± SEM) mRNA expression in arbitrary units compared to the housekeeping gene, Gapdh; n=3–4 mice/strain. *p<0.05; ***p<0.001. b) As predicted, Avpr1a−/− mice (−/−) display more late-phase formalin pain behavior than wildtype mice (+/+); heterozygous mice (+/−) were intermediate. Bars represent mean (± SEM) % positive samples (n=9–17 mice/genotype); *p<0.05 compared to +/+. c) Unique among a variety of other pain modalities examined 23, Avpr1a−/− mice (−/−) display increased capsaicin pain behavior. Bars represent mean (± SEM) time spent licking the injected paw (s) (n=12–14 mice/genotype); *p<0.05 compared to +/+.
This would predict that a null mutant of Avpr1a should be more sensitive to late-phase formalin nociception than wildtype (B6), and that is what we observed (F2,38 = 3.4, p<0.05) (Fig. 2b), providing causal evidence of the gene's involvement in the trait. There were no genotype differences in early-phase behavior or edema (not shown). We tested the Avpr1a−/− mice on a battery of nociceptive assays (data reported in 23), and no other significant genotype differences were observed, except on the capsaicin (2.5 μg) test, another chemical assay featuring spontaneous licking behavior, in which null mutants again displayed higher pain-related behavior (F2,36 = 4.1, p<0.05) (Fig. 2c). We have not formally excluded a role for Ppm1h in inflammatory pain, but this gene is not known to be expressed in any pain-relevant loci (http://biogps.gnf.org/).
AVPR1A Associations Interact with Sex and Stress
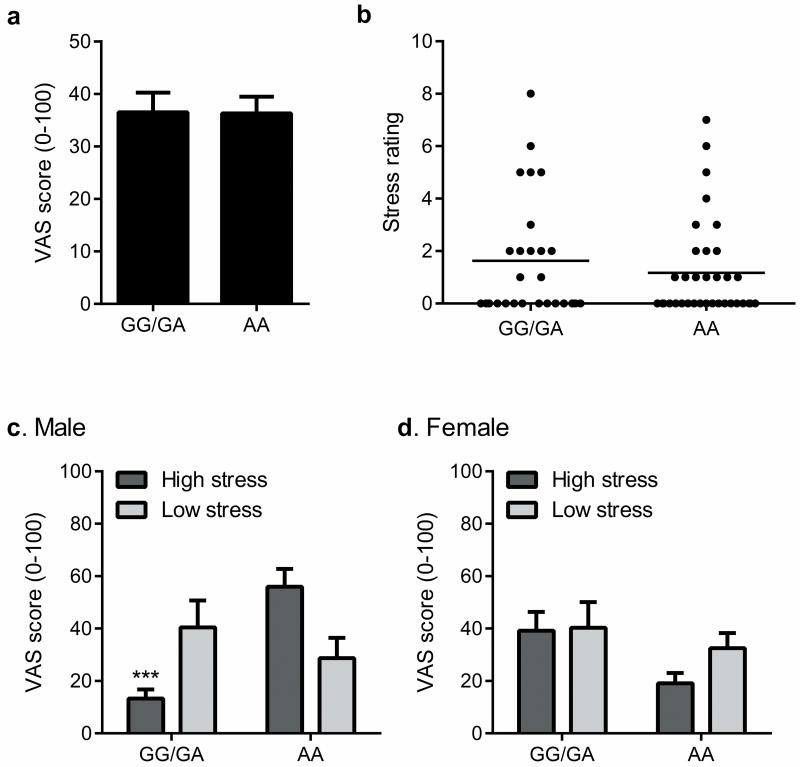
To investigate whether the human AVPR1A gene plays a role in variable responses to pain in our species, we genotyped two SNPs within the small (<6.4 kb) gene (rs1042615 and rs10877969; contained in the single haplotype block covering the AVPR1A region) in available cohorts of human volunteers (n=104) with recorded capsaicin pain ratings and banked genomic DNA samples. No genetic association with either SNP was seen in overall mean pain ratings (Fig. 3a and data not shown), nor in ratings of stress (n=61) at the time of testing (Fig. 3b and data not shown). However, for the promoter SNP, rs10877969, we observed a significant three-way interaction between genotype, stress level and sex (F1,53 = 5.2, p<0.05), such that in male subjects reporting stress, pain ratings were lower in those with the GG/GA genotype compared to the AA genotype (Fig. 3c). We saw no effects of stress or genotype in women (Fig. 3d). Allele frequencies of rs10877969 differed in Caucasian and Asian versus African-American subjects, and the lower pain ratings of male GG/GA subjects was not observed among the latter racial group (Fig. S4). The overall results remained significant regardless of whether data from African-American subjects were included. Factor analysis of components explaining the temporally dynamic capsaicin pain response 24 revealed a factor representing the influence of stress on capsaicin pain; we observed a significant sex × genotype interaction (genotype effect in males only) within this “stress-induced analgesia” factor (Fig. S5).
Fig. 3.
Genetic association of a SNP within AVPR1A (rs10877969) to capsaicin pain ratings. Because of the paucity of subjects with GG genotypes (n=12), they were grouped with AG heterozygotes (n=33) for statistical comparison to AA homozygotes (n=59). a) No association of rs10877969 to overall capsaicin VAS scores. Bars represent mean (± SEM) VAS score averaged over the 50-min testing period. b) Subjective stress ratings (0–10 scale) in the subset of subjects (n=61) in which these data were collected, stratified by genotype. c) Genotype at rs10877969 interacts with stress to affect capsaicin pain in male subjects. Bars represent mean (± SEM) VAS score. The stress groups were defined as those above and below the means shown in b. ***p<0.001 compared to analogous AA group. d) No significant effect of stress (p=0.21) or genotype (p=0.14) on capsaicin pain in female subjects. Bars as in c.
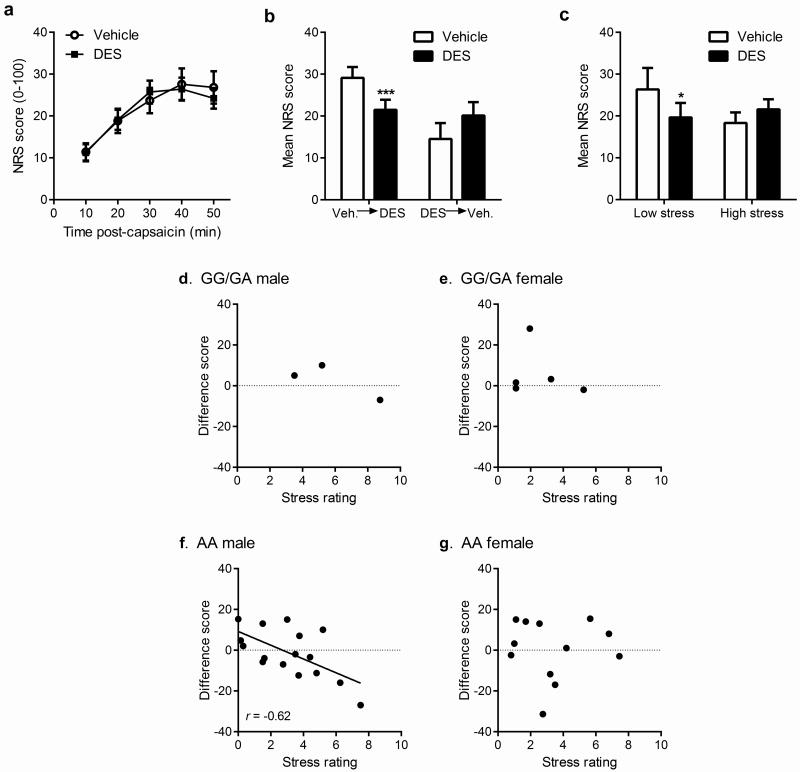
The demonstrated involvement of AVPR1A in capsaicin pain suggested that vasopressin may have analgesic properties against this pain modality. We administered the synthetic vasopressin analog, desmopressin (1-desamino-8-d-arginine vasopressin; 50 μg), or saline (0.5 ml volume) intranasally to human subjects 25 min prior to pain testing with topical capsaicin (8%, applied to a 3-cm2 area overlying the ventral forearm). Subjects gave ratings on a 0-100 scale every 10 min post-capsaicin administration for 50 min, and these ratings were averaged. We also measured pressure and heat pain thresholds, and heat pain tolerance, before and after drug administration. Critically, the experiment occurred on two testing days per subject; the design was randomly counterbalanced such that half the subjects received desmopressin on the first day and half on the second day. Overall, there was absolutely no effect of desmopressin on capsaicin pain ratings compared to saline (Fig. 4a). However, we noticed a striking and robust interaction between the apparent efficacy of desmopressin and the order in which subjects completed the experiment (F1,36 = 15.4, p=0.0004): in those receiving saline on the first day and desmopressin on the second, desmopressin produced significant analgesia, whereas desmopressin was completely inefficacious in those receiving desmopressin on the first day and saline on the second (Fig. 4b). The interaction between pain and stress noted previously suggested a hypothesis. On the first testing day, subject stress levels might be higher—since subjects had no prior experience with capsaicin pain and might fear its intensity—leading to stress-induced analgesia. On the second testing day, having already experienced capsaicin and realizing that pain levels are modest (<30/100 at peak), stress levels would be low and thus no stress-induced analgesia would be present. Indeed, regardless of drug, stress ratings (immediately before capsaicin) decreased significantly (F1,37 = 5.7, p<0.05) from the first to second testing day (Day 1: 3.4±0.4/10; Day 2: 2.3±0.3/10). We thus reanalyzed the data using a median split of stress ratings irrespective of testing order, and again we observed that desmopressin produced significant analgesia when stress levels were low, but not when stress levels were high (F1,37 = 7.1, p=0.01) (Fig. 4c). These effects were similar in both sexes, with the exception of a strongly trending (p=0.06) interaction between drug and sex such that males given desmopressin on the first testing day displayed the highest stress levels (4.8/10) of all groups, in accordance with findings that intranasal vasopressin elevated anxiety to neutral stimuli in men but not women 5. We found the relationship between stress and desmopressin analgesia to also interact with sex and genotype, with a significant correlation between stress and analgesia (r = −0.62, p<0.01) only observed in men with the AA genotype of rs10877969 (Fig. 4d–g). All these effects were specific to capsaicin pain; we observed no analgesic effects of desmopressin nor interactions with sex, stress or genotype, for heat or pressure pain (Fig. S6).
Fig. 4.
Evidence for the interaction of stress with desmopressin analgesia in 38 human subjects. a) Numerical rating scale (NRS) ratings of capsaicin pain (0–100) at 10-min intervals for desmopressin versus saline reveal that the overall effect of desmopressin (DES) was non-significant. Symbols represent mean (± SEM) NRS score. b) The order of drug administration interacted significantly with drug condition, such that desmopressin was highly efficacious in those receiving it in the second session (Veh.→DES), but not among those receiving it in the first session (DES→Veh.). Bars represent mean (± SEM) NRS scores averaged over the 50-min testing period; ***p<0.001 compared to Veh. c) Using a median split, subjects were divided into high- versus low-stress groups, which revealed a significant stress × drug interaction, such that desmopressin produced significant analgesia for low-stress subjects, but no effect of desmopressin emerged among high-stress subjects; *p<0.05 compared to vehicle. Bars as in b. d–g) The correlation of desmopressin analgesia (expressed as saline–desmopressin difference scores in mean NRS scores; positive values represent desmopressin analgesia) with stress levels (0–10) is only seen in male subjects with the TT genotype at rs10877969 within AVPR1A.
AVP- and Stress-Induced Analgesia in Mice
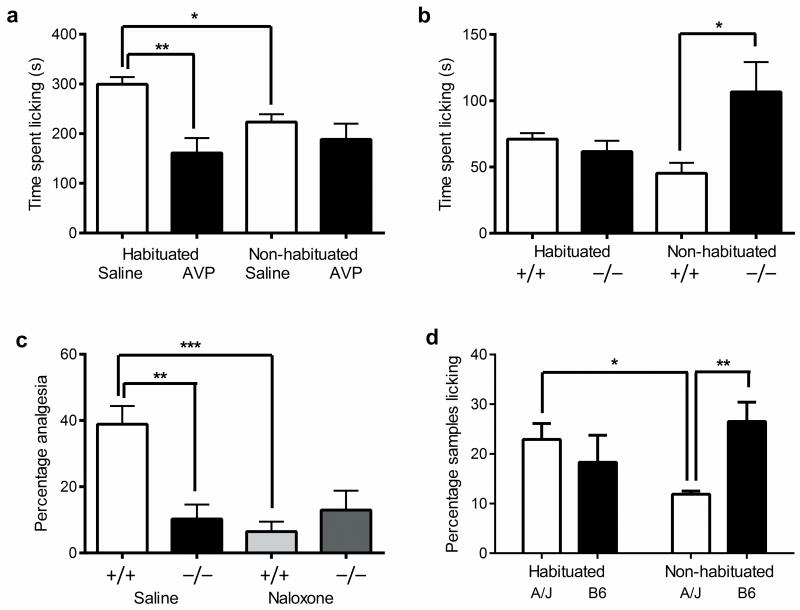
We then designed experiments to investigate whether vasopressin analgesia interacted with stress and genotype in mice as well as in humans. It is well known in the rat that the novelty of the testing situation can produce stress-induced analgesia that can be ameliorated by prior habituation to the environment 25-27, and this struck us as highly analogous to the apparent situation in the human desmopressin study. We thus explicitly compared the ability of AVP (0.1 mg/kg, s.c.) to inhibit capsaicin-induced licking behavior in male mice that were either habituated to the testing room and observation cylinders six times (each for 60 min) or were naïve to the testing room (i.e., not habituated). In a striking parallel to the human study, AVP was highly efficacious in habituated mice but produced no significant analgesia in non-habituated mice (drug × habituation: F1,23 = 4.7, p<0.05) (Fig. 5a). The AVP analgesia was mediated via the V1AR, as no analgesia could be evinced in Avpr1a−/− mice (Fig. S7). Decreased licking behavior of saline-treated mice in the non-habituated condition compared to the habituated condition revealed the existence of stress-induced analgesia in the former (Fig. 5a). The influence of Avpr1a gene deletion on capsaicin pain behavior (and formalin pain behavior; data not shown) depended on habituation status; we replicated the increased sensitivity of knockout mice in a new cohort of non-habituated male mice, but no genotype differences were seen in habituated mice (genotype × habituation: F1,13 = 5.3, p<0.05) (Fig. 5b). That habituation reduces stress was demonstrated by changes in fecal boli, plasma corticosterone levels and plasma vasopressin levels (Fig. S8).
Fig. 5.
Interaction of AVP/V1AR-mediated analgesia and stress in male mice. a) Male CD-1 mice (n=7 mice/drug/condition) either habituated or not habituated to the testing environment were tested for capsaicin (2.5 μg)-induced pain behavior following s.c. injection of AVP (0.1 mg/kg) or saline (10 ml/kg). Bars represent mean (± SEM) time spent licking the injected paw (s); *p<0.05, **p<0.01. b) Increased capsaicin sensitivity only in non-habituated Avpr1a-/- mice (−/−) compared to wildtype mice (+/+) (n =4–5 mice/genotype/condition). Bars represent mean (± SEM) time spent licking the injected paw (s); *p<0.05. c) Absence of opioid-mediated (i.e., reversible by 10 mg/kg naloxone) swim stress-induced analgesia in Avpr1a-/- mice (−/−). Bars represent mean (± SEM) percentage of maximum possible analgesia on the 56 °C hot-plate test (n=6–7 mice/genotype/drug); **p<0.01; ***p<0.001. d) A/J versus B6 strain differences in formalin sensitivity are dependent on habituation. Bars represent mean (± SEM) percentage of positive samples in the late-phase formalin test (n=4–6 mice/genotype/condition); *p<0.05. For analogous female data, see Fig. S8.
The finding that only non-habituated Avpr1a mutants would differ from wildtypes would be predicted if mutant mice were unable to produce stress-induced analgesia that would otherwise decrease pain behavior. Accordingly, we demonstrated that male Avpr1a−/− mice were deficient in the opioid-mediated (i.e., naloxone-reversible) stress-induced analgesia on the 56 °C hot-plate test 28 produced by forced swimming in 32 °C water (genotype × drug: F1,21 = 8.2, p<0.01) (Fig. 5c). Finally, we retested male A/J and B6 mice on the formalin test with and without habituation; the expected strain difference was observed in the non-habituated condition, but no strain difference was seen if mice were habituated (genotype × habituation: F1,17 = 5.3, p<0.05) (Fig. 5d). As predicted by results in the null mutants, the high Avpr1a-expressing A/J strain displayed evidence of stress-induced analgesia in the non-habituated condition, whereas the lower-expressing B6 strain did not. We have previously demonstrated a relative deficit in opioid-mediated stress-induced analgesia in male B6 mice 29. Thus, ironically, the elucidation of Avpr1a as the gene underlying Nociq2 was wholly reliant on testing-related stress; if habituation had been used originally no QTL would have been apparent. Nociq2 is not actually a pain QTL, but rather a stress-induced analgesia QTL. Notably, the interaction between AVP/V1AR and stress appeared in every case described above to be specific to male mice: in female mice, AVP analgesia was seen regardless of habituation status; capsaicin pain and stress-induced analgesia were unaltered in Avpr1a−/− mice; and the A/J versus B6 strain difference was observed in both the habituated and non-habituated conditions (Fig. S9).
Discussion
Taken together, these results suggest that—in both mice and humans—vasopressin, acting on the V1AR, produces inhibition of capsaicin-induced pain by activating endogenous (stress-induced) analgesic systems unless those same systems have already been activated by stress itself. The likelihood of stress-induced analgesia being present, affecting both pain and desmopressin inhibition of that pain, is in turn strongly influenced by sex and AVPR1A genotype. An explanatory model is provided as Fig. S10. It appears, therefore, that desmopressin analgesia in males results from a three-way interaction between genotype, drug, and acute stress. Similarly, capsaicin (and in mice, formalin) pain sensitivity is also influenced, in males, by an interaction between genotype and stress.
We believe this is the first explicit evidence of the dependence of analgesic efficacy with the acute emotional state of the subject at the time of administration. If the pharmacological efficacy of drugs interacts with both genotype and stress (especially chronic stress), this could have widespread implications for both pharmacotherapy and the design of pharmacological studies. Moreover, these findings strikingly demonstrate the utility of the bidirectional (bench to bedside and back to bench) translational approach, since all three research components (murine genetics, human genetics, human pharmacology) provided a crucial piece of information clarifying the analysis of the others, and successfully predicted the results of new, more complex experiments. Although we observe the role of V1ARs in pain to be specific to the chemical/inflammatory modality, most chronic pain states feature inflammation, and the capsaicin test is thought to be an excellent model of human clinical pain 30. Finally, these data represent yet another dramatic example of qualitative differences in the operation of pain mechanisms between the sexes 31.
Supplementary Material
Acknowledgments
This study was supported by NIH grant NS41670 (R.B.F., J.S.M.) and the Louise and Alan Edwards Foundation (J.S.M.). We thank Pfeiffer of America Inc. for providing metered spray pumps.
Footnotes
Author Contributions: R.E.S., M.L.L.-F., S.G.S., J.R., J.-S.A., A.S.-P., K.M., J.C., R.A.B., J.B.M., and W.F.S. conducted experiments. J.S.M., S.B.S., A.F., W.R.L., C.D.B., I.B., and R.B.F. performed data analyses. J.K.N., C.M.C., R.R.E., J.N.C., M.R.W., I.B. and R.B.F. collected human phenotypic and genotypic data. J.N.C. provided transgenic mice and advice on their use. J.S.M., I.B., and R.B.F. supervised the project. J.S.M. wrote the manuscript.
References
- 1.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2009;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tribollet E, Arsenijevic Y, Barberis C. Vasopressin binding sites in the central nervous system: distribution and regulation. Prog Brain Res. 1998;119:45–55. doi: 10.1016/s0079-6123(08)61561-7. [DOI] [PubMed] [Google Scholar]
- 4.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walum H, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemppainen P, Pertovaara A, Huopaniemi T, Hamalainen O, Gronblad M. Human pain thresholds after the application of lypressin, a vasopressin analogue. Pharmacol Toxicol. 1987;61:16–19. doi: 10.1111/j.1600-0773.1987.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 8.Pohl J, et al. Modulation of pain perception in man by a vasopressin analogue. Peptides. 1996;17:641–647. doi: 10.1016/0196-9781(96)00034-4. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Takano Y. New topics in vasopressin receptors and approach to novel drugs: involvement of vasopressin 1a and V1b receptors in nociceptive responses and morphine-induced effects. J Pharmacol Sci. 2009;109:38–43. doi: 10.1254/jphs.08r30fm. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Chen JM, Liu WY, Snog CY, Lin BC. Through V2, not V1 receptor relating to endogenous opiate peptides, arginine vasopressin in periaqueductal gray regulates antinociception in the rat. Regul Pept. 2006;137:156–161. doi: 10.1016/j.regpep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, et al. Through the central V2, not V1 receptors influencing the endogenous opiate peptide system, arginine vasopressin, not oxytocin in the hypothalamic paraventricular nucleus involves in the antinociception in the rat. Brain Res. 2006;1069:127–138. doi: 10.1016/j.brainres.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 12.LaCroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogil JS, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costigan M, et al. Multiple chronic pain states are associated with a common amino acid–changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tegeder I, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 16.Nissenbaum J, et al. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 2010;20:1180–1190. doi: 10.1101/gr.104976.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogil JS, et al. Heritability of nociception. I. Responses of eleven inbred mouse strains on twelve measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 18.Lariviere WR, et al. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 19.Mogil JS, Lichtensteiger CA, Wilson SG. The effect of genotype on sensitivity to inflammatory nociception: characterization of resistant (A/J) and sensitive (C57BL/6) inbred mouse strains. Pain. 1998;76:115–125. doi: 10.1016/s0304-3959(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SG, et al. Identification of quantitative trait loci for chemical/inflammatory nociception in mice. Pain. 2002;96:385–391. doi: 10.1016/S0304-3959(01)00489-4. [DOI] [PubMed] [Google Scholar]
- 21.Nair HK, et al. Genomic loci and candidate genes underlying inflammatory nociception. Pain. 2011;152:599–606. doi: 10.1016/j.pain.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortin A, et al. Recombinant congenic strains derived from A/J and C57BL/6J: a tool for genetic dissection of complex traits. Genomics. 2001;74:21–35. doi: 10.1006/geno.2001.6528. [DOI] [PubMed] [Google Scholar]
- 23.Schorscher-Petcu A, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaban CD, McBurney DH, Affeltranger MA. Three distinct categories of time course of pain produced by oral capsaicin. J Pain. 2005;6:315–322. doi: 10.1016/j.jpain.2005.01.346. [DOI] [PubMed] [Google Scholar]
- 25.Abbott FV, Franklin KBJ, Connell B. The stress of a novel environment reduces formalin pain: possible role of serotonin. Eur J Pharmacol. 1986;126:141–144. doi: 10.1016/0014-2999(86)90750-8. [DOI] [PubMed] [Google Scholar]
- 26.Fanselow MS, Sigmundi RA. Species-specific danger signals, endogenous opioid analgesia, and defensive behavior. J Exp Psychol: ABP. 1986;12:301–309. [PubMed] [Google Scholar]
- 27.Gamble GD, Milne RJ. Repeated exposure to sham testing procedures reduces reflex withdrawal and hot-plate latencies: attenuation of tonic descending inhibition? Neurosci Lett. 1989;96:312–317. doi: 10.1016/0304-3940(89)90397-2. [DOI] [PubMed] [Google Scholar]
- 28.Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. Opioid and non-opioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996;59:123–132. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 29.Mogil JS, Belknap JK. Sex and genotype determine the selective activation of neurochemically-distinct mechanisms of swim stress-induced analgesia. Pharmacol Biochem Behav. 1997;56:61–66. doi: 10.1016/S0091-3057(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 30.Schmelz M. Translating nociceptive processing into human pain models. Exp Brain Res. 2009;196:173–178. doi: 10.1007/s00221-009-1809-2. [DOI] [PubMed] [Google Scholar]
- 31.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. 2010;186:141–157. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 32.Frazer KA, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nat. 2007;448:1050–1055. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 33.Wong GT. Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides. 2002;36:230–236. doi: 10.1054/npep.2002.0905. [DOI] [PubMed] [Google Scholar]
- 34.Hu SB, et al. Vasopressin receptor 1a-mediated negative regulation of B cell receptor signaling. J Neuroimmunol. 2003;135:72–81. doi: 10.1016/s0165-5728(02)00442-3. [DOI] [PubMed] [Google Scholar]
- 35.Campbell CM, et al. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141:114–118. doi: 10.1016/j.pain.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi MM, Myrand SP, Bleavins MR, de la Iglesia FA. High throughput genotyping for the detection of a single nucleotide polymorphism in NAD(P)H quinine oxidoreductase (DT diaphoresis) using TaqMan probes. Mol Pathol. 1999;52:295–299. doi: 10.1136/mp.52.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purcell S, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lariviere WR, McBurney DH, Frot M, Balaban CD. Tonic, phasic, and integrator components of psychophysical responses to topical capsaicin account for differences of location and sex. J Pain. 2005;6:777–781. doi: 10.1016/j.jpain.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Stern RA. Visual Analog Mood Scale. Psychological Assessment Resources; 1997. [Google Scholar]
- 40.Fillingim RB, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.