Abstract
Significant sex differences have been demonstrated in clinical and preclinical studies of cocaine addiction, with some of the most consistent differences noted in regards to the role of stress and craving. The current study examined stress-induced reinstatement of cocaine seeking in male and female rats in an animal model of relapse using corticotropin-releasing factor (CRF) administration. Both male and female rats demonstrated increased cocaine seeking in response to CRF. CRF-induced reinstatement was highly variable across both male and female rats, and further analysis revealed a subpopulation that was particularly sensitive to CRF (high responders). Female high responders displayed significantly increased responding to CRF compared to males. Individual differences in stress responsivity could thus contribute to the likelihood of relapse, with females showing greater heterogeneity to stress-induced relapse.
Keywords: cocaine, females, males, reinstatement, relapse, stress
1. Introduction
Differences in the progression from initial drug exposure to more compulsive patterns of use may contribute to the severity of addiction, susceptibility to relapse, and responsiveness to treatment. Increasing evidence suggests prominent sex differences exist at both early and late stages of addiction [1]. Although the incidence of cocaine addiction remains higher among males [2, 3], women begin using cocaine at a younger age and display higher rates of use [2, 4] than men. A number of reported sex differences after acute exposure to cocaine suggest that the subjective effects of cocaine differ between males and females [5]. Women report higher anxiety levels after oral or intranasal cocaine [6, 7], and report more euphoria [8] and less dysphoria than males [9], as well as taking longer to detect the presence of the drug [10]. Women also display shorter periods of abstinence from cocaine use than men [6] and seek treatment more quickly after initiating use [11], which supports the idea that women may transition more quickly from casual use to addiction [12].
After progression to the addicted state has occurred, identifying differences in withdrawal symptoms, treatment adherence, and circumstances of relapse is critical to the successful treatment of addiction. During abstinence, females report more craving and depressive symptoms than males [13], greater craving in response to social stress [14], and may be more likely to relapse after periods of stress or depression [13, 15]. Evidence suggests that an enhanced sensitivity of the stress system in female cocaine dependent subjects [16] and dysregulation of female stress systems may contribute to the progression of the disease and likelihood of relapse [5].
Animal models of cocaine addiction have shown significant sex differences in cocaine reward and reinforcement. Female rats display greater behavioral sensitization to cocaine [17] and demonstrate conditioned place preference for cocaine at lower doses than males [18]. Female rats also acquire cocaine self-administration more quickly than males [19], show more sensitivity to the reinforcing effects of cocaine [20], and display greater cocaine intake on long-access self-administration schedules [21]. Finally, female rats display greater cocaine-primed reinstatement of cocaine seeking than males [19], an effect that is estrous cycle dependent [22].
While of great interest in clinical studies of addiction [5], female sensitivity to stress-induced reinstatement of cocaine seeking has generally not been explored in animal models. We recently reported that females displayed enhanced reinstatement to the pharmacological stressor, yohimbine [23]. Considering the extensive data on sensitivity of females to stress-related disorders, stress-related withdrawal symptoms, and stress-triggered relapse, investigation of sex differences and their underlying mechanisms in appropriate animal models of relapse is of clear interest. Thus, in the current study, we examined reinstatement of cocaine seeking in male and female rats after intracerebroventricular infusions of corticotropin-releasing factor (CRF). We predicted that females would show greater CRF-induced reinstatement than males.
2. Materials and Methods
2.1. Subjects
Male (n=22) and female (n=20) Sprague–Dawley rats (initial weight 275–300 g; Charles River, Wilmington, MA, USA) were individually housed in a temperature- and humidity controlled vivarium on a reverse 12 h light–dark cycle (lights off 06:00–18:00). Male and female rats were housed in separate rooms. Animals received water and standard rat chow (Harlan, Indianapolis, IN, USA) ad libitum, with the exception of 2–3 days of food restriction during initial cocaine self-administration. Housing and care of the rats were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP) followed by equithesin (0.5 ml/kg, IP). Ketorolac (2.0 mg/kg, IP) was given just prior to surgery as an analgesic. Surgical procedures were conducted using aseptic techniques. Catheters were constructed using previously described methods [24] and consisted of external guide cannulae with screw-type connectors (Plastics One Inc., Roanoke, VA, USA), Silastic tubing (10 cm; i.d. = 0.64 mm; o.d. = 1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (2 cm diameter, Atrium Medical Corporation, Hudson, NH, USA), and cranioplastic cement. The end of the catheter was inserted into the right jugular vein, secured with suture, and exited on the rat’s back, posterior to the shoulder blades. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL, USA). Bilateral stainless steel guide cannulae (26 gauge; Plastics One, Inc.) were inserted dorsal to the lateral ventricle (−0.8 A, −2.2 L, −1.8 V, 10° angle). Three small screws and cranioplastic cement secured the guide cannulae to the skull. Stylets (Plastics One, Inc.) were placed into the guide cannulae and catheter to prevent occlusions.
To maintain patency, catheters were flushed once daily for 4 days after surgery with 0.1 ml each of an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA) and then heparinized saline (70 U/ml). For the duration of the experiment, each subject received 0.1 ml of heparinized saline (10 U/ml) immediately prior to self-administration and the cefazolin and 70 U/ml heparinized saline regimen following each session. To verify catheter patency, rats occasionally received a 0.10–0.12 ml infusion of methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
2.3. Cocaine self-administration
Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% sterile saline; 0.5 mg/kg per 50 ul infusion; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2 h sessions according to an FR 1 schedule of reinforcement. At the start of each session, the catheter was connected to a swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). Self-administration occurred in standard operant conditioning chambers (30×20×20 cm) linked to a computerized data collection program (MED-PC, Med Associates Inc., St. Albans, VT, USA). The chambers were equipped with two levers, a stimulus light above each lever, a tone generator (ENV-223HAM, Med Associates), and a house light on the back wall of the chamber. The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active lever resulted in a 2-s infusion and a 5-s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 4.5 kHz). Following each infusion, responding on the active lever had no consequences during a 20-s time-out period. Inactive lever presses had no consequences, but were recorded. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of 10 sessions with at least 10 infusions per session.
2.4. Extinction and Reinstatement Testing
Following chronic self-administration and before the first reinstatement test, rats underwent daily 2 h extinction sessions, during which active lever presses had no programmed consequences (no cocaine infusions, no light-tone stimulus presentations). Rats received sham infusions on Extinction days 4 and 5 to acclimate them to the infusion procedure. Once extinction criterion was reached (a minimum of seven extinction sessions, with ≤15 active lever responses per session for the last two consecutive days before testing), each rat underwent three separate reinstatement tests for CRF-induced reinstatement (1.0, 1.5, and 2.0 ug doses). The doses of CRF were based on a pilot experiment performed in our laboratory, as well as previously published studies [25, 26]. Prior studies have successfully utilized similar repeated reinstatement testing designs [27, 28]. Animals were extinguished to criterion between reinstatement tests (≤15 active lever responses per session for two consecutive days). All reinstatement tests were given in a counterbalanced order for CRF dose.
2.5. Intracerebroventricular infusions
For intracerebroventricular CRF infusions, stainless steel injection cannulae (33 gauge, Plastics One) were inserted to a depth of 2 mm below the tip of the guide cannulae 60 min prior to placement into the chamber. The injection cannulae were connected to 10-ul Hamilton syringes (Hamilton Co., Reno, NV, USA) mounted on an infusion pump (Harvard Apparatus, South Natick, MA, USA). CRF (1.0–2.0 ug; 2.5 ul volume) or phosphate-buffered saline vehicle (pH=7.0 for both solutions) was infused bilaterally over a 2 min time period. The injection cannulae were left in place for 1 min prior to and after the infusion.
2.6. Angiotensin drinking test and histology
Accurate cannulae placement into the lateral ventricles was verified via two means. One week after intracranial surgery, angiotensin drinking tests were administered to all rats. Injectors were lowered into the cannulae, and angiotensin (50 ng/ml) was administered bilaterally over a period of 60 sec. Immediately following administration, stylets were replaced and the animals were given access to drinking water for 10 min. Rats that drank less than 5 ml of water were excluded from the study (n=6). After completion of reinstatement testing, rats were deeply anesthetized with equithesin and transcardially perfused with PBS and 10% formaldehyde solution. The brains were dissected and stored in 10% formaldehyde solution prior to sectioning. Using a vibratome (Technical Products International, St. Louis, MO, USA), brains were sectioned in the coronal plane (75 μm thickness), mounted on gelatin-coated slides, and stained for Nissl substance with cresyl violet (Kodak, Rochester, NY, USA). The sections were examined with light microscopy using 10x magnification. The paths of the microinjectors targeting the lateral ventricles were mapped onto schematics from a rat brain atlas [29].
2.7. Data analysis
For each experiment, total cocaine intake (mg/kg) was compared for males and females using paired t-tests. Active lever responding over the ten days of self-administration and extinction were each analyzed using a repeated measures analysis of variance (ANOVA), with day as the within factor and sex as the between factor. Male and female responding during extinction was also compared for the number of active lever responses on the last extinction day before testing, as well as the total days it took to reach extinction criterion using paired t-tests.
Reinstatement testing was analyzed using a two-way ANOVA (sex by CRF dose) to examine significant differences in active lever presses during each reinstatement test with post-hoc comparisons for any main effects. Pronounced variability has been reported in response to stress, in particular in the reinstatement paradigm [30]. Since such heterogeneity was also evident in the current data, we analyzed whether or not there was a significant difference in variability between males and females using the F test to compare variance. Further, we accounted for individual variability by establishing criterion for “high responders” as the 6 animals that displayed the highest average level of active lever pressing across the 3 CRF reinstatement tests with a minimum criterion as twofold higher lever responding on the last day of extinction before testing. This method established a clear criterion that could be consistently applied to both male and female populations, and we chose 6 subjects, as this represents the top third of all responders, and animals below these 6 did not comprise a consistent population that responded strongly to more than a single dose of CRF. After division, the reinstatement levels in the high responders were analyzed separately with an ANOVA. We also examined high and low responders on several measures of self-administration and extinction behavior with either ANOVAs or t-tests, and examined relationships between this behavior and reinstatement responding with Pearson correlations. All F and p-values are shown for main effects, but only statistically significant interactions are reported. Analyses were considered statistically significant at p<0.05 and data are reported as the mean±SEM.
3. Results
3.1. Histology
Only those animals with a positive result in the angiotensin drinking test (>5 ml of water in 10 min) were used. Inspection of injection cannulae tip locations after completion of the experiment verified that all animals that passed the angiotensin drinking test had correct lateral ventricle placements.
3.2. Self-administration
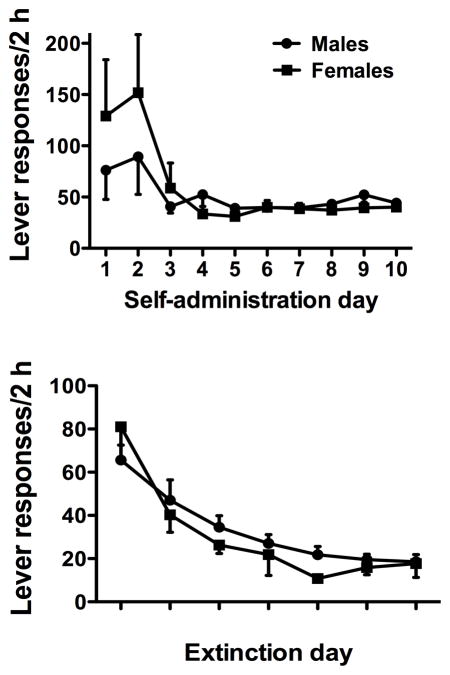
Both male and female rats readily acquired cocaine self-administration, discriminated between active and inactive levers, and displayed stable patterns of active lever responding and cocaine intake throughout the maintenance phase of the experiment. There were no significant differences between males and females in total cocaine intake across the self-administration period (males=174±10.6 mg/kg; females =197±10.0 mg/kg), although this measure approached significance (p=0.1). Repeated measures ANOVA revealed a significant effect of day on active lever responding (Fig. 1, F(9,379)=3.96, p<0.05), with responding on days 1 and 2 showing high levels that stabilized to maintenance levels after day 3 of self-administration. There was no significant overall effect of sex and no significant sex × day interaction. Inactive lever responding was minimal throughout the duration of the study and showed no significant differences between groups or over time (data not shown).
Figure 1.
Active lever responding in males and females during cocaine self-administration (top) and extinction (bottom). Significant differences (*p<0.05) are noted for self-administration days 1–2 relative to self-administration days 3–10, and extinction day 1 relative to extinction days 2–7.
3.3. Extinction
Both male and female rats readily extinguished lever pressing upon removal of all cocaine reinforcement and cue presentation (Fig. 1). There were no significant sex differences in the number of days to reach extinction criterion or for the number of lever responses on the final day of extinction before testing. Repeated measures ANOVA revealed a significant effect of extinction day (F(6,264)=18.48, p<0.001), with days 1 and 2 of extinction significantly greater than final extinction levels (p<0.05), but no significant effect of sex or sex × day interaction.
3.4.1 Reinstatement
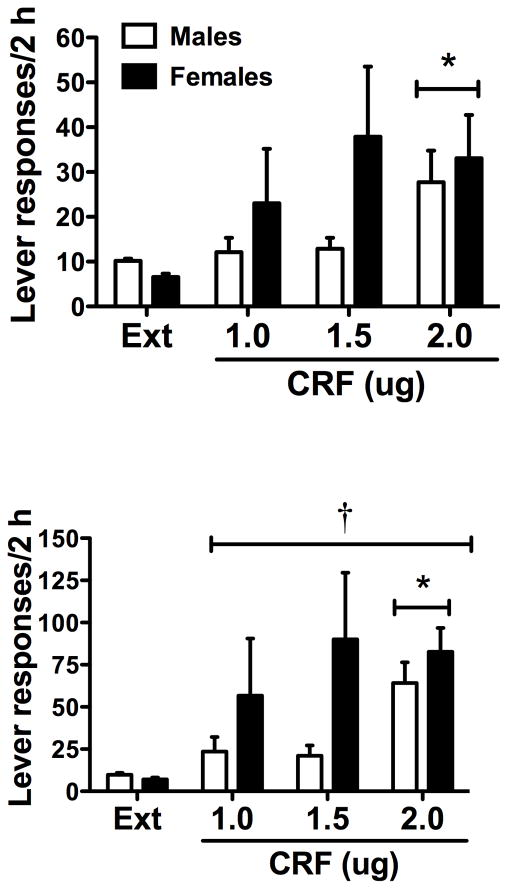
As seen in Fig. 2 (top), CRF significantly reinstated cocaine seeking (F(3,143)=2.88, p<0.05). Post-hoc analysis revealed statistically significant differences between responding after vehicle infusion and the high dose of CRF (p<0.05). There was no significant effect of sex or any significant interaction between sex and CRF.
Figure 2.
Active lever responses in males and females for the last day of extinction before testing (Ext) and on CRF-induced reinstatement tests for all subjects (top) or in high responders (bottom). Significant differences are noted for responding over extinction (*p<0.05) and for females relative to males (†p<0.05).
3.4.2 High responders
The response to CRF was highly variable (range of 0 to 278 active lever responses); therefore, we performed additional analyses in these populations. Female rats displayed significantly greater variability in response to CRF than male rats at both the low (F(17,19)=35.77, p<0.0001) and medium doses (F(17,19)=12.84, p<0.0001). A subset of rats in each sex was designated as “high responders” (see Methods). There were no significant differences in total cocaine intake for male or female high responder rats compared to the others (males=176±13.0 mg/kg; females =212±15.9 mg/kg). Active lever responding across days of self-administration was not significantly different between high responders and the rest of the population. In the subpopulation of high responders of male and female rats (Fig. 2 – bottom), a two-way ANOVA analyzing the effect of CRF and sex on active lever pressing revealed a significant effect of CRF (F(3,40)=3.82, p<0.01) and a significant effect of sex (F(1,40)=4.37, p<0.05). Post-hoc analyses revealed that responding after the high dose of CRF was significantly different from extinction (p<0.05), and females displayed higher levels of responding than males.
4. Discussion
Here, we demonstrated significant CRF-induced reinstatement of cocaine seeking in both male and female rats. Importantly, responsivity to CRF showed a high degree of individual variability, in that high responder females showed significantly greater reinstatement to CRF than high responder males and females showed more variable responses to CRF. The enhanced sensitivity to CRF in a subpopulation of females is consistent with the notion that some females may be more prone to stress-induced relapse, and that individual differences play a key role in stress activation of motivated cocaine seeking.
The variability in CRF-induced reinstatement occurred with both male and female rats. Heterogeneous and variable response patterns have been reported in prior studies of stress-induced reinstatement in males [31–34], but this is the first such report in females. The heterogeneity of reinstatement was more pronounced in females, as the range of active lever responding to CRF greatly exceeded that of males (range for females=0–278, males=0–114), and females displayed significantly more variable responding than males. Since individual sensitivity to stressful stimuli is well documented in other paradigms [35–38], the individual variability to the effects of CRF on cocaine seeking is not surprising. Previous reports on CRF-induced reinstatement in males have demonstrated variable and modest results [39, 40]. Mantsch et al. [26] reported that CRF at a dose of 1.0 ug failed to produce reinstatement unless animals had experienced long daily access regimens of cocaine self-administration. The current data also found a similar lack of effect at this dose, except in a few subjects. We chose to examine a slightly higher dose of CRF as such doses (and beyond) are often used to examine anxiety and stress-related responses using other behavioral paradigms ([41–43], Further, some evidence suggests higher doses of CRF may be necessary to detect sex differences or exaggerated responses to CRF after drug exposure [41, 44]. However, previous studies have demonstrated activation of similar populations of neurons in response to ascending doses of CRF (0.5–2.0ug), including regions previously implicated in stress-induced reinstatement such as the central amygdala and bed nucleus of the stria terminalis [45]. Therefore, it is likely that we achieved comparable circulating levels of CRF as lower dose studies, resulting in activation of similar limbic neurocircuitry.
Enhanced responsivity to CRF-induced reinstatement may be a result of cocaine exposure and related to self-administration or extinction behavior. However, we compared male and female high responders, both separately by sex and across the entire population, on measures of days to acquisition, overall cocaine intake and intake during acquisition and maintenance, active lever responding during initial and late stages of self-administration and extinction, and days to extinction criterion. We found no significant differences on any of these measures. We also examined correlations between reinstatement behavior and several of these measures, none of which revealed significant relationships with active lever responding in response to CRF.
A critical role for CRF in addiction and relapse has emerged over the past several years. Clinical studies reveal that CRF is elevated in patients withdrawn from alcohol [46], but decreased in patients withdrawn from opiates. CRF also causes cocaine craving [47], and other studies have suggested a key role for the CRF-HPA axis in stress and drug cue-induced cocaine craving [48, 49]. CRF-induced reinstatement in the current study was modest when measured across the population. However, some animals displayed very dramatic reinstatement to cocaine seeking after CRF infusion. Therefore, while large sex differences across the entire population may appear subtle, high-risk portions of the population may be particularly susceptible to stress activation, as demonstrated by CRF in females. Interestingly, clinical data has shown a more dramatic effect of CRF on heart rates in females than males, as well as a more severe dysregulation of stress-related systems in female cocaine addicts when compared to males [47]. This effect has also been reported in alcohol dependent women [50] and female smokers [51].
Female rats display enhanced responsivity to cocaine priming injections [19, 22] and yohimbine-induced reinstatement [23]. We hypothesize that female rats may be more responsive to “internal” stressors, as CRF, yohimbine, and cocaine all exert their effects via direct pharmacological mechanisms. Support for this possibility comes from studies in which males reinstate more to conditioned cues than female rats; however, other studies suggest that the stress system is integral in both cue [52] and stress-induced, but not cocaine-induced reinsatement [53]. Recent reports demonstrate that differences in CRF receptor signaling and trafficking in female rats may lead to heightened responsivity and decreased adaptation to stress compared to male rats [54]. Also, female rats show higher plasma ACTH and CRF mRNA levels in the paraventricular hypothalamus and central amygdala after footshock stress than males [55]. Such differences could contribute to the current results.
Enhanced responsivity to yohimbine in females may be related to the current results, as previous studies demonstrate that activation of CRF receptors likely occurs downstream of noradrenergic systems [56]. However, other reports vary on whether yohimbine effects are independent of CRF systems [56–58]. Further studies are necessary to determine the exact way in which yohimbine, noradrenergic, and CRF systems interact to mediate stress-induced reinstatement.
The current results suggest that sex, as well as individual differences, are important to consider in the evaluation and treatment of cocaine addiction. Recent studies indicate that the degree of stress-induced cocaine craving is predictive of time to relapse in abstinent addicts, and stress-induced hormone release is related to the amount of cocaine intake during subsequent cocaine-taking episodes [59]. Therefore, monitoring drug craving and physiological responses to stress in cocaine addicts could be critical to ultimate treatment success, particularly given the potential for a highly variable response pattern. Individualized treatment plans that take into account addiction severity, stress responsivity, and comorbidity with other stress and anxiety-related illnesses are likely to have the greatest chance of success at combating addiction.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants DA16511 and DA21690 (RES), 1F32 DA025411 (DMB), and NIH grant C06 RR015455. The authors thank Shannon Ghee, Alisha Henderson, Bernard Smalls, and Sarah Wade Boatwright for technical assistance and data collection.
References
- 1.Quinones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Res. 2006;1126:200–3. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- 2.SAMHSA. NSDUH Series H-36, HHS Publication No SMA 09–4434. Office of Applied Studies; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 3.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–6. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 5.Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, et al. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–8. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 8.McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–28. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- 9.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 10.Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–54. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- 11.McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8:300–11. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- 12.Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–35. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- 13.Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- 14.Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, et al. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 35:798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–22. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- 17.Haney M, Castanon N, Cador M, Le Moal M, Mormede P. Cocaine sensitivity in Roman High and Low Avoidance rats is modulated by sex and gonadal hormone status. Brain Res. 1994;645:179–85. doi: 10.1016/0006-8993(94)91651-9. [DOI] [PubMed] [Google Scholar]
- 18.Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–20. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- 19.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 21.Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, et al. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–52. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- 23.Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–10. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–20. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- 26.Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 28.Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–7. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. New York: Academic Press; 1997. [Google Scholar]
- 30.Brown ZJ, Erb S. Footshock stress reinstates cocaine seeking in rats after extended post-stress delays. Psychopharmacology (Berl) 2007;195:61–70. doi: 10.1007/s00213-007-0846-4. [DOI] [PubMed] [Google Scholar]
- 31.Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–7. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–26. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 35.Taylor J, Weyers P, Harris N, Vogel WH. The plasma catecholamine stress response is characteristic for a given animal over a one-year period. Physiol Behav. 1989;46:853–6. doi: 10.1016/0031-9384(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 36.Marquez C, Nadal R, Armario A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–12. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 37.DeTurck KH, Vogel WH. Factors influencing plasma catecholamine levels in rats during immobilization. Pharmacol Biochem Behav. 1980;13:129–31. doi: 10.1016/0091-3057(80)90132-x. [DOI] [PubMed] [Google Scholar]
- 38.Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Res Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 39.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–14. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:4RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blatchford KE, Choi EA, McNally GP. Altered responsivity to central administrations of corticotropin-releasing factor in rats with a history of opiate exposures. Behav Neurosci. 2006;120:1169–74. doi: 10.1037/0735-7044.120.5.1169. [DOI] [PubMed] [Google Scholar]
- 42.Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–55. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Tejeda HA, Chefer VI, Zapata A, Shippenberg TS. The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacology (Berl) 210:231–40. doi: 10.1007/s00213-010-1799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriel KI, Yu CL, Osborn JA, Weinberg J. Prenatal ethanol exposure alters sensitivity to the effects of corticotropin-releasing factor (CRF) on behavior in the elevated plus-maze. Psychoneuroendocrinology. 2006;31:1046–56. doi: 10.1016/j.psyneuen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–56. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15:288–95. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- 47.Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, et al. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–30. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 106:21–7. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 50.Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003;27:410–23. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- 51.Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, et al. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33:560–8. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–32. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 53.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–36. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 15:877, 96–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–30. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- 57.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 59.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]