Abstract
As more adults take the stimulant medication methylphenidate to treat attention deficit hyperactivity disorder (ADHD) residual type, the risk arises with regard to the potential risks of early developmental exposure if people taking the medication become pregnant. We studied the neurobehavioral effects of methylphenidate in zebrafish. Zebrafish offer cellular reporter systems, continuous visual access and molecular interventions such as morpholinos to help determine critical mechanisms underlying neurobehavioral teratogenicity. Previously, we had seen that persisting neurobehavioral impairment in zebrafish with developmental chlorpyrifos exposure was associated with disturbed dopamine systems. Because methylphenidate is an indirect dopamine agonist, it was thought that it might also cause persistent behavioral impairment after developmental exposure. Zebrafish embryos were exposed to the ADHD stimulant medication methylphenidate 0-5 days post fertilization (12.5-50 mg/l). They were tested for long-term behavioral effects as adults. Methylphenidate exposure (50 mg/l) caused significant increases in dopamine, norepinepherine and serotonin on day 6 but not day 30 after fertilization. In the novel tank diving test of predatory avoidance developmental methylphenidate (50 mg/l) caused a significant reduction in the normal diving response. In the three-chamber spatial learning task early developmental methylphenidate (50 mg/l) caused a significant impairment in choice accuracy. These data show that early developmental exposure of zebrafish to methylphenidate causes a long-term impairment in neurobehavioral plasticity. The identification of these functional deficits in zebrafish enables further studies with this model to determine how molecular and cellular mechanisms are disturbed to arrive at this compromised state.
Keywords: Methylphenidate, Neurotoxicity, Zebrafish, Learning
1. Introduction
Methylphenidate (Ritilin®) is one of the most widely prescribed stimulant medications used to combat the symptoms of attention deficit hyperactivity disorder (ADHD) (Dopheide and Pliszka, 2009) ADHD is classically a disorder of childhood and adolescence but ADHD-residual type is recognized in adulthood (DSM-IV-Task-Force, 2000). In a group of 18-44 year old adults examined the incidence of ADHD was 4.4% overall with 3.2% females and 5.4% males (Kessler et al., 2006). The 3.2% of women of child-bearing years who take methylphenidate presents the likelihood that substantial numbers of babies born each year run the risk of being exposed to methylphenidate during early development. Most of these adults with ADHD are untreated, however as their identification as having ADHD increases, it is likely that treatment rates will increase.
Methylphenidate is effective in reducing attentional impairment in adults with ADHD (Biederman and Spencer, 2002). Considerable research has focused on potential risk of methylphenidate toxicity during juvenile and adolescent periods in humans and animal models (Achat-Mendes et al., 2003; Adriani et al., 2006; Bizot et al., 2007; Bolanos et al., 2003; Brandon et al., 2001; Brandon et al., 2003; Garland, 1998; Gray et al., 2007; Greydanus et al., 2002; Moll et al., 2001; Smith et al., 2000; Soileau, 2008; Vendruscolo et al., 2008; Zhu et al., 2010). But as adults are increasingly taking methylphenidate, the potential risk of early developmental exposure must be considered because some women taking methylphenidate to treat ADHD become pregnant. In terms of morphological indices methylphenidate was found to have low teratological potential in rat and rabbit models (Beckman et al., 2008; Teo et al., 2003; Teo et al., 2002). Given that methylphenidate has its key actions of increasing concentrations of norepinepherine and dopamine in the neocortex (Berridge et al., 2006), a likely target organ for methylphenidate-induced teratology is the brain.
However, there is very limited literature background concerning the persisting neurobehavioral effects of embryonic methylphenidate exposure. In one rodent study, methylphenidate (5 mg/kg) was administered to pregnant mice on embryonic days 8-10, 12-14, and 16-18. In adulthood, mice exposed to methylphenidate on E8-10 exhibited a decrease in anxiety-related behaviors and a concomitant increase in exploratory behavior. Prenatal methylphenidate exposure during these brief prenatal periods was not found to alter water maze learning (McFadyen-Leussis et al., 2004).
Zebrafish provide considerable advantages for the study of early and subsequent neurodevelopment. With fertilization outside the mother, their clear chorion, and transparent body during early development provides visual access along with fluorescent reporter systems to label particular cell types zebrafish providing a lucid window into the processes of neurodevelopment as well as disturbances of development with chemical exposure. However, to study neurodevelopment completely the model needs to provide information about the output of the nervous system, behavior.
In the past decade our laboratory like others have developed behavioral tests of zebrafish to assess sensorimotor processes, exploratory and escape behavior as well as more complex cognitive function (Arthur and Levin, 2001; Bencan and Levin, 2008; Bencan et al., 2009; Eddins et al., 2009; Levin et al., 2007; Levin and Cerutti, 2008; Levin and Chen, 2004; Levin et al., 2006). Our zebrafish behavior battery focuses on three tasks. The tactile startle and habituation test indexed the elementary sensory-motor response in the zebrafish and the diminution of that response with added experience with the tactile stimulus. The novel tank diving test indexes the normal reaction of zebrafish to a novel environment which is to dive to the bottom, a reaction which would leave them less vulnerable to attack from predators. The three-chamber choice apparatus with a central start chamber and two lateral choice chambers provides a way to efficiently assess spatial and non-spatial learning and memory in which the zebrafish learns and remembers the correct response to avoid a restriction in swimming space.
The zebrafish model has been particularly useful in studying developmental neurotoxicity. Zebrafish have proven to be very useful in the study of the developmental neurotoxicity of the organophosphate pesticide chlorpyrifos. Chlorpyrifos exposure during the first five days after fertilization caused significant persistent impairment in learning accompanied by hyperactivity and decreased dopamine turnover (Eddins et al., 2010; Levin et al., 2003; Levin et al., 2004; Sledge et al., 2009). Disruptions of dopamine systems during development by methylphenidate may have persisting effects on behavioral function.
The current study was conducted to determine whether the persisting neurobehavioral impairments would be seen in zebrafish after early developmental exposure during the first five days after fertilization during embryonic and early larval development. If reliable behavioral expression of developmental methylphenidate exposure can be determined, then the advantages of the zebrafish model can be used to help determine the molecular and cellular mechanisms of the impairment.
2. Methods
2.1. Subjects and Methylphenidate Exposure
Zebrafish embryos (AB strain Danio rerio) were kept at approximately 28.5 °C on a 10 hour dark and a 14 hour light cycle. They were exposed to methylphenidate HCl (0, 12.5, 25 and 50 mg/l) in egg water from fertilization for the first five days after fertilization. Methylphenidate HCl is freely soluble in water at these concentrations. The kinetics of methylphenidate across the chorion is not currently known. We did not see increased lethality or overt dysmorphogenisis at doses up to and including 50 μg/ml of methylphenidate. The larvae were fed brine shrimp and as they matured flake fish food (Tetra Fish Food, Blacksburg, VA, USA) were added. The mature zebrafish were housed 3-liter tanks in an aquatic habitats flow through housing system. Tanks were housed on a 6-tier dual filtration and constantly aerated rack unit. The tank water was made from deionized H2O, sodium bicarbonate, and sea salts (Instant Ocean, 1.2 g/20 l of water). Adult behavioral testing of pre-exposed embryos took place at the third month of life. The adult fish were kept on a Experiments were performed during the light phase between 8:00 a.m. and 5:00 p.m.
2.2. Neurochemistry
Dopamine, serotonin, and norepinepherine were assayed in groups of whole embryos one day after the end of methylphenidate (0 or 50 mg/l) over 0–5 day postfertilization period exposure on day 6 after fertilization (39-60 embryos/sample with N=7 control samples and 6 methylphenidate samples) to observe short-term neurochemical effects and in other sets of fish one month after fertilization to test for persisting effects in processed head samples. There were 8 zebrafish per treatment group in the one-month-old group. Zebrafish were anesthetized by submersion in 4 °C aquarium water and were euthanized by decapitation. The heads were rapidly removed and homogenized (25 × volume per weight) in a solution. Next the excised samples underwent column purification and were diluted in a mobile 1:10 solution. 20 μl were analyzed for neurotransmitters levels.
The methods are the same as we have used previously to determine monoamine levels in the zebrafish brain (Eddins et al., 2010; Eddins et al., 2008). The HPLC system used consists of an isocratic pump (model LC1120, GBC Separations, Hubbardston, MA), a Rheodyne injector (model 7725i) with a 20 μl PEEK loop, and an INTRO Amperometric detector (Antec Leyden, Zoeterwoude, Netherlands). The electrochemical flow cell (model VT 03, Antec Leyden) had a 3 mm carbon working electrode with a 25 μm spacer. An Ag/AgCl served as reference electrode. The cell potential was set at 700 mV. The signal was filtered with a low pass in-line noise killer, (LINK) set at a 14 seconds peak width and a cut off frequency of 0.086 Hz. The signal was integrated and aligned using the EZ Chrom elite chromatography software by Scientific Software Inc. The injector flow cell and analytical column were placed in the Faraday shielded compartment of the detector where the temperature was maintained at 30 °C. The stationary phase was a reverse phase BDS Hypersil C18 column often used to minimize peak tailing. The column expanded 100 mm × 2.1 mm, with 5 μm particle size and 120 Å pore size (Keystone Scientific). The mobile solution for experimental phase consist of 50 mM H3PO4, 50 mM citric acid, 100 mg/l 1-octanesulfonic acid (sodium salt), 40 mg/l EDTA, 2 mM KCl and 3% methanol. pH was obtained to 3.0 with NaOH. The mobile phase was continually degassed with a Degasys Populaire, an on-line degasser (Sanwa Tsusho Co., Tokyo, Japan.). Delivery was set at a flow rate of 0.26 ml/min. The limit of quantitation was approximately 1.56 pg/mg tissue. The limit of detection was approximately 1.07 pg/mg tissue. There were external standards ran with this procedure. The standard curve was run at concentrations of 2.5, 10, 40 and 160 pg/20 μl. Data was stored and profile by computerized software.
2.3 Novel Tank Dive Test
The novel tank diving task is a paradigm that indexes the normal tendency of zebrafish to dive to the bottom of a novel environment upon first introduction and dwell near the bottom until sufficient time without disturbance has elapsed and they proceed to explore the upper levels of the tank (Bencan and Levin, 2008; Bencan et al., 2009; Levin, 2011; Levin et al., 2007). This diving response is an effective strategy for predatory avoidance inasmuch as it removes the possibility of being consumed from below. This is similar to the phenomenon of thigmotaxis (attraction to the wall) seen in rodents in a novel open field environment. In addition to avoiding predation, the zebrafish benefits from completely exploring the environment in search of food. Over time in the novel environment as the predatory threat is not realized, the searching strategy becomes more prominent evidenced by increasing time spent at the upper levels of the tank. Zebrafish were singly tested in 1.5-liter plastic tanks filled with 1350 ml of tank water. The trapezoid shaped tanks were 22.9 cm along the bottom and 27.9 cm along the top. The diagonal side of the tank was 15.9 cm and the opposite vertical side was 15.2 cm, the same design as used previously (Levin et al., 2007). The video image was divided into lower, middle, and top thirds using the EthoVision™ program (Noldus Information and Technology, Wageningen, Netherlands). The trial was five-minutes in duration. The video signal was transmitted through a Samsung Camcorder that was positioned approximately 88 cm away from the horizontal facing tanks. The video signal was transmitted to the computer for analysis. There were 17-31 per group.
2.4. Three-Chamber Learning Test
The three-chamber task was developed in our laboratory to assess spatial and non-spatial learning and memory in zebrafish (Arthur and Levin, 2001; Eddins et al., 2009; Levin and Cerutti, 2008; Levin and Chen, 2004; Levin et al., 2003; Levin et al., 2006). It consists of a central start chamber and two lateral choice chambers with movable partitions between. The apparatus was made of a cylindrical pipe cut in half length-wise and was divided into three chambers, the central start chamber and two choice chambers are on the right and left. Rotatable plastic partitions were movable along Plexiglas rods through both sides of the apparatus. On the inner part of each of the two rods were that allowed passage for the zebrafish. The partitions were circular and stood vertical on both side within the test chamber. The partitions were attached to the 12.7 cm long rod rails. The two rails could be moved back toward the wall or completely forward toward the central chamber. Three thick black lines along the back of the chamber provided a visual cue to provide an axis of orientation for right–left orientation so that the fish can readily discriminate right from left. Prior to training, 5 preliminary trials were performed to establish a preferred side. After preliminary testing, 10 trials were performed to test zebrafish learning against their preferred side.
One zebrafish at a time was placed in the start chamber. After 60 seconds, the partitions to each of the choice chambers were simultaneously opened. If the fish swam into the correct choice chamber, the partition was closed and the fish was left alone in that chamber unpunished for one minute. But, if the fish swam into the incorrect side, the partition was closed and then moved back to the wall 1 cm to reduce swimming space as punishment for ten seconds. If the fish did not make a choice within 20 seconds, a plastic beaker was dropped just above the start chamber to induce a selection. Choice accuracy and response latency were recorded. There were 31-57 fish per group.
2.5. Tap Startle and Habituation Test
The startle test apparatus consisted of flat white 20.4 cm × 38.1 cm surface with white 12.7 cm × 15.2 cm frontal and rear blocking barriers attached. On the flat surface there were arranged eight 5.1 cm × 7.6 cm clear cylindrical arenas made of Plexiglas in a 2 × 4 array. Each arena was filled with 30 ml of tank water. The apparatus was positioned between two white opaque barriers, which faced each other and projected a bare white screen. Mechanical solenoids were positioned beneath each arena. A Samsung camcorder was located approximately 71 cm above the apparatus. The solenoids were used to administer taps to the bottom of the cylindrical arena under control of the computer. Eight zebrafish were taken from the holding tanks and each one was placed into a cylindrical arena on the tap apparatus. Zebrafish were allowed to acclimate in the arena for 3 minutes prior to testing. After the adjustment period, the tap test was started and solenoids tapped the testing arenas at 1 minute intervals for 10 consecutive trials. The zebrafish response in distance traveled was recorded 5 seconds after the tap. The total testing time for the initial phase was 10 minutes. The image of the fish movement in response to the repeated taps was indexed by the EthoVision™ program (Noldus Information and Technology, Wageningen, Netherlands). There were 26-28 fish per treatment group.
2.6. Statistics
The dependent measures for each behavioral and neurochemical test were assessed by analysis of variance for between subjects factors and repeated measures. A p-value less than 0.05 was the threshold for significance. The Dunnett's test was used for comparisons of each methylphenidate group to control.
3. Results
3.1. Neurochemistry
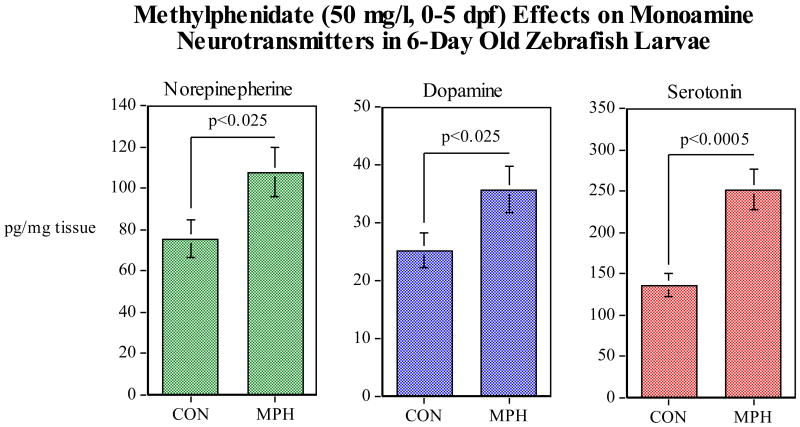
Analysis of the monoamine neurotransmitters in 6-day old larvae after exposure to 50 mg/l methylphenidate during the first five days after fertilization showed significant increases in dopamine, norepinepherine and serotonin (Fig. 1). Methylphenidate exposure relative to control significantly increased norepinepherine (F(1,11)=7.97, p<0.025), dopamine (F(1,11)=7.25, p<0.025) and serotonin (F(1,11)=26.31, p<0.0005) whole body concentrations in the larvae one day after withdrawal of the methylphenidate exposure. These neurochemical effects were not seen to be persistent inasmuch as no significant effects were seen at 30 days after fertilization.
Figure 1.
Early developmental methylphenidate exposure effects on whole larva concentrations of monoamine neurotransmitters one day after methylphenidate (50 mg/l) exposure during the first five days after fertilization (mean±sem). Methylphenidate exposure relative to control significantly increased norepinepherine (p<0.025), dopamine (p<0.025) and serotonin (p<0.0005).
3.2. Novel Tank Diving Test
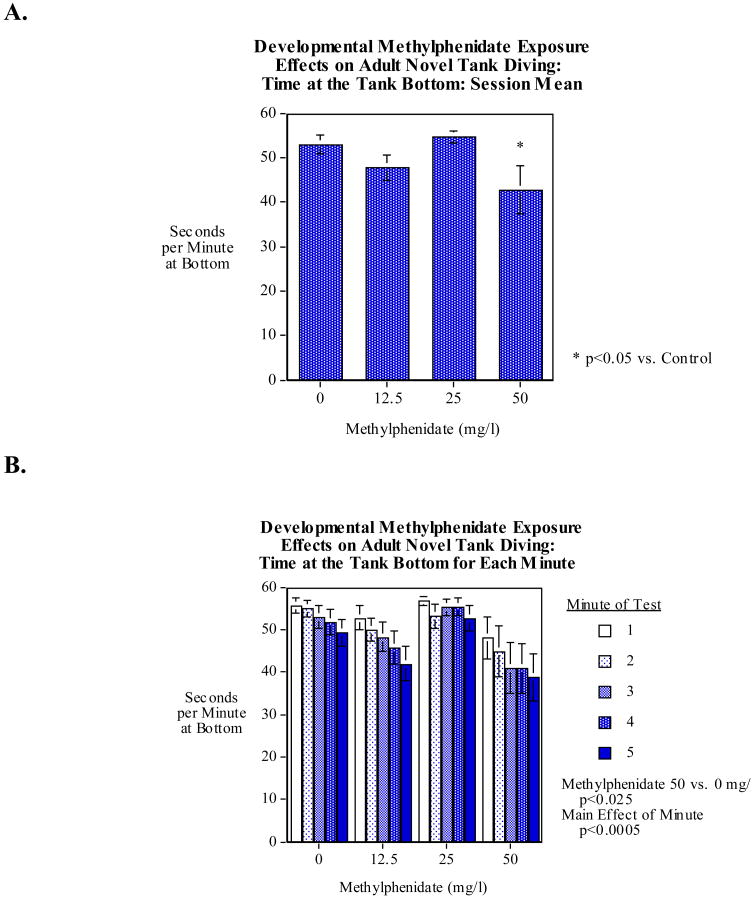
Early developmental methylphenidate exposure for the first five days after fertilization had long-term effects on the novel tank diving test in adult zebrafish. As shown in figure 2A, the mean time spent in the bottom third of the tank, mean across the five-minute test was significantly (Dunnett's test, p<0.05) decreased by the 50-mg/l methylphenidate exposure relative to vehicle treated controls. The lower dose methylphenidate exposure was not found to significantly affect the novel tank diving response. Figure 2B shows the detailed diving data for each of the five minutes of the test. As we have seen in many earlier studies the bottom dwelling time decreased over the five minutes of the session. There was a significant main effect of minute (F(4,356)=7.20, p<0.0005) reflect less bottom dwelling as the test progressed. There was no significant interaction of the methylphenidate effect with this effect of time (Fig. 2B). With swimming speed there was a significant (F(4,356)=8.45, p<0.0005) increase in speed cm/min from 57.3±5.6 (mean±sem) during the first minute to 90.8±7.2 during the last minute. There was no significant effect of methylphenidate exposure on swimming speed in the novel tank diving test (data not shown) and there was no significant interaction of methylphenidate exposure with time.
Figure 2.
A) Early developmental methylphenidate exposure effects on the novel tank diving test in adult zebrafish, mean time spent in the bottom third of the tank, mean across the five-minute test (mean±sem). 50 mg/l methylphenidate exposures for five days after fertilization significantly (p<0.025) decreased bottom dwelling in the novel tank diving task.
B) Early developmental methylphenidate exposure effects on the novel tank diving test in adult zebrafish, time spent in the bottom third of the tank in each of the five minutes of the test (mean±sem). There was a significant main effect of minute (p<0.0005) reflect less bottom dwelling as the test progressed. There was no significant interaction of the methylphenidate effect with the effect of time.
3.3. Three-Chamber Spatial Learning
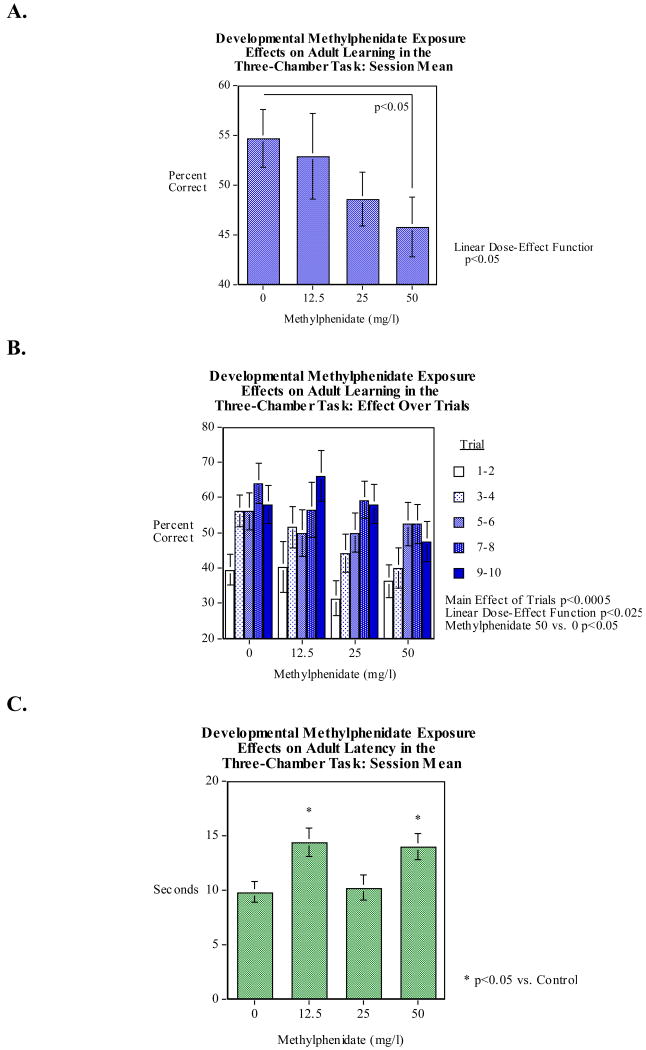
Early developmental methylphenidate exposure during the first five days after fertilization had a significant effect on choice accuracy on the three-chamber spatial learning task. Figure 3A shows the choice accuracy averaged across the ten trials in the training session (mean±sem). 50-mg/l methylphenidate exposure for five days after fertilization significantly (Dunnett's test, p<0.05) decreased choice accuracy relative to control and there was a significant linear (F(1,167)=5.16 p<0.025) decline in accuracy over the dose methylphenidate dose range tested. Figure 3B shows the detailed data of choice accuracy for each of the five two-trial blocks in the training period. There was a significant (F(4,668)=10.58, p<0.0005) effect of trials reflecting the improvement of the fish with training. There was no significant interaction of methylphenidate exposure with training trial block. All the methylphenidate exposure groups and controls started with choice accuracy approximately 35-40%. This was below the chance level of 50% for the two-choice task because the fish were trained against the side of their initial preference. All the groups rose from this low initial level with continued training. Figure 3C shows the mean response latency per trial in seconds averaged over the trials of the session (mean±sem). The 12.5 and 50-mg/l methylphenidate exposures for the first five days after fertilization significantly (Dunnett's test, p<0.01) increased response latency relative to controls. Curiously, the 25-mg/l methylphenidate exposure group did not show a significant effect on response latency.
Figure 3.
A) Early developmental methylphenidate exposure effects on choice accuracy on the three-chamber spatial learning task, mean choice accuracy across the ten trials in the training session (mean±sem). 50 mg/l methylphenidate exposures for five days after fertilization significantly (p<0.05) decreased choice accuracy relative to control in the three-chamber spatial learning task and there was a significant linear (p<0.05) decline in accuracy over the dose methylphenidate dose range tested.
B) Early developmental methylphenidate exposure effects on choice accuracy on the three-chamber spatial learning task, choice accuracy in two-trial blocks during the training session (mean±sem). There was a significant main effect of trials (p<0.0005) reflecting the improvement in accuracy with continued training. There was no interaction of trials with the methylphenidate-induced impairment in overall choice accuracy.
C) Early developmental methylphenidate exposure effects on response latency on the three-chamber spatial learning task, mean response latency in seconds over the trials of the session (mean±sem). The 12.5 and 50 mg/l methylphenidate exposures for the first five days after fertilization significantly (p<0.01) increased response latency.
3.4. Tap Startle Response and Habituation
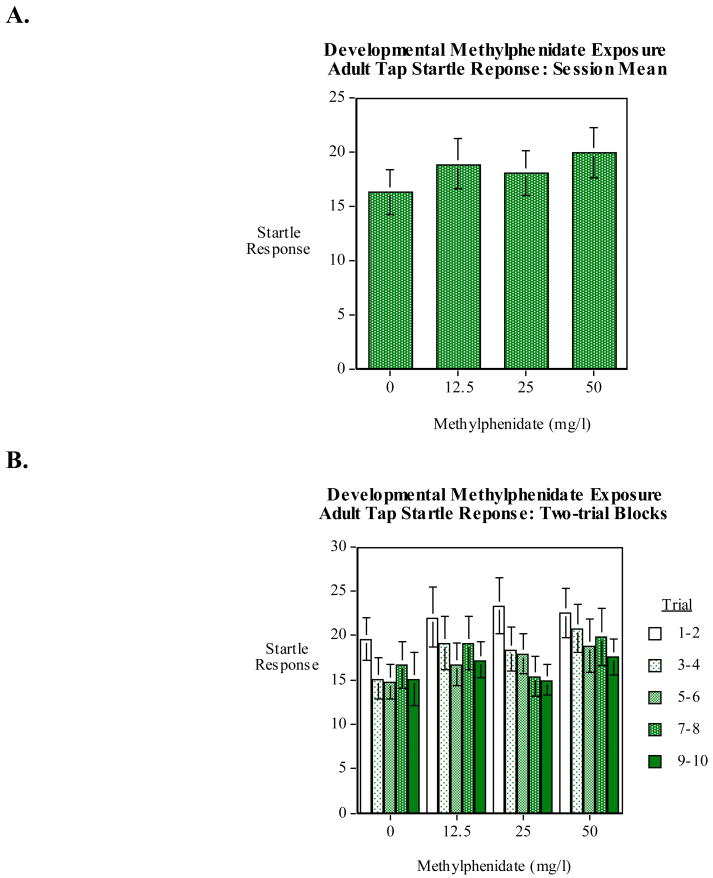
Startle response for the five seconds after a tap and habituation of that response in adult zebrafish were not found to be significantly affected by early developmental methylphenidate exposure. As shown in figure 4A there was a suggestion of increased in the mean startle response across the ten-trial session after 50-mg/l methylphenidate, but this was not significant. There was a very pronounced and significant (F(4,416)=7.50, p<0.0005) decrease in response across the ten trials of the session (Fig. 4B). However, there was no significant interaction of this effect with early developmental methylphenidate exposure.
Figure 4.
A) Early developmental methylphenidate exposure effects on startle response in adult zebrafish, mean across ten trials (mean±sem).
B) Early developmental methylphenidate exposure effects on startle response in adult zebrafish, habituation of response over the ten-trial session (mean±sem).
4. Discussion
Methylphenidate exposure in zebrafish during the first five days after fertilization was found to cause short-term neurochemical disruption and long-term behavioral dysfunction. Short-term effects of methylphenidate were seen with increasing levels of the monoaminergic neurotransmitters, norepinepherine, dopamine and serotonin. These increases in monoaminergic levels were transient, not being apparent by one-month of age. This early developmental methylphenidate exposure did cause long-term behavioral dysfunction. The 50-mg/l methylphenidate exposure caused a significant deficit in predatory escape response in the novel tank diving test and impaired choice accuracy in the three-chamber spatial learning task. These behavioral effects were specific inasmuch as tactile startle response and its inhibition was not found to be significantly affected by this dose range of developmental methylphenidate exposure during the first five days after fertilization. Recent increases in the use of methylphenidate by adults diagnosed with attention deficit hyperactivity disorder-residual type have raised the risk of embryonic and fetal exposure to methylphenidate. The current results raise the concern that methylphenidate use during pregnancy may pose a risk of long-term behavioral toxicity in the offspring.
The neurochemical effects of exposure to 50 mg/l of methylphenidate were pronounced. All three of the monoamine neurotransmitter levels assessed, dopamine, norepinepherine and serotonin were significantly increased when assessed on the day after the end of exposure during the first five days after fertilization. This neurochemical effect was transient. By 30 days after fertilization there was no effect of the early methylphenidate exposure on monoamine levels seen. This early neurochemical disruption though transient caused persisting behavioral impairment.
In contrast to the transient neurochemical effects seen there were significant long-term behavioral effects of early developmental methylphenidate exposure. The short-term effect of methylphenidate of significantly increasing monoamine levels during early development with the monoamine levels returning to control values after withdrawal from the exposure is not surprising. This dynamic change together with long-term behavioral effects is not a flaw in the study. Rather it provides important information concerning the mechanisms of the induction and expression of the developmental neurotoxic effects. From the early measurements we know that as in mammals methylphenidate causes increases in dopamine and the other monoaminergic transmitters in zebrafish. These monoaminergic systems are connected with a variety of other neural systems. Early over-activity of the monoamine systems appear to disrupt related non-monoaminergic systems in a persistent way so as to compromise behavioral function even as these monoamine levels return to normal. The discussion has been revised to address this issue.
The threshold for the significant long-term behavioral impairment caused by early developmental methyphenidate is 50-mg/l. The novel tank diving response was significantly decreased, swimming speed in the novel tank was significantly increased and choice accuracy in the three-chamber learning task was significantly impaired in adult fish by exposure to 50-mg/l during early development while lower doses were not found to have these effects.
The significant decrease choice accuracy in the three-chamber task is similar to what we have found after early developmental exposure by zebrafish to the organophosphate pesticide chlorpyrifos (Levin et al., 2003). However, developmental chlorpyrifos exposure was also found to cause significant elevation of tap startle response, an effect not seen after methylphenidate exposure in the current study.
Methylphenidate (50 mg/l) caused an overall decrease in percent correct in the three-chamber spatial learning task. There was not a significant interaction with trials, but as can be seen in figure 5, both the control and the 50-mg/l methylphenidate treated group began at just under 40% correct. This initial percent correct level of lower than random chance level of 50% resulted from training the fish against their initial preferred side. The control group quickly learned by trials 3-4 to choice accuracy more than fifteen percentage points higher than the initial rate, while the 50-mg/l methylphenidate treated group show very little sign of learning until later in the training session for a significant (p<0.05) difference between the control and 50 mg/l groups.
The behavioral effects were not pervasive. Startle response and habituation were not found to be affected. The methylphenidate treated fish had an overall lower percent correct in the three-chamber task, however they did not show significantly less improvement across the ten trials of training. The methylphenidate treated fish also showed normal within session decreases in the diving response and increase in swimming speed in the novel tank diving task.
There is only limited literature concerning the persisting neurobehavioral effects of early developmental methylphenidate. The current study provides information that the long-term neurobehavioral toxicity in zebrafish. The advantages of the zebrafish model with their clear chorion, molecular tools and reporter systems offer the ability to conduct detailed mechanistic studies. These results serve as a caution that there may be concern about the neurotoxic risk of methylphenidate use during pregnancy. It is reasonable to consider that drugs designed to have effects on brain function may pose neurotoxic risk to the developing brain.
Highlights.
Increasing adults use of methylphenidate raises the risk of exposure in pregnancy
Embryonic methylphenidate exposure in zebrafish caused a transient increase in dopamine, norepinepherine and serotonin.
Embryonic methylphenidate exposure in zebrafish caused a long-term impairment in the predatory avoidance response.
Embryonic methylphenidate exposure in zebrafish caused a long-term impairment in spatial learning.
Acknowledgments
Supported by the Duke University Superfund Research Center ES10356 and NIEHS ARRA grant ES016554.
Footnotes
Conflict of Interests Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131. [Google Scholar]
- Beckman DA, Schneider M, Youreneff M, Tse FL. Developmental toxicity assessment of d,l-methylphenidate and d-methylphenidate in rats and rabbits. Birth Defects Research Part B, Developmental and Reproductive Toxicology. 2008;83:489–501. doi: 10.1002/bdrb.20168. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology, Biochemistry and Behavior. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T. Methylphenidate in treatment of adults with Attention-Deficit/Hyperactivity Disorder. Journal of Attention Disorders. 2002;6 doi: 10.1177/070674370200601s12. [DOI] [PubMed] [Google Scholar]
- Bizot JC, Chenault N, Houze B, Herpin A, David S, Pothion S, Trovero F. Methylphenidate reduces impulsive behaviour in juvenile Wistar rats, but not in adult Wistar, SHR and WKY rats. Psychopharmacology. 2007;193:215–223. doi: 10.1007/s00213-007-0781-4. [DOI] [PubMed] [Google Scholar]
- Bolanos C, Barrot M, Berton O, Wallace-Black D, Nestler E. Methylphenidate Treatment during Pre- and Periadolescence Alters Behavioral Responses to Emotional Stimuli at Adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29:656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- DSM-IV-Task-Force. Diagnostic and Statistical Manual for Mental Disorders: DSM-IV, Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Developmental chlorpyrifos causes behavioral and neurochemical defects in zebrafish. Neurotoxicology and Teratology. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallothionein knockout mice. Neurotoxicology and Teratology. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology. 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- Garland EJ. Pharmacotherapy of adolescent attention deficit hyperactivity disorder: challenges, choices and caveats. Journal of Psychopharmacology. 1998;12:385–395. doi: 10.1177/026988119801200410. [DOI] [PubMed] [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, Zupan B, Menzer D, Rice J, Drake CT, Romeo RD, Brake WG, Torres-Reveron A, Milner TA. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. Journal of Neuroscience. 2007;27:7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greydanus DE, Sloane MA, Rappley MD. Psychopharmacology of ADHD in adolescents. Adolescent Medicine State of the Art Reviews. 2002;13:599–624. [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Beiderman J, Conner CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustiun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: Filling the gap between in vitro and rodent models for drug development. Reviews in the Neurosciences. 2011;22:75–84. doi: 10.1515/RNS.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology and Behavior. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Levin ED, Cerutti DT. Behavioral neuroscience of zebrafish. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; New York: 2008. pp. 293–310. [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- McFadyen-Leussis MP, Lewis SP, Bond TL, Carrey N, Brown RE. Prenatal exposure to methylphenidate hydrochloride decreases anxiety and increases exploration in mice. Pharmacol Biochem Behav. 2004;77:491–500. doi: 10.1016/j.pbb.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. Journal of Child & Adolescent Psychopharmacology. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Sledge D, Yen J, Morton T, Dishaw L, Shuler K, Donerly S, Linney E, Levin ED. Critical windows of exposure for developmental chlorpyrifos effects on behavioral function in zebrafish. Society of Toxicology, Annual Meeting Baltimore, MD 2009 [Google Scholar]
- Smith BH, Waschbusch DA, Willoughby MT, Evans S. The efficacy, safety, and practicality of treatments for adolescents with attention-deficit/hyperactivity disorder (ADHD) Clinical Child & Family Psychology Review. 2000;3:243–267. doi: 10.1023/a:1026477121224. [DOI] [PubMed] [Google Scholar]
- Soileau EJ., Jr Medications for adolescents with attention-deficit/hyperactivity disorder. Adolescent Medicine. 2008;19:254–267. [PubMed] [Google Scholar]
- Teo SK, Stirling DI, Hoberman AM, Christian MS, Thomas SD, Khetani VD. D-methylphenidate and D,L-methylphenidate are not developmental toxicants in rats and rabbits. Birth Defects Research Part B, Developmental and Reproductive Toxicology. 2003;68:162–171. doi: 10.1002/bdrb.10018. [DOI] [PubMed] [Google Scholar]
- Teo SK, Stirling DI, Thomas SD, Hoberman AM, Christian MS, Khetani VD. The perinatal and postnatal toxicity of D-methylphenidate and D,L-methylphenidate in rats. Reprod Toxicol. 2002;16:353–366. doi: 10.1016/s0890-6238(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Izidio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behavioural Pharmacology. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. The multifaceted effects of oral administration of methylphenidate in juvenile rats: Anxiety, activity, and attention. European Neuropsychopharmacology. 2010;20:236–244. doi: 10.1016/j.euroneuro.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]