Abstract
Trimethyltin chloride (TMT) is a neurotoxicant that is widely present in the aquatic environment, primarily from the manufacture of PVC plastic, but few studies have evaluated aquatic neurotoxicity. We have examined TMT dose-dependent malformation and neurobehavioral toxicity in the embryonic zebrafish model. Exposure of embryos to TMT (0–10 μM) from 48–72 hours post fertilization (hpf) elicited a concentration-related increase (0–100%) in malformation incidence with an EC25 of 5.55 μM. TMT also significantly modulated the frequency of tail flexion, the earliest motor behavior observed in developing zebrafish, and the ability to respond to a mechanical tail touch. Exposure to 5 μM TMT from 48–72 hpf modulated the photomotor response at 4 and 5 days post fertilization and significantly promoted apoptosis in the tail. Our study demonstrates the morphological and behavioral sensitivity of the developing zebrafish to TMT and establishes a platform for future identification of the affected pathways and chemical modulators of TMT toxicity.
Keywords: zebrafish, TMT, malformation, neurobehavioral toxicity, photomotor response
1. Introduction
Trimethyltin chloride (TMT) occurs in polyvinyl chloride (PVC) and silicone products, such as kitchen utensils, food packages, and fungicides (Gomez et al., 2007; Hoch, 2001). TMT is detected in domestic water systems, drinking water supplies, marine environments and aquatic specimens (Gomez et al., 2007; Shawky and Emons, 1998). As a byproduct of plastic stabilizers, TMT has caused 67 poisoning accidents worldwide, affecting 1,849 people between 1978–2008 (Tang et al., 2010). The most common, acute exposures to TMT appear to be occupational during plastic manufacture or other industrial processes that require heating of plastic. Ingestion of organotin polluted food or water in areas heavily affected by plastics manufacturing is also likely to be of increasing concern (Fonger et al., 2000; Tang et al., 2010). Intoxication with TMT leads to profound behavioral and cognitive deficits in both humans (Fortemps et al., 1978) and experimental animals (Fonger et al., 2000; Hlinak et al., 2008; Ishida et al., 1997; Kim et al., 2009; Niittykoski et al., 1998). The neurotoxic presentation most frequently reported in human cases is a limbic-cerebellar syndrome, the manifestations of which include memory defects, confusion, seizures, tinnitus, insomnia, and depression (Saary and House, 2002). Despite the documented presence of TMT in aquatic environments (Jiang et al., 2004; Jiang et al., 2001; Liu and Jiang, 2002; Shawky and Emons, 1998; Zhou et al., 2001) its potential risk to aquatic organisms is largely unknown.
TMT has been widely used as a model toxicant for studying central nervous system (CNS) toxicity (El-Fawal and O’Callaghan, 2008; Trabucco et al., 2009; Zuo et al., 2009). In mice and rats TMT was shown to induce lesions in the hippocampus and impair spatial memory (Ishida et al., 1997). TNFα-induced, PKC-dependent stannin expression has been hypothesized to play a role in the selective neurotoxicity of TMT (Reese et al., 2005; Toggas et al., 1992). Some have hypothesized from experiments in the marine fish Sebastiscus marmoratus that glutamatergic receptor NMDAR and its signaling pathway components are important for TMT neurotoxicity (Zuo et al., 2009). In another report, TMT-induced neurotoxicity and memory impairment in adult rats were reversed by sigma 1 receptor antagonists, and TMT exposure reduced the amount of sigma 1 receptor in the hippocampus suggesting that TMT acts, at least in part, through sigma 1 receptor stimulation (Shin et al., 2007). Much remains to be learned about the mechanism of organotin neurobehavioral toxicity. Though numerous studies describe a consensus phenotype of TMT intoxication in the adult rat and mouse, only a few studies have addressed the outcomes of developmental TMT exposure in vertebrates. No reports have appeared from a vertebrate model amenable to genetic screens for modulators of TMT toxicity. Several prior studies in the developing rat have demonstrated that TMT significantly impaired olfactory learning, spatial memory and learning in adulthood (Stanton, 1991; Stanton et al., 1991), elicited obvious hyperactivity in the preweaning stage, impaired the acoustic startle response in preweaning and adult rats and resulted in the significant loss of pyramidal neurons in the hippocampus (Miller et al., 1982; Miller and O’Callaghan, 1984; Ruppert et al., 1983, 1985). Despite these significant findings from 20–30 years ago, almost no additional investigations of developmental processes affected by TMT have been reported to date.
An excellent vertebrate model, zebrafish (Danio rerio) shares many developmental, cellular, signaling and physiological characteristics with mammals (Cole and Ross, 2001; Haendel et al., 2004). The inherent advantages of embryonic zebrafish stem from their small size, transparency, and rapid developmental cycle (Kimmel et al., 1995). The transparent embryos develop externally, which can be continuously observed, facilitating developmental time course studies. Commercial platforms for recording and analyzing larval zebrafish photomotor behavior in a 96 well format have enabled efficient screening for a once tedious endpoint (Huang et al.; Rihel et al., 2010). We routinely use a semi-automated approach to evaluate larval zebrafish swimming behavior in response to sudden, visible light-to-dark transition (Huang et al., 2010; Wang et al., 2011).
Our objectives were to more precisely define the neurotoxicity of TMT in the developing zebrafish and gain insight into the developmental processes affected.
2. Materials and methods
2.1. Fish husbandry and embryo collection
Tropical 5D and transgenic NBT zebrafish were reared and kept at the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University under standard laboratory conditions of temperature 28 ± 0.5°C, pH 7.2 ± 0.2, on a 14:10 dark/light photoperiod according to standard zebrafish breeding protocols (Westerfield, 1995). Zebrafish embryos were obtained from spawning adults with a sex ratio of 1:1. Embryos were collected within 0.5 h of spawning and rinsed in embryo medium (EM) (Westerfield, 1995). Fertilized and normal embryos were staged under a stereomicroscope (Nikon, Japan) according to the descriptions of Kimmel et al. (Kimmel et al., 1995).
2.2. TMT stock solutions and exposure protocols
Trimethyltin chloride (TMT; Sigma PN#146498; purity >97%) was purchased from Sigma-Aldrich. A TMT stock solution of 1mM in water was stored at 4°C until further dilution with embryo medium (EM)(Westerfield, 1995). Embryos were individually waterborne-exposed to TMT at 0, 2, 5, 10 μM in plastic 96-well plates (200 μL/ well) at 8 hours post fertilization (hpf), and covered with parafilm to prevent evaporation. At specific developmental stages, spontaneous movement, touch response, and cell death in vivo were assessed. The short 24 – 36 hour TMT exposures were used to evaluate the effect of transient TMT exposure on effects observed at 96 and 120 hpf. An earlier study also identified 48–72 hpf as a sensitive, transient window for TMT exposure. In order to identify the malformation dose for this window, 48 hpf larvae were waterborne-exposed to TMT (1–10 μM) as above until 72 hpf. At the end of the exposure period, the zebrafish were rinsed three times with EM and allowed to continue development until 96 hpf when the incidence of malformation and type were recorded.
2.3. Effects of TMT on embryo tail flexion and touch response
To determine whether exposure to TMT affected embryo spontaneous tail flexion and touch response, embryos were exposed in 96-well plates as in 2.2 to TMT at 0, 2, 5, 10 μM beginning at 8 hpf. From 19 to 27 hpf, the spontaneous movement of 8 embryos for each TMT concentration was video recorded for a 1 min duration, and repeated 3 times. The time to complete the plate was approximately 12 minutes. For the touch response test, exposed and control embryos were manually dechorionated at 24hpf using fine forceps and at 27 and 36 hpf, the touch response of 8 embryos per treatment was recorded, and repeated 3 times.
2.4 Behavior assessment
To determine the effect of TMT on the 4 and 5 dpf larval swimming response to sudden light to dark transitions we used a variation of the photomotor response test. Morphologically normal larvae exposed to 5 μM TMT from 48 to 72 hpf were assayed as one larva per well per 2ml of EM in a 24 well plate using a ZebraBox behavior monitoring station (ViewPoint Life Sciences, Inc., Montreal, Canada). The 48–72 hpf exposure window was the same as for the gene expression work (2.6) to better facilitate addressing the gene expression changes underlying the behavior phenotype. Larvae were allowed to adapt for 20 min in visible light of intensity similar to that of ambient, overhead fluorescent lighting in the laboratory before recording the photomotor response as the average swimming speed in each well per minute. The lighting parameters were 10 min light (visible light) followed by a 10min dark (infrared light) interval, and repeated for 50 min. The behavior testing was conducted between 11:00 am and 3:00 pm. Each photomotor experiment had 12 TMT exposed animals and 12 control animals in one 24-well plate. Three biological replicates (embryos collected from different batches on separate days) were conducted.
2.5 Cell apoptosis assay using acridine orange staining
In zebrafish, live embryos can be used to detect and quantify cell death in the whole animal with the utilization of acridine orange. An earlier TMT exposure window was used because we surmised that TMT-elicited apoptosis, if occurring during the body patterning and neuronal development phases of embryogenesis, would be highly detrimental to development. To visualize cell death in vivo, control and TMT (5μM) exposed embryos (from 8 to 24 hpf) were manually dechorionated and rinsed twice with EM and incubated with 5 μg/ml acridine orange dissolved in EM for 45 min at room temperature in the dark (Usenko et al., 2007). The embryos were then washed with EM three times for 5 min each. Before examination, the live animals were anesthetized with 0.02% MS-222 for 5 min and mounted laterally in 1% w/v low melt agarose on a microscope slide. A rhodamine filter set mounted to a motorized stage on a Zeiss Axiovert 200 M microscope (Carl Zeiss, Germany) was used to examine all embryos (n = 24 for each group) and capture the same magnification and orientation pictures of the head and tail region for control and TMT treated larvae. Acridine orange- positive cells were quantified from images using Image Pro Plus software program (Media Cybernetics, Inc., Silver Spring, MD).
2.6. TMT behavior associated gene expression
We have included here a small, vision-related subset of the unpublished expression data as it potentially relates to effects of TMT on behavior reported in the present study (Table 1), details of TMT effects will be published elsewhere. We found that a susceptible developmental stage to TMT exposure was between 48–72 hpf, with 10 μM TMT producing pericardial edema in 100% of the larvae. Embryos at 48 hpf were exposed in 96 well plates in three replicate experiments to 10 μM TMT or embryo medium and collected and pooled (40 individuals/pool) for RNA isolation at 60 and 72 hpf. Double strand cDNA was labeled with Cy5 and hybridized to 12 × 135K zebrafish arrays (Roche Nimblegen, Madison, WI). The human orthologs of the zebrafish mRNA sequences were subjected to Ingenuity Pathways Analysis (IPA, Ingenuity® Systems, www.ingenuity.com) to generate a plausible network of affected genes.
Table 1.
Vision associated transcriptional changes elicited by TMT in the developing zebrafish. 48 hpf embryos were exposed to 10 μM TMT or embryo medium and collected at 60 and 72 hpf, in replicate pools of 40 individuals. Double strand cDNA was labeled with Cy5 and samples were hybridized to 12 × 135K zebrafish arrays (Roche Nimblegen, Madison, WI).
| Symbol | Entrez Gene Name | Fold Change | Location | Family |
|---|---|---|---|---|
| ARR3 | arrestin 3, retinal (X-arrestin) | −1.97 | Cytoplasm | arrestin |
| CRX | cone-rod homeobox | −1.24 | Nucleus | transcription regulator |
| G protein α | G protein α | −1.88 | Plasma Membrane | G protein |
| Gpcr | G Protein Coupled Receptor | −2.42 | Plasma Membrane | G-protein coupled receptor |
| OPN1LW | long-wave-sensitive | −2.46 | Plasma Membrane | G-protein coupled receptor |
| Opsin | short-wave-sensitive | −2.00 | Plasma Membrane | G-protein coupled receptor |
| RHO | rhodopsin | −4.64 | Plasma Membrane | G-protein coupled receptor |
2.7. Data analysis
Sigmoidal regression for concentration–response curves for estimation of the EC25 was performed in Origin 8.0 (OriginLab, Northampton, MA, USA). For gene expression, one-way ANOVA for unequal variance with Tukey’s post hoc test (p<0.05) in GeneSpring GX v11.0 (Agilent Technologies, Santa Clara, CA) was used to generate significant gene lists. For tail flexion and larval behavior data, we used a repeated-measures ANOVA followed by Bonferroni t-tests to identify significant differences between control and each TMT dose at each timepoint. For the non-parametric touch response data, we used Friedman’s two-way ANOVA for repeated-measures followed by Cochran’s Q test to identify significant differences between control and each TMT dose for both timepoints. Tests were run in Base SAS 9.2 (SAS, Cary, NC, USA). The apoptosis data, as background subtracted pixel values above an intensity threshold, were statistically assessed by a t-test. All the graphed data were reported as means ± standard error (SEM) unless otherwise stated.
3. Results
3.1. TMT affects motor behavior in zebrafish embryos
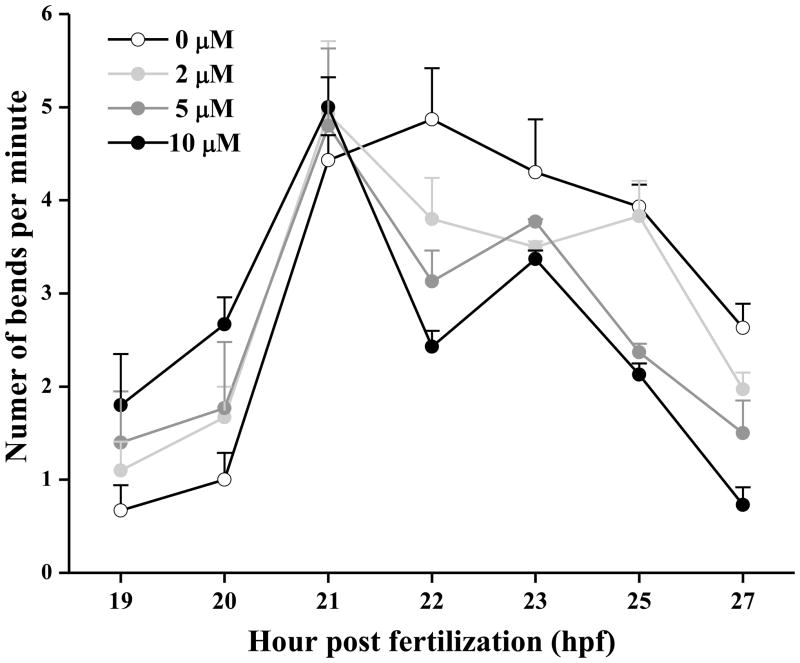
The first observable behavior in early development of zebrafish is spontaneous movement (periodic tail bending) from the presence of functional neurons adjoining the somites (Saint-Amant and Drapeau, 1998). At 20 hpf, 10 μM TMT significantly (P < 0.05) stimulated tail bending (2.67 ± 0.29 bends/min) compared to the control embryos (1.0 ± 0.29 bends/min) (Fig. 1.). The spontaneous movement frequency between 19–27 hpf for control embryos peaked at 4.87 ± 0.55 bends/min at 22 hpf and slowly declined thereafter, while all three groups of TMT treated larvae reached a similar peak frequency at 21 hpf, then rapidly declined by 22 hpf to frequencies significantly less (P < 0.01) than the control peak. Both 5 and 10 μM TMT significantly decreased (P < 0.05) tail bending frequency at 25 and 27 hpf.
Figure 1.
TMT promoted early spontaneous tail flexions (Mean ± S.E.M.) in zebrafish embryos followed by a rapid decline in flexions (n = 8 embryos per group). Tail flexions were recorded for 1 min, hourly from 19–27 hpf.
3.2. TMT affects the embryo’s response to tail touch
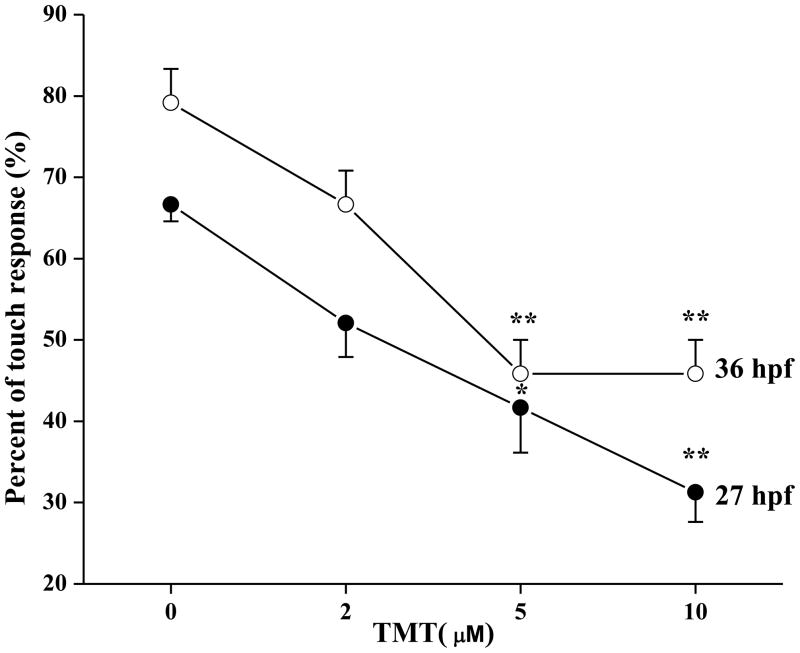
At 27 and 36 hpf, tactile stimulation (rounded probe touch) to the tail of TMT treated embryos elicited fewer responses than the control group. At 27 hpf, the percent of animals displaying a touch response was lower in 5 μM (41.7 ± 5.5; P < 0.05) or 10 μM TMT treated larvae (31.3 ± 3.6; P < 0.001) than in control larvae (66.7 ± 2.08) (Fig. 2). At 36 hpf, exposure to 5 and 10 μM TMT resulted in 45.8 ± 4.2 (%) touch response incidence compared with 79.2 ± 4.2 (%) in the control group (P < 0.001; Fig. 2).
Figure 2.
TMT reduced the tail touch response (mean ± SEM) of zebrafish embryos at 27 and 36 hpf (n = 8). The data indicate the percentage of animals at each TMT concentration that displayed a touch response. *= P < 0.05 and **= P < 0.001 for significant differences from the control group.
3.3. TMT elicits malformations and aberrant photomotor behavior in zebrafish larvae
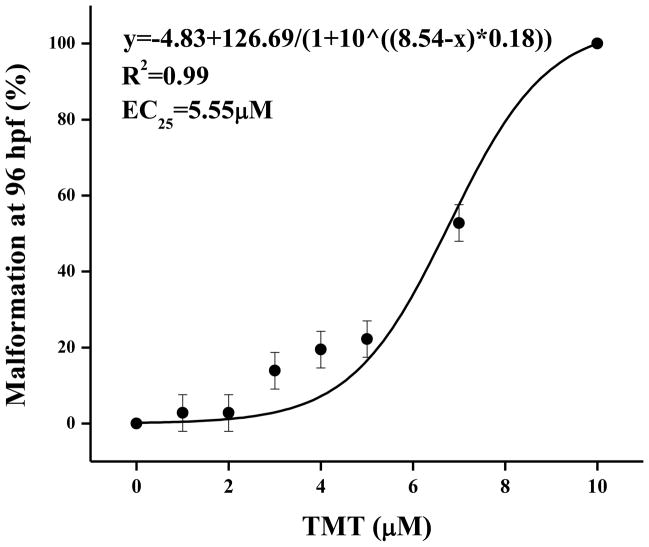
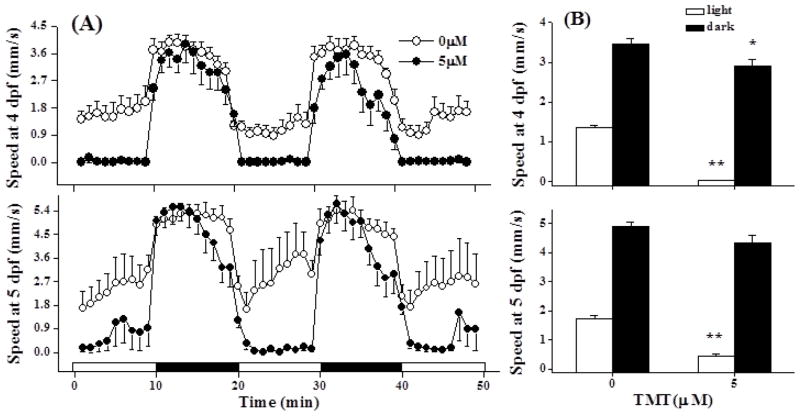
The EC25 for TMT exposure from 48 to 72 hpf (a sensitive window, data not shown) was 5.55 μM when assessed at 96 hpf (Fig. 3). Yolk sac and pericardial edemas comprised <25% of the malformations elicited by 5 μM TMT. Larvae that did not develop visible malformations when exposed to 5 μM TMT were used to evaluate the effect of TMT on photomotor behavior. At 4 and 5 dpf, TMT exposed embryos displayed obvious hypoactivity (P < 0.001) in the visible light periods (Fig 4A and B). At 4dpf the TMT exposed larvae were also weakly hypoactive in the dark (P<0.05; Fig. 4B) though this weak effect was insignificant at 5dpf.
Figure 3.
TMT induced dose related malformations between 48 and 72 hpf (n = 36 zebrafish larvae per treatment).
Figure 4.
TMT induced hypoactivity in the light. (A) Photomotor responses were recorded at 4 and 5 dpf for larvae exposed to 5 μM TMT from 48 to 72 hpf. Each light or dark interval was 10 min and 12 larvae were assessed per group. White intervals on the x axis were visibly lighted periods and dark intervals were IR lighted (dark) periods. (B) Comparison of mean light and dark responses between control and TMT exposed larvae at 4 and 5 dpf. **The TMT responses in the light at 4 and 5 dpf were hypoactive (P < 0.001) relative to the control responses. *The TMT dark response at 4 dpf was weakly hypoactive (P < 0.05) and this effect was not significant at 5 dpf.
3.4. TMT induces localized apoptosis
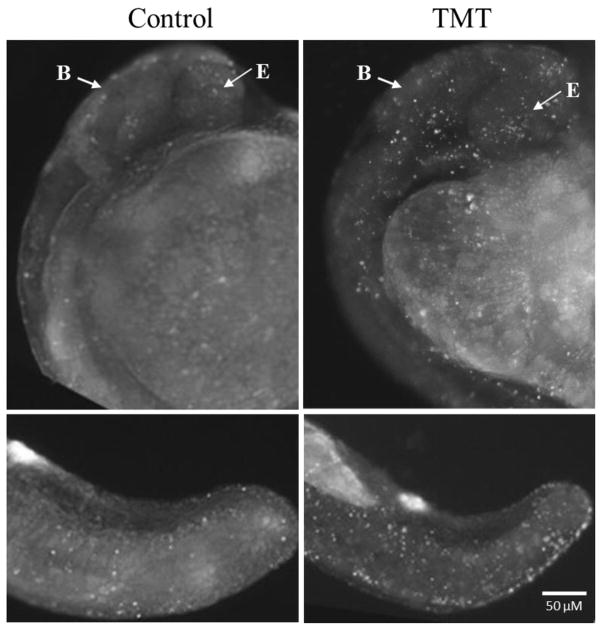
Cellular death assays were performed to determine if TMT affected apoptosis in specific cells or tissues. Embryos exposed to 5 μM TMT from 8 to 24 hpf were dechorionated manually before being exposed to acridine orange for 45 min. Only a few apoptotic cells were observed in the control larvae. While considerable apoptotic activity was consistently localized in the brain, eye, ear and tail region of TMT exposed embryos (Fig. 5), the apoptotic difference between the control and TMT exposed fish in the head region was not statistically significant (P = 0.063; n=3). The difference in tail region was significant (P < 0.05; n=3).
Figure 5.
TMT promoted localized apoptosis. Representative images of apoptotic cells (bright white spots) observed in whole mount embryos at 24 hpf after exposure to 5 μM TMT (n = 24). The top two panels are the head and trunk wrapping around the large yolk sac (center, right). The bottom two panels are of the tail region. B: Brain; E: eye. The apoptotic difference between the control and TMT exposed fish in the head region was apparent, but not statistically significant (P = 0.063; n=3). The difference in tail region was significant (P < 0.05; n=3).
4. Discussion
In vitro, TMT appears to have a direct effect on neurons and is often used as a model neurotoxicant for studying neuronal degeneration (Philbert et al., 2000) and delayed neuronal cell death (Thompson et al., 1996). There has been less focus on the effects of TMT exposure on aquatic organisms. We have shown evidence that developmental TMT exposure attenuated secondary motorneuron dependent tail bending at 24 hpf. TMT exposure in a later window (48–72 hpf) still elicited a hypoactive photomotor behavior response in the light at 4 and 5 dpf. Early (body patterning and neurogenesis phase) developmental TMT exposure promoted localized apoptosis.
Understanding of the mechanisms of organotin developmental toxicity will require much further study, but our experiments have shed light on some of the developmental processes affected by TMT, and in a vertebrate model that is amenable to high throughput genetic screening. The developing zebrafish will allow researchers to capitalize on screens for modulators of organotin toxicity that are anchored to easily assayed endpoints of the central nervous system. Tail flexion and the touch response are very early and robust central nervous system phenotypes. TMT treated embryos showed increased frequency of spontaneous tail flexion relative to control animals, peaking an hour earlier in development than in untreated embryos, followed by a rapid and dramatic decline in tail bending frequency relative to the control embryos. The response pattern is suggestive of a relatively potent stimulation of an unknown neuroreceptor by TMT, immediately followed by a saturated or desensitized period. This may occur in caudal primary motorneurons in each trunk muscle hemisegment, or some other class of spinal neuron. TMT exposed embryos also presented significantly decreased tail touch responses at both 36 and 48 hpf. For the early embryo, Pietri et al. (Pietri et al., 2009) emphasized the critical roles of the primary mechanosensory Rohon-Beard cell (RB) and the primary ascending commissural interneuron (CoPA) in the touch response circuit. By combining the touch assay with observation of swimming ability, one can identify mutations that affect the sensory but not motor side of the underlying circuit (Granato and Nusslein-Volhard, 1996). The touch response is governed by spinal sensory inputs that activate the primary motorneurons (Saint-Amant and Drapeau, 1998). As the only sensory neurons in the tail, RBs mediate tactile sensitivity between ~24–60 hpf. (Kuwada et al., 1990; Metcalfe et al., 1990). Specific examination of TMT effects on RB responses is still needed.
Our study sought to examine the effects of TMT on simple photomotor behavior in the developing zebrafish. Untreated zebrafish larvae typically are more active in darkened conditions than in the light, soon after a rapid light to dark transition (Prober et al., 2006). However, we observed that 4 and 5 dpf zebrafish larvae exposed to TMT were hypoactive in the visibly lighted periods but displayed the same heightened photomotor response as the control larvae after the transition to darkness. TMT-elicited hypoactivity may result from a heightened sense of vulnerability and instinctual predation avoidance. In rodents and humans, photophobia is an observed effect of TMT exposure (Candura, 1998; Halladay et al., 2006). The observed photomotor response in larvae, and future examination of TMT-affected adult zebrafish behavior, will be important clues toward identifying the neurologic targets of TMT.
Cell apoptosis plays a critical role in developmental modeling, immune repertoires, and homeostasis maintenance. And it is a tightly controlled physiological process regulated by a well-tuned balance of inducer and repressor factors. The balance of apoptosis and proliferation is a critical part of normal development and susceptible to perturbation by toxicant exposure (Ahmadi et al., 2003; Cole and Ross, 2001). Exposure to TMT resulted in visually apparent increases in apoptotic cell death both in the head and tail region at 24 hpf. Enhanced apoptosis in the tail may partly explain the attenuated tail touch response in the present study. Several studies support the hypothesis that TMT induces cell death, particularly in the limbic system. While the mechanisms by which TMT induces neurodegeneration are still not understood, many hypotheses suggest that unwanted neuronal apoptosis could be largely due to calcium overload (Mattson and Chan, 2003; Mundy and Freudenrich, 2006; Wang et al., 2008).
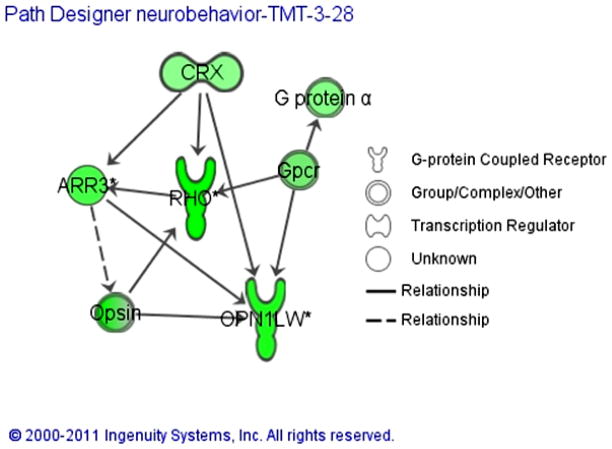
Vision-related transcript down-regulation by TMT served as a preliminary identifier of potential targets of TMT toxicity (Table 1). Pathway analysis (Fig. 6) suggested that potential TMT targets span locomotor behavior, cell proliferation, neurogenesis, learning and memory. Future studies involving systematic repression of these targets with antisense technology, as well as chemical genetic screening for compounds that modulate the effects of TMT, will define the mechanism of developmental toxicity and illuminate potential therapeutic approaches to the adverse effects of organotins.
Figure 6.
Pathway model based on vision related TMT-associated gene expression changes indicating a possible mechanism for TMT elicited neurobehavioral changes. Overarching regulation by the cone-rod homeobox protein (CRX) in this scenario might account for the observed abnormal photomotor response of TMT exposed zebrafish larvae.
Highlights.
Trimethyltin chloride (TMT) is a putative neurotoxicant
The mechanism of toxicity is largely unstudied
We evaluated the neurobehavioral TMT toxicity in the zebrafish for the first time
TMT exposure elicits a concentration -related increase (0 – 100%) in malformation incidence with an EC25of 5.55 μM.
TMT exposure significantly modulated the frequency of tail flexion, the earliest motor behavior observed in developing zebrafish.
TMT leads to a reduced touch response.
Exposure to 5 μM TMT from 48–72 hpf significantly modulates the photomotor response
TMT exposure promoted region specific apoptosis
This manuscripts establishes a zebrafish model for mechanistic studies
Acknowledgments
This work was supported in part by funding from the project of National Natural Science Foundation of China (20977068), the National Environmental Protection Public Welfare Science and Technology Research Program of China (200909089), the International Collaboration Project from Wenzhou City Government (H20070037) and the National Institutes of Health, Environmental Health Sciences grant #P3000210.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. Journal of Neurochemistry. 2003;87:914–921. doi: 10.1046/j.1471-4159.2003.02068.x. [DOI] [PubMed] [Google Scholar]
- Candura SM, Manzo L, Costa LG, editors. Exposure to Chemicals and Neuropsychiatric Effects. CRC Press; Boca Raton, FL: 1998. [Google Scholar]
- Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Developmental Biology. 2001;240:123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- El-Fawal HAN, O’Callaghan JP. Autoantibodies to neurotypic and gliotypic proteins as biomarkers of neurotoxicity: Assessment of trimethyltin (TMT) NeuroToxicology. 2008;29:109–115. doi: 10.1016/j.neuro.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Fonger GC, Stroup D, Thomas PL, Wexler P. TOXNET: A computerized collection of toxicological and environmental health information. Toxicol Ind Health. 2000;16:4–6. doi: 10.1177/074823370001600101. [DOI] [PubMed] [Google Scholar]
- Fortemps E, Amand G, Bomboir A, Lauwerys R, Laterre EC. Trimethyltin poisoning report of two cases. International Archives of Occupational and Environmental Health. 1978;41:1–6. doi: 10.1007/BF00377794. [DOI] [PubMed] [Google Scholar]
- Gomez FD, Apodaca P, Holloway LN, Pannell KH, Whalen MM. Effect of a series of triorganotins on the immune function of human natural killer cells. Environmental Toxicology and Pharmacology. 2007;23:18–24. doi: 10.1016/j.etap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Granato M, Nusslein-Volhard C. Fishing for genes controlling development. Curr Opin Genet Dev. 1996;6:461–468. doi: 10.1016/s0959-437x(96)80068-2. [DOI] [PubMed] [Google Scholar]
- Haendel MA, Tilton F, Bailey GS, Tanguay RL. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish. Toxicological Sciences. 2004;81:390–400. doi: 10.1093/toxsci/kfh202. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Wilson DT, Wagner GC, Reuhl KR. Trimethyltin-induced alterations in behavior are linked to changes in PSA-NCAM expression. Neurotoxicology. 2006;27:137–146. doi: 10.1016/j.neuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Hlinak Z, Krejci I, Hynie S, Klenerova V. Dipeptide “alaptide” prevented impairments in spontaneous behavior produced with trimethyltin in male rats. Neuro Endocrinol Lett. 2008;29:917–923. [PubMed] [Google Scholar]
- Hoch M. Organotin compounds in the environment -- an overview. Applied Geochemistry. 2001;16:719–743. [Google Scholar]
- Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, Tanguay RL, Dong Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS) Aquatic Toxicology. 2010;98:139–147. doi: 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Akaike M, Tsutsumi S, Kanai H, Masui A, Sadamatsu M, Kuroda Y, Watanabe Y, Mewen BS, Kato N. Trimethyltin syndrome as a hippocampal degeneration model: temporal changes and neurochemical features of seizure susceptibility and learning impairment. Neuroscience. 1997;81:1183–1191. doi: 10.1016/s0306-4522(97)00220-0. [DOI] [PubMed] [Google Scholar]
- Jiang GB, Liu JY, Zhou QF. Search for the contamination source of butyltin compounds in wine: agglomerated cork stoppers. Environ Sci Technol. 2004;38:4349–4352. doi: 10.1021/es049787+. [DOI] [PubMed] [Google Scholar]
- Jiang GB, Zhou QF, Liu JY, Wu DJ. Occurrence of butyltin compounds in the waters of selected lakes, rivers and coastal environments from China. Environ Pollut. 2001;115:81–87. doi: 10.1016/s0269-7491(01)00088-4. [DOI] [PubMed] [Google Scholar]
- Kim JK, Bae H, Kim MJ, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, Kim EK, Kim HK, Kim BY, Shin DH. Inhibitory effect of Poncirus trifoliate on acetylcholinesterase and attenuating activity against trimethyltin-induced learning and memory impairment. Biosci Biotechnol Biochem. 2009;73:1105–1112. doi: 10.1271/bbb.80859. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. American Journal of Anatomy. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kuwada JY, Bernhardt RR, Nguyen N. Development of spinal neurons and tracts in the zebrafish embryo. The Journal of Comparative Neurology. 1990;302:617–628. doi: 10.1002/cne.903020316. [DOI] [PubMed] [Google Scholar]
- Liu JY, Jiang GB. Survey on the presence of butyltin compounds in chinese alcoholic beverages, determined by using headspace solid-phase microextraction coupled with gas chromatography-flame photometric detection. J Agric Food Chem. 2002;50:6683–6687. doi: 10.1021/jf025712i. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development. 1990;110:491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- Miller DB, Eckerman DA, Krigman MR, Grant LD. Chronic neonatal organotin exposure alters radial-arm maze performance in adult rats. Neurobehav Toxicol Teratol. 1982;4:185–190. [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Biochemical, functional and morphological indicators of neurotoxicity: effects of acute administration of trimethyltin to the developing rat. J Pharmacol Exp Ther. 1984;231:744–751. [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM. Apoptosis of cerebellar granule cells induced by organotin compounds found in drinking water: Involvement of MAP kinases. NeuroToxicology. 2006;27:71–81. doi: 10.1016/j.neuro.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Niittykoski M, Lappalainen R, Jolkkonen J, Haapalinna A, Riekkinen P, Sr, Sirvio J. Systemic administration of atipamezole, a selective antagonist of alpha-2 adrenoceptors, facilitates behavioural activity but does not influence short-term or long-term memory in trimethyltin-intoxicated and control rats. Neurosci Biobehav Rev. 1998;22:735–750. doi: 10.1016/s0149-7634(98)00002-5. [DOI] [PubMed] [Google Scholar]
- Philbert MA, Billingsley ML, Reuhl KR. Mechanisms of Injury in the Central Nervous System. Toxicologic Pathology. 2000;28:43–53. doi: 10.1177/019262330002800107. [DOI] [PubMed] [Google Scholar]
- Pietri T, Manalo E, Ryan J, Saint-Amant L, Washbourne P. Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Dev Neurobiol. 2009;69:780–795. doi: 10.1002/dneu.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/Orexin overexpression induces an insomnia-like phenotype in zebrafish. The Journal of Neuroscience. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Davidson C, Billingsley ML, Yun J. Protein Kkinase C epsilon regulates tumor necrosis factor-alpha-induced stannin gene expression. Journal of Pharmacology and Experimental Therapeutics. 2005;314:61–69. doi: 10.1124/jpet.105.084236. [DOI] [PubMed] [Google Scholar]
- Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert PH, Dean KF, Reiter LW. Developmental and behavioral toxicity following acute postnatal exposure of rat pups to trimethyltin. Neurobehav Toxicol Teratol. 1983;5:421–429. [PubMed] [Google Scholar]
- Ruppert PH, Dean KF, Reiter LW. Development of locomotor activity of rat pups exposed to heavy metals. Toxicol Appl Pharmacol. 1985;78:69–77. doi: 10.1016/0041-008x(85)90306-0. [DOI] [PubMed] [Google Scholar]
- Saary MJ, House RA. Preventable exposure to trimethyl tin chloride: a case report. Occupational Medicine. 2002;52:227–230. doi: 10.1093/occmed/52.4.227. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. Journal of Neurobiology. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Shawky S, Emons H. Distribution pattern of organotin compounds at different trophic levels of aquatic ecosystems. Chemosphere. 1998;36:523–535. doi: 10.1016/s0045-6535(97)10011-x. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Nah SY, Chae JS, Bing G, Shin SW, Yen TPH, Baek IH, Kim WK, Maurice T, Nabeshima T, Kim HC. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via [sigma]1 receptor activation in rats. Neurochemistry International. 2007;50:791–799. doi: 10.1016/j.neuint.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Neonatal exposure to triethyltin disrupts olfactory discrimination learning in preweanling rats. Neurotoxicol Teratol. 1991;13:515–524. doi: 10.1016/0892-0362(91)90059-6. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Jensen KF, Pickens CV. Neonatal exposure to trimethyltin disrupts spatial delayed alternation learning in preweanling rats. Neurotoxicol Teratol. 1991;13:525–530. doi: 10.1016/0892-0362(91)90060-a. [DOI] [PubMed] [Google Scholar]
- Tang X, Yang X, Lai G, Guo J, Xia L, Wu B, Xie Y, Huang M, Chen J, Ruan X, Sui G, Ge Y, Zuo W, Zhao N, Zhu G, Zhang J, Li L, Zhou W. Mechanism underlying hypokalemia induced by trimethyltin chloride: Inhibition of H+/K+-ATPase in renal intercalated cells. Toxicology. 2010;271:45–50. doi: 10.1016/j.tox.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Thompson TA, Lewis JM, Dejneka NS, Severs WB, Polavarapu R, Billingsley ML. Induction of apoptosis by organotin compounds in vitro: neuronal protection with antisense oligonucleotides directed against stannin. Journal of Pharmacology and Experimental Therapeutics. 1996;276:1201–1216. [PubMed] [Google Scholar]
- Toggas SM, Krady JK, Billingsley ML. Molecular neurotoxicology of trimethyltin: identification of stannin, a novel protein expressed in trimethyltin-sensitive cells. Molecular Pharmacology. 1992;42:44–56. [PubMed] [Google Scholar]
- Trabucco A, Di Pietro P, Nori SL, Fulceri F, Fumagalli L, Paparelli A, Fornai F. Methylated tin toxicity a reappraisal using rodents models. Arch Ital Biol. 2009;147:141–153. [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen J, Lin K, Chen Y, Hu W, Tanguay RL, Huang C, Dong Q. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ Toxicol Chem. 2011;30:2073–2080. doi: 10.1002/etc.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cai J, Zhang J, Wang C, Yu A, Chen Y, Zuo Z. Acute trimethyltin exposure induces oxidative stress response and neuronal apoptosis in Sebastiscus marmoratus. Aquatic Toxicology. 2008;90:58–64. doi: 10.1016/j.aquatox.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Zhou QF, Jiang GB, Liu JY. Small-scale survey on the contamination status of butyltin compounds in seafoods collected from seven Chinese cities. J Agric Food Chem. 2001;49:4287–4291. doi: 10.1021/jf010438y. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Cai J, Wang X, Li B, Wang C, Chen Y. Acute administration of tributyltin and trimethyltin modulate glutamate and N-methyl-d-aspartate receptor signaling pathway in Sebastiscus marmoratus. Aquatic Toxicology. 2009;92:44–49. doi: 10.1016/j.aquatox.2009.01.008. [DOI] [PubMed] [Google Scholar]