Abstract
The purpose of this study was to evaluate the potential of cellulose nanofibers (also referred as microfibrillated cellulose, nanocellulose, nanofibrillated, or nanofibrillar cellulose) as novel tabletting material. For this purpose, physical and mechanical properties of spray-dried cellulose nanofibers (CNF) were examined, and results were compared to those of two commercial grades of microcrystalline cellulose (MCC), Avicel PH101 and Avicel PH102, which are the most commonly and widely used direct compression excipients. Chemically, MCC and CNF are almost identical, but their physical characteristics, like mechanical properties and surface-to-volume ratio, differ remarkably. The novel material was characterized with respect to bulk and tapped as well as true density, moisture content, and flow properties. Tablets made of CNF powder and its mixtures with MCC with or without paracetamol as model compound were produced by direct compression and after wet granulation. The tensile strength of the tablets made in a series of applied pressures was determined, and yield pressure values were calculated from the measurements. With CNF, both wet granulation and direct compression were successful. During tablet compression, CNF particles were less prone to permanent deformation and had less pronounced ductile characteristics. Disintegration and dissolution studies showed slightly faster drug release from direct compression tablets with CNF, while wet granulated systems did not have any significant difference.
Key words: cellulose nanofibers, characterization, excipient, microcrystalline cellulose, tabletting
INTRODUCTION
Cellulose is biodegradable, renewable, and the most abundant natural polymer in the world. It has highly ordered hierarchical structure starting from the parallel glucan chains forming nanosized fibrils, which are further organized in fibril aggregates (1). The fibrils and fibril aggregates are quite often denoted cellulose nanofibers (CNF) or microfibrils (2–6) and have a diameter of 4–20 nm depending on species and history (2,6). Recently, separation of cellulose nanofibers from macroscopic cellulose fibers has become the subject of much attention due to their characteristics such as high surface-to-volume ratio and outstanding mechanical properties.
Since the cellulose microfibrils consist of both amorphous and crystalline regions, traditional method of production of microcrystalline cellulose (MCC) that includes strongly acidic conditions leads to extensive hydrolysis of amorphous fractions and formation of short rod-like MCC fibril bundles with low aspect ratio. On the other hand, milder enzymatic hydrolysis, absence of hydrolysis step, and application of strong mechanical force in cellulose treatment result in production of the long nanoscale partly amorphous fibrils referred as CNF. In the literature, terms like microfibrillated cellulose, nanocellulose, nanofibrillated, or nanofibrillar cellulose have also been used instead of CNF (7). The production of CNF and their application in different areas have gained increasing attention recently due to their high strength and stiffness, combined with low weight (high porosity), biodegradability, and renewability (8). Method of production of CNF that was reported for the first time by Turbak et al. (2) required high-energy input. However, new methods to disintegrate cellulose fibers to the level of nanofibers have been proposed. Those methods utilize mechanical force using super grinder treatment or high-pressure refiner (4,9), microfluidizer (10), and high-pressure homogenizer treatment (3,11,12). Preparation of CNF combining enzymatic hydrolysis with mechanical shearing and high-pressure homogenization has been also reported (7). All these methods lead to a production of gel-like water suspension with high water content, which can be further processed into powder by spray drying. CNF are at the moment subject of continuing research. Their excellent mechanical properties have been reported, and thus, application of CNF as a material for nanocomposite reinforcement has been studied (10,13–15). However, application of CNF in the pharmaceutical field has not been reported so far.
Thus, the aim of this study was to evaluate the usability of spray-dried CNF as novel tabletting material. For that purpose, the CNF material was compared to two commercially available grades of MCC: Avicel PH 101 and Avicel PH 102. MCC was chosen for comparison due to its similarity to CNF in chemical structure. Besides, Avicel PH 101 and Avicel PH 102 are the two most commonly grades of MCC used in tabletting. Determination of powders main physical characteristics was done as well as examination of their compression behavior. Both direct compression and wet granulation methods were tested.
MATERIALS AND METHODS
Materials
The spray-dried CNF particles were obtained from UPM-Kymmene Corporation (Finland). Avicel PH 101 and Avicel PH 102 were purchased from FMC Bio Polymer (Ireland). Paracetamol (Acetaminophen) was used as model drug compound and was purchased from Hawkins, Inc. (USA). For direct compression, Aerosil 200 (Orion Pharma, Finland) was used to improve the flowability of powder mixtures. For wet granulation process, Ac-Di-Sol (sodium salt of carboxymethylcellulose, FMC Bio Polymer, Ireland) was used as a disintegrant, and aqueous solution of Kollidon 30 (polyvinylpyrrolidone, BASF Corporation, Germany) was used as a binder.
Methods
Preparation of Powder Mixtures for Direct Compression
For direct compression studies, powder mixtures containing Avicel PH 102 or spray-dried CNF as well as mixtures of Avicel PH 102 and increasing amounts of spray-dried CNF were prepared (Table I). Further powder mixtures containing Avicel PH 102 or spray-dried CNF and paracetamol as model API were also prepared (Table I). Powders were blended using a blender Turbula, System Schatz (Willy A. Bachofen AG, Switzerland) for 2 min.
Table I.
Composition (Weight/Weight Percent) of Powder Mixtures for Direct Compression
| Avicel PH 102 | Spray-dried CNF | Paracetamol | |
|---|---|---|---|
| A2 | 100 | ||
| C | 100 | ||
| A2C1 | 90 | 10 | |
| A2C2 | 80 | 20 | |
| A2C3 | 70 | 30 | |
| A2P | 50 | 50 | |
| CP | 50 | 50 |
Production of Granules
Eight batches of granules containing different ratios of Avicel PH 101, Avicel PH 102, spray-dried CNF, and paracetamol (Table II) were prepared manually (using mortar and pestle) using 4% aqueous solution of Kollidon K30 as a binder and Ac-Di-Sol as disintegrant. Granulation process was performed by adding the binder solution drop by drop until a granulation mass with desirable properties was obtained. The wet granulation mass was forced through a 1.6-mm sieve and dried on trays at 21°C and 50% relative humidity. The dry granules were passed again through the 1.6-mm sieve. The obtained granules were sieved trough a 0.5-mm sieve, and granules bigger than 0.5-mm were collected for further studies.
Table II.
Composition (Weight/Weight Percent) of Avicel PH 101, Avicel PH 102, and Spray-Dried CNF Granules
| Paracetamol | Avicel PH 101 | Avicel PH 102 | Spray-dried CNF | Kollidon K30 | Ac-Di-Sol | |
|---|---|---|---|---|---|---|
| GA1P6 | 60 | 36 | 2 | 2 | ||
| GA2P6 | 60 | 36 | 2 | 2 | ||
| GCP6 | 60 | 36 | 2 | 2 | ||
| GA1CP6 | 60 | 18 | 18 | 2 | 2 | |
| GA1P4 | 40 | 56 | 2 | 2 | ||
| GA2P4 | 40 | 56 | 2 | 2 | ||
| GCP4 | 40 | 56 | 2 | 2 | ||
| GA1CP4 | 40 | 28 | 28 | 2 | 2 |
Scanning Electron Microscopy
Micrographs of the studied powders were obtained using scanning electron microscope Zeiss DSM 962 (Carl Zeiss, Oberkochen, Germany). The samples were fixed onto two-sided carbon tape and sputtered with platinum for 25 s with an Agar sputter device (Agar Scientific Ltd., Stansted, UK). The micrographs were used for morphological characterization and particle size determination.
Moisture Content
The moisture content of powders and granules was examined using moisture balance (Sartorius MA 100, UK) at 105°C and calculated from the weight loss after heating until constant weight using approximately 1 g of the material.
Densities and Porosity
The bulk and tapped density, Pbulk and Ptap, respectively, were determined according to the Ph.Eur. method (16), using tapped density tester (Erweka, SVM, Apparatebau, Germany), with the exception that smaller quantities of the substances were used. True density Ptrue was determined by helium pycnometry (Multivolume Pycnometer 1305, Micromeritica, USA). Results were calculated as an average value of three measurements. Porosity of the powder mixtures, ε, was determined using the relationship:
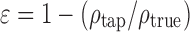 |
1 |
Flow Properties
Flow properties of the materials were assessed by flow-through-an-orifice, Carr’s index (CI), and Hausner ratio (HR) methods. The flow of powders through an orifice was measured using Flow Properties Tester (KERN, KB 200-3N, Germany) with a constant cylinder volume of 4.69 ml and diameter of cylinder hole 3 mm. Results were calculated as an average value of three measurements. The CI (17) and the HR (18) were determined from poured and tapped densities according to the relationships 2 and 3:
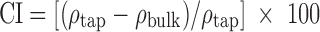 |
2 |
and
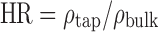 |
3 |
Tabletting Studies
Compression behavior of powders was studied by producing tablets by direct compression method (Table I), as well as by compression of granules prepared by wet granulation process (Table II). The powder mixtures for direct compression and granules were stored for 24 h at relative humidity of 50% before use. The same humidity conditions were employed during the compression study. Compacts were compressed with an instrumented eccentric tablet machine (Korsch, EK-0, Erweka Apparatebau, Germany) using flat-faced punches with 9 mm diameter. The adjustments of the tablet machine were kept constant in all compressions in order to ensure the same speed of the upper punch and the same thickness of the tablets in all cases. The compression force was controlled by changing the amount of material in the die.
Tablet Evaluation
The thickness of the tablets was measured 24 h after compression. The porosity of the tablet was calculated according to the Eq. 4:
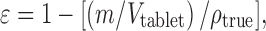 |
4 |
where m and Vtablet are weight and volume of the tablet, respectively. Vtablet was calculated using the values of tablet diameter and thickness and assuming that tablets have cylindrical shape. The Heckel plots were constructed by plotting the natural logarithm of the inverse of the compact porosities as a function of the compression pressures. Regression analysis was performed on the linear portion of the curve. The slope values obtained were converted to yield pressures (Py) using the relationship:
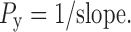 |
5 |
Tablet crushing strengths were determined according to the European Pharmacopoeia as a force needed to break the tablet diametrically using apparatus Schleuniger-2E 803 (Mood 2E/205, Switzerland). Results were calculated as an average value of three measurements. Crushing strength was then used to calculate the tensile strength, σx, according to the Newton equation (19)
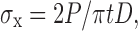 |
6 |
where P, t, and D are the crushing strength, tablet thickness, and tablet diameter, respectively.
Disintegration Studies
Disintegration tests were performed according to the European Pharmacopoeia using purified water at 37°C (Sotax DT3, Sotax, Basel, Switzerland). The disintegration times reported are averages of six determinations.
Dissolution Studies
Dissolution tests were performed using a paddle-type dissolution apparatus (Erweka DT-D6, Germany) with rotation speed of 50 rpm and 900 ml of purified water at 37 ± 0.5°C as a medium. After each sample (500 μl), the volume was replaced by the same amount of medium. The samples were filtered through 0.45-μm filters and analyzed using UV spectrophotometer (Pharmacia LKB Ultrospec III, Sweden) at 243 nm wavelength (20). Results were calculated as average value of three measurements.
RESULTS AND DISCUSSION
Powder Characterization
SEM
Commercially available MCC is known to have porous, irregularly shaped particles and broad particle size distribution, 50 μm mean diameter for Avicel PH 101 and 100 μm for Avicel PH 102. The scanning electron microscopy (SEM) micrographs of Avicel PH 101 and CNF powder are shown in Fig. 1. CNF particles were more regular and had spherical shape and smaller particle size, roughly 5–10 μm. Differences in surface morphologies were also evident. The higher magnifications revealed the surface structure of the CNF particles to be porous, formed by a web of fibrils. The fact that such size and textural differences are apparent leads us to expect differences in particle rearrangement and, consequently, flow and compression behavior.
Fig. 1.
a–c SEM images of Avicel PH 101 with ×1,000 magnification (a), spray-dried CNF with magnification ×2,000 (b), and ×10,000 (c)
Densities and Porosity
The bulk, tapped, and true densities as well as porosity of powders and granules are listed below (Table III). The bulk and tapped densities of CNF were higher compared to the Avicel. This is attributed to the smaller particle size of CNF powder and its spherical shape (Fig. 1b) that allows better packing. Generally, the tap density is a measure of particles ability to pack in certain space and could be used to predict the propensity of the powder to rearrange in the die during the compaction. Even though the CNF powder was expected to be highly cohesive as a consequence of its small particle size, higher values of tapped density show that the new excipient material possesses better consolidation properties than the two types of MCC.
Table III.
Powder Characteristics of Avicel PH 101, Avicel PH 102, and Spray-Dried CNF
| Moisture content (%) | Densities (g/ml) | Porosity | Carr index | Hausner ratio | Flow (mg/s) | |||
|---|---|---|---|---|---|---|---|---|
| Bulk | Tapped | True | ||||||
| Avicel PH 101 | 3.51 | 0.353 | 0.416 | 1.574 | 0.736 | 16.25 | 1.19 | 12.55 |
| Avicel PH 102 | 3.59 | 0.329 | 0.393 | 1.574 | 0.750 | 15.20 | 1.18 | 38.07 |
| Spray-dried CNF | 3.48 | 0.488 | 0.602 | 1.556 | 0.613 | 18.87 | 1.23 | 16.17 |
The obtained value of true density for CNF is a bit lower than for Avicel PH 101 and Avicel PH 102. Considering that the values of bulk and tapped densities of CNF are higher and the true density is lower than in the case of Avicel PH 101 and Avicel PH 102, it can be concluded that the intraparticulate porosity is higher in the case of CNF powder. This is most likely a consequence of the production method. CNF particles are produced by spray drying aqueous slurry of cellulose fibers whose width is in the nanometer scale. During the drying process, water evaporates leaving the pores between dry cellulose fibers (Fig. 1c). Values of powder porosity (Table III) confirm that the CNF particles are in close contact and have better packing due to better rearrangement of small spherical particles (Fig. 1b).
Flow Properties
The Hausner ratio and the Carr index have been widely used to estimate the flow properties of powders. A Hausner ratio of less than 1.20 indicates good flowability of the material, whereas a value of 1.5 or higher suggests poor flow characteristics of the material (18,21). The Carr index values of 5–10, 12–16, 18–21, and 23–28 indicate excellent, good, fair, and poor flow properties of the material, respectively (17).
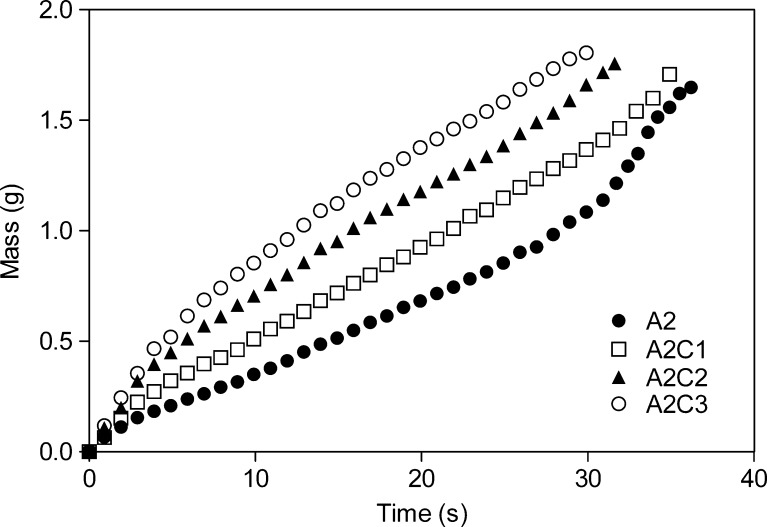
The values of CI and HR are given in Table III. Higher values of both parameters for the spray-dried CNF powder than for the MCCs indicated poorer flow properties of the CNF than the MCCs. At relatively high humidity, van der Waals forces between small particles are higher than between larger particles causing poorer flowability of powders consisted of small particles (22,23). However, it seems that the uniform spherical particle shape of the CNF particles (Fig. 1b) helps in overcoming the previously mentioned problems and provides actually better experimental flowability of the new excipient than that of Avicel PH 101 (Table III). Furthermore, an addition of spray-dried CNF powder to Avicel PH 102 improved its flow properties, increasing the slope of the mass-time curves with increasing amounts of the CNF powder in the mixtures (Fig. 2). This is due to the rearrangement of small CNF particles between larger Avicel PH 102, thus CNF acting as a glidant. CNF particles are also likely to be located on the surface of the Avicel PH 102 particles filling the pits of the high roughness areas and decreasing in this way the particle surface roughness.
Fig. 2.
Flow properties of Avicel PH102 (closed circle) and mixtures containing Avicel PH102 and 10% (open square), 20% (closed triangle), and 30% (open circle) of CNF
Granules Characterization
Table IV shows the main characteristics of produced granules. From these values, it could be concluded that the batches, which contained 40% of paracetamol, were more porous than the batches containing 60% of the same API that could be consequence of variations in production methods since granules were produced manually. All batches had values of CI between 5 and 10 and HR less than 1.2 indicating excellent flow properties.
Table IV.
Characteristics of Avicel PH 101, Avicel PH 102, and Spray-Dried CNF Granules
| Moisture content (%) | Densities (g/ml) | Porosity | Carr index | Hausner ratio | |||
|---|---|---|---|---|---|---|---|
| Bulk | Tapped | True | |||||
| GA1P6 | 2.42 | 0.357 | 0.385 | 1.367 | 0.718 | 7.14 | 1.08 |
| GA2P6 | 2.35 | 0.364 | 0.400 | 1.371 | 0.708 | 9.09 | 1.10 |
| GCP6 | 3.78 | 0.323 | 0.357 | 1.371 | 0.740 | 9.68 | 1.11 |
| GA1CP6 | 3.01 | 0.333 | 0.357 | 1.356 | 0.737 | 6.67 | 1.07 |
| GA1P4 | 3.28 | 0.313 | 0.345 | 1.415 | 0.756 | 9.38 | 1.10 |
| GA2P4 | 2.16 | 0.303 | 0.345 | 1.418 | 0.757 | 9.09 | 1.10 |
| GCP4 | 3.43 | 0.313 | 0.357 | 1.409 | 0.747 | 9.38 | 1.10 |
| GA1CP4 | 2.87 | 0.313 | 0.357 | 1.399 | 0.745 | 9.68 | 1.11 |
Direct Compression Studies
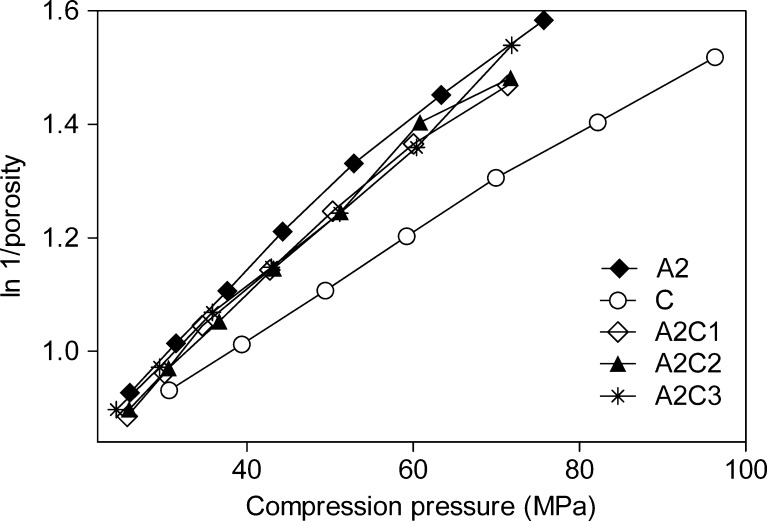
Heckel plots (Fig. 3) were constructed for five batches of tablets containing different ratios (Table I) of Avicel PH 102 and spray-dried CNF. They were produced by applying compression pressures in the range of 25–100 MPa in order to evaluate the effect of applied pressure on the deformation process (Fig. 3). The dominant linear region in the Heckel curve is characteristic for materials that undergo plastic deformation during the compression. The slope indicates the extent of the plastic deformation where a steeper curve is obtained in the case of more ductile materials. MCC has been known by its plastic deformation (22,24–26). Since plastic deformation of materials during the compression results in close contact between the particles and bond formation, it has been proposed that crystals of MCC come close enough that hydrogen bonding can occur during the compression process (24). From the curves shown in the Fig. 3, it can be concluded that spray-dried CNF is less ductile than Avicel PH 102. Table V gives compression pressure ranges over which the regression analysis was performed, as well as yield pressure values (Py), which were calculated using the relationship 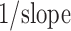 . During the compression process, particles undergo rearrangement followed by elastic deformation. When elastic limit is exceeded, brittle or plastic deformation takes place. It has been reported (27) that materials with Py values less than 80 MPa go through the plastic deformation during the compaction process and increase in Py values indicates that the material is more brittle. For example, for lactose that is known to undergo fragmentation during the compression process (25), these values are in range from 85 up to 200 MPa (28–30), depending on crystallinity and moisture content. From the Py values (Table V), it can be concluded that Avicel PH 102 as well as its mixtures with CNF undergo plastic deformation, while the new excipient alone showed more pronounced brittle characteristics during the compression. It has been shown that the compression behavior is not dependent on the particle size (25,26). Since MCC and CNF are chemically almost identical, the differences between the studied materials must be a consequence of material structure and properties.
. During the compression process, particles undergo rearrangement followed by elastic deformation. When elastic limit is exceeded, brittle or plastic deformation takes place. It has been reported (27) that materials with Py values less than 80 MPa go through the plastic deformation during the compaction process and increase in Py values indicates that the material is more brittle. For example, for lactose that is known to undergo fragmentation during the compression process (25), these values are in range from 85 up to 200 MPa (28–30), depending on crystallinity and moisture content. From the Py values (Table V), it can be concluded that Avicel PH 102 as well as its mixtures with CNF undergo plastic deformation, while the new excipient alone showed more pronounced brittle characteristics during the compression. It has been shown that the compression behavior is not dependent on the particle size (25,26). Since MCC and CNF are chemically almost identical, the differences between the studied materials must be a consequence of material structure and properties.
Fig. 3.
Heckel plots for Avicel PH102 (closed diamonds), spray-dried CNF (open circle), and mixtures containing Avicel PH102 and 10% (open diamond), 20% (closed triangle), and 30% (star) of spray-dried CNF
Table V.
Regression Analysis of Mechanical Properties of Avicel PH 102, Spray-Dried CNF, and Their Mixtures
| Compression pressure range (MPa) | R 2 | Slope | Yield pressure P y (MPa) | |
|---|---|---|---|---|
| A2 | 25–76 | 0.9935 | 0.0133 | 75 |
| C | 30–97 | 0.9984 | 0.0090 | 111 |
| A2C1 | 25–72 | 0.9905 | 0.0128 | 78 |
| A2C2 | 25–72 | 0.9920 | 0.0131 | 76 |
| A2C3 | 25–72 | 0.9973 | 0.0131 | 76 |
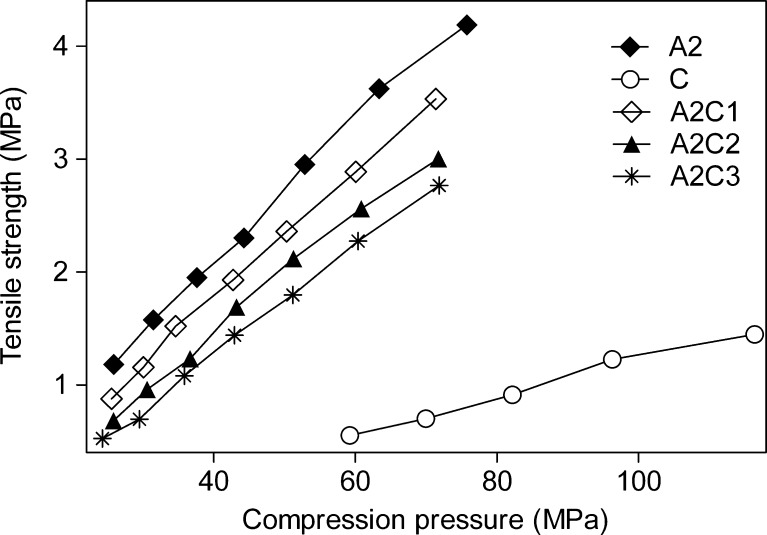
The relationship between tablet strength and compression pressure applied is shown in Fig. 4. A linear relation between tensile strength and compression force was observed for all tablet batches under the test conditions. The obtained results clearly show that the CNF formed the weakest tablets, while Avicel PH 102 formed the strongest tablets with the same applied pressure. Higher compression pressure is needed for the production of spray-dried CNF tablets than Avicel PH 102 tablets with the same tensile strength. Also an increase in the amount of spray-dried CNF in Avicel PH 102/spray-dried CNF mixtures causes a decrease in tablet strength, although the changes are quite small. It has been shown that high ductility, low brittleness, as well as low elasticity are parameters that favor the formation of strong compacts (31,32). CNF powder is less ductile than Avicel PH 102, deforms mainly by fragmentation, and has pronounced elastic component. All these properties cause the compacts made from new tabletting material not to be as hard as Avicel PH 102 compacts.
Fig. 4.
Effect of compression pressure on the tensile strength of tablets made of Avicel PH102 (closed diamond), spray-dried CNF (open circle), and mixtures containing Avicel PH102 and 10% (open diamond), 20% (closed triangle), and 30% (star) of spray-dried CNF
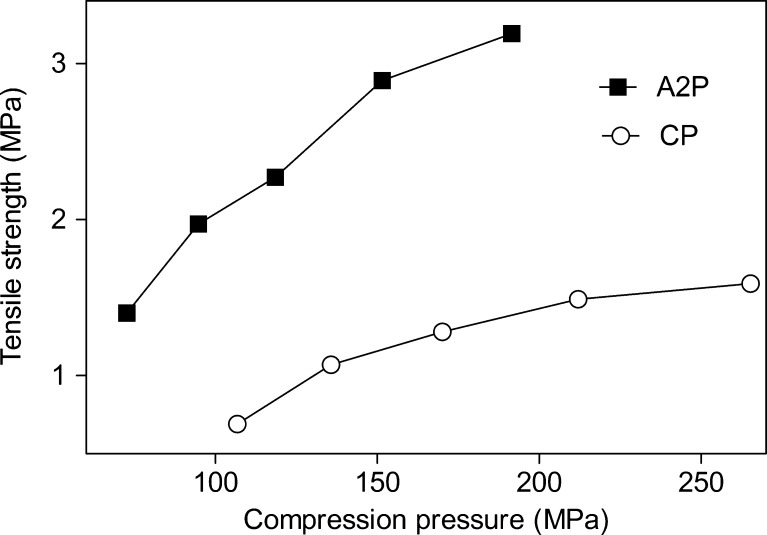
Dependence of the tablet strength on compression pressure was also studied for tablets containing paracetamol as a model drug compound (Fig. 5). The curves followed the same trend as in the case of compacts without paracetamol. Tablets containing Avicel PH 102 were much stronger than tablets containing spray-dried CNF. Here difference was even more pronounced compared to the tablets without paracetamol.
Fig. 5.
Effect of compression pressure on the tensile strength of Avicel PH102 (closed square) and spray-dried CNF (open circle) tablets containing paracetamol produced by direct compression
Compression of Granules
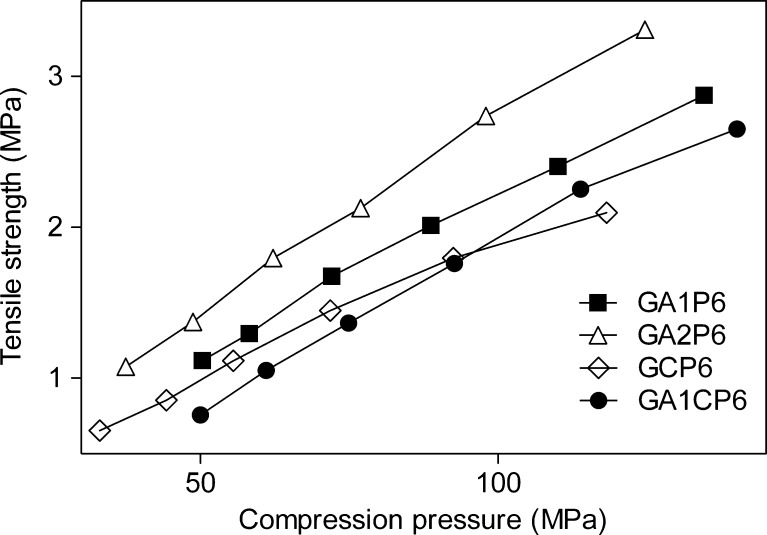
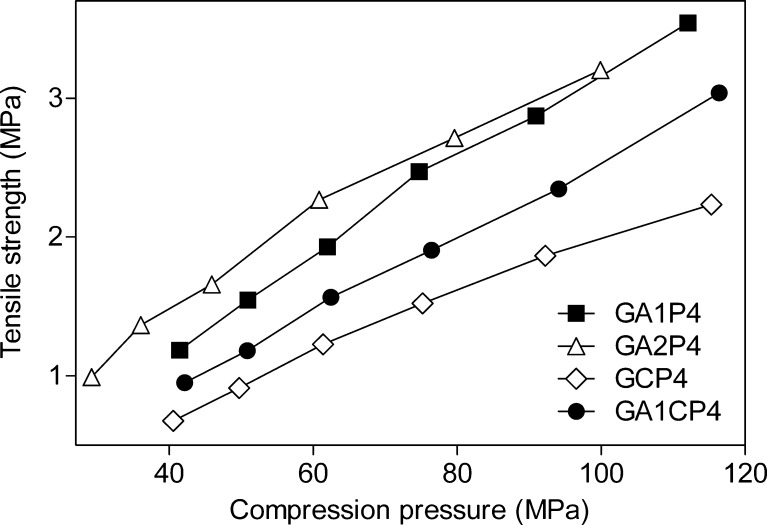
The results of tensile strength tests for tablets made from granules containing 60% of paracetamol are shown in Fig. 6. In all cases, the results showed a linear relationship between the tablet tensile strength and the compression force. Tablets made from granules containing CNF were less hard than tablets made from granules with Avicel PH 101 and Avicel PH 102, after the same compression force was applied. The difference in hardness increased with increasing compression force (Fig. 6). The curves follow a similar trend as in case of tablets produced by direct compression. Such behavior is consequence of properties of CNF powder and its characteristic to deformation by fragmentation. However, it can be noticed that the difference in the tensile strength between the CNF and MCC tablets is less pronounced when wet granulation process has been applied. This is due to the fact that MCC significantly loses plasticity after the wet granulation process (22). The same trend was observed during the tensile strength test for tablets made from granules containing 40% of paracetamol (Fig. 7). A further explanation for the different slopes of the tensile strength–compression pressure relationships for the CNF and MCC concerns the possibility that the materials differ in degree of elastic deformation during compression. Although the new tabletting material had generally lower compactibility than MCC in all cases studied, it was still able to form tablets of satisfactory tensile strengths for ordinary handling.
Fig. 6.
Effect of compression pressure on the tensile strength of tablets made from granules containing 60% of paracetamol and Avicel PH101 (closed square), Avicel PH102 (open triangle), spray-dried CNF (open diamond), and mixture of Avicel PH101 and spray-dried CNF (closed circle)
Fig. 7.
Effect of compression pressure on the tensile strength of tablets made from granules containing 40% of paracetamol and Avicel PH101 (closed square), Avicel PH102 (open triangle), spray-dried CNF (open diamond), and mixture of Avicel PH101 and spray-dried CNF (closed circle)
Dissolution Studies
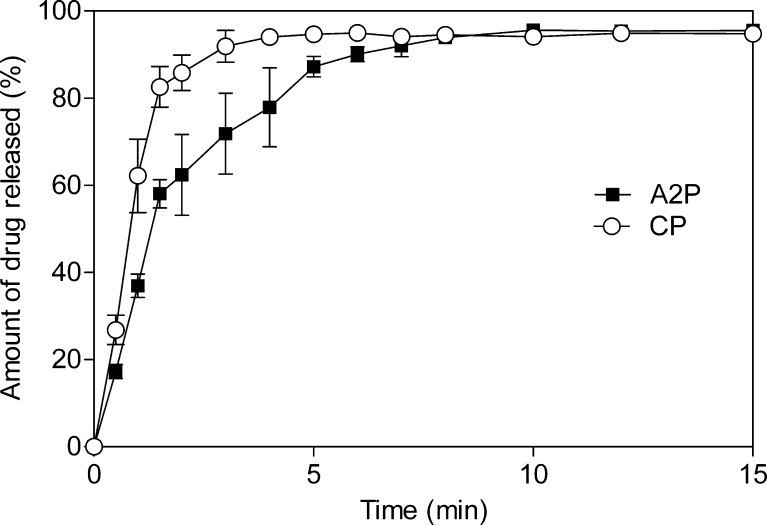
Tablets with tensile strength of 1.5 MPa were chosen for dissolution studies. Results of the dissolution studies for the tablets made by direct compression method are shown in Fig. 8. Both formulations released maximum amount of drug in 10 min and met the USP requirement for immediate release tablets (20), 80% drug release within 60 min. Avicel PH 102 tablets displayed slightly slower release rate, and the obtained dissolution curves show that the Avicel PH 102 tablets released 78% of loaded drug, whereas tablets containing CNF released 94% of the drug after 4 min. These results are a consequence of faster disintegration of the spray-dried CNF tablets than Avicel PH 102 tablets. During the disintegration test, it was observed that all the tablets were disintegrated within 30 s, and the CNF tablets showed slightly shorter disintegration times. Disintegration process of tablets containing MCC is described as breaking of the hydrogen bonds that were formed between MCC particles during plastic deformation (24). Since this step in compaction is less pronounced during the deformation of CNF powder, fewer hydrogen bonds are present causing faster tablet disintegration.
Fig. 8.
Dissolution profiles of Avicel PH102 (closed square) and spray-dried CNF (open circle) tablets containing paracetamol made by direct compression
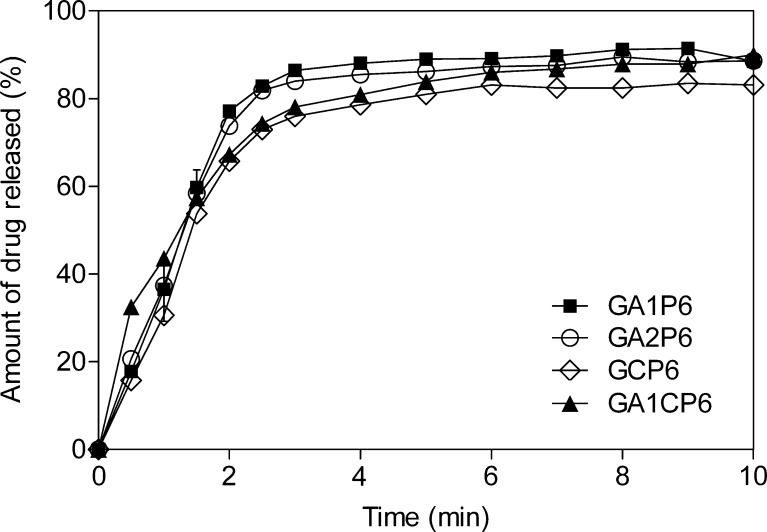
Results of the dissolution studies of tablets made by compression of granules containing 60% of paracetamol are shown in Fig. 9. Similar results were obtained from the dissolution tests of tablets made from granules containing 40% of paracetamol. All the batches had similar release profiles, which means that the granules containing spray-dried CNF have approximately the same disintegration time as granules made of the two types of MCC and, consequently, have the same release kinetics. Tablets composed of MCC might not always exhibit complete release of the active substance because of the large particle surface area and adsorptive characteristics (24). This phenomenon can be noticed in the tablets analyzed, especially in the tablets made from the granules. Since spray-dried CNF powder has higher intraparticulate porosity than the MCC grades, paracetamol release percentage was smaller from the tablets made from the CNF powder. However, all the analyzed batches gave fast drug release due to the fast tablet disintegration. These results illustrate that the material structure does not affect disintegration properties and consequently dissolution profiles.
Fig. 9.
Dissolution profiles of tablets made from granules containing 60% of paracetamol and Avicel PH101 (closed square), Avicel PH102 (open circle), spray-dried CNF (open diamond), and mixture of Avicel PH101 and spray-dried CNF (closed triangle)
CONCLUSIONS
The powder and mechanical properties of spray-dried CNF were examined and compared to two grades of commercial MCC. The results show that new excipient has a better ability to pack with lower powder porosity than commercial MCC. The studied material has good flow properties, and its addition to commercial MCC improves the flowability of the obtained mixtures. CNF is more brittle than MCC. As a consequence, compression behavior of new tabletting material requires higher compression forces for deformation. Furthermore, CNF tablets disintegrate slightly faster, which causes faster drug release form tablets made by direct compression. In conclusion, this study shows that spray-dried CNF can be used as an excipient for tablet production.
Acknowledgments
The authors would like to thank The Finnish Centre for Nanocellulosic Technologies, Finland as well as Panu Lahtinen and Hanna-Mari Sinilehto, VTT, Finland for the preparation of trial materials.
References
- 1.Hult EL, Larsson PT, Iversen T. Cellulose fibril aggregation—an inherent property of kraft pulps. Polymer. 2001;42:3309–3314. doi: 10.1016/S0032-3861(00)00774-6. [DOI] [Google Scholar]
- 2.Turbak AF, Snyder FW, Sandberg KR. Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci. 1983;37:815–827. [Google Scholar]
- 3.Herrick FW, Casebier RL, Hamilton JK, Sandberg KR. Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci. 1983;37:797–813. [Google Scholar]
- 4.Chakraborty A, Sain M, Kortschot M. Cellulose microfibrils: a novel method of preparation using high shear refining and cryocrushing. Holzforschung. 2005;59:102–107. doi: 10.1515/HF.2005.016. [DOI] [Google Scholar]
- 5.Quiévy N, Jacquet N, Sclavons M, Deroanne C, Paquot M, Devaux J. Influence of homogenization and drying on the thermal stability of microfibrillated cellulose. Polym Degrad Stabil. 2010;95:306–314. doi: 10.1016/j.polymdegradstab.2009.11.020. [DOI] [Google Scholar]
- 6.Svagan AJ, Azizi Samir MAS, Berglund LA. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules. 2007;8:2556–2563. doi: 10.1021/bm0703160. [DOI] [PubMed] [Google Scholar]
- 7.Pääköö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules. 2007;8:1934–1941. doi: 10.1021/bm061215p. [DOI] [PubMed] [Google Scholar]
- 8.Siro I, Plackett D. Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose. 2010;17:459–494. doi: 10.1007/s10570-010-9405-y. [DOI] [Google Scholar]
- 9.Yano H, Sugiyama J, Nakagaito A, Nogi M, Matsuura T, Hikita M, et al. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv Mater. 2005;17:153–155. doi: 10.1002/adma.200400597. [DOI] [Google Scholar]
- 10.Zimmermann T, Pohler E, Geiger T. Cellulose fibrils for polymer reinforcement. Adv Eng Mater. 2004;6:754–761. doi: 10.1002/adem.200400097. [DOI] [Google Scholar]
- 11.Dufresne A, Cavaille JY, Vignon MR. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J Appl Polym Sci. 1997;64:1185–1194. doi: 10.1002/(SICI)1097-4628(19970509)64:6<1185::AID-APP19>3.0.CO;2-V. [DOI] [Google Scholar]
- 12.Nakagaito AN, Yano H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl Phys A. 2005;80:155–159. doi: 10.1007/s00339-003-2225-2. [DOI] [Google Scholar]
- 13.Lu J, Wang T, Drzal LT. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos Part A. 2008;39:738–746. doi: 10.1016/j.compositesa.2008.02.003. [DOI] [Google Scholar]
- 14.Nakagaito AN, Fujimura A, Sakai T, Hama Y, Yano H. Production of microfibrillated cellulose (MFC)-reinforced polylactic acid (PLA) nanocomposites from sheets obtained by a papermaking-like process. Compos Sci Technol. 2009;69:1293–1297. doi: 10.1016/j.compscitech.2009.03.004. [DOI] [Google Scholar]
- 15.Gabr MH, Elrahman MA, Okubo K, Fujii T. Effect of microfibrillated cellulose on mechanical properties of plain-woven CFRP reinforced epoxy. Compos Struct. 2010;92:1999–2006. doi: 10.1016/j.compstruct.2009.12.009. [DOI] [Google Scholar]
- 16.European Pharmacopeia. 7th ed. Strasbourg: Council of Europe; 2010
- 17.Carr RL. Classifying flow of solids. Chem Eng. 1967;72:69–72. [Google Scholar]
- 18.Hausner HH. Friction conditions in a mass of metal powder. Int J Powder Metall. 1967;3:7–13. [Google Scholar]
- 19.Fell JT, Newton JM. Determination of tablet strength by the diametral compression test. J Pharm Sci. 1970;59:668–691. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- 20.USP 27/NF 22 (United States Pharmacopeia 27/National Formulary 22), Washington, DC; 2003
- 21.Wells JI. Pharmaceutical preformulation: the physicochemical properties of drug substances. New York: Wiley; 1988. [Google Scholar]
- 22.Doelker E. Comparative compaction properties of various microcrystalline cellulose types and generic products. Drug Dev Ind Pharm. 1993;19:2399–2471. doi: 10.3109/03639049309047196. [DOI] [Google Scholar]
- 23.Forsyth AJ, Hutton S, Rhodes MJ. Effect of cohesive interparticle force on the flow characteristics of granular material. Powder Technol. 2002;126:150–154. doi: 10.1016/S0032-5910(02)00046-3. [DOI] [Google Scholar]
- 24.Reier GE, Shangraw RF. Microcrystalline cellulose in tableting. J Pharm Sci. 1966;55:510–514. doi: 10.1002/jps.2600550513. [DOI] [Google Scholar]
- 25.McKenna A, McCafferty DF. Effect of particle size on the compaction mechanism and tensile strength of tablets. J Pharm Pharmacol. 1982;34:347–351. doi: 10.1111/j.2042-7158.1982.tb04727.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts RJ, Rowe RC. The effect of the relationship between punch velocity and particle size on the compaction behaviour of materials with varying deformation mechanisms. J Pharm Pharmacol. 1986;38:567–571. doi: 10.1111/j.2042-7158.1986.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 27.Podczeck F, Revesz P. Evaluation of the properties of microcrystalline and microfine cellulose powders. Int J Pharm. 1993;91:183–193. doi: 10.1016/0378-5173(93)90338-G. [DOI] [Google Scholar]
- 28.Haware RV, Tho I, Bauer-Brandl A. Multivariate analysis of relationships between material properties, process parameters and tablet tensile strength for α-lactose monohydrates. Eur J Pharm Biopharm. 2009;73:424–431. doi: 10.1016/j.ejpb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Sebhatu T, Ahlneck C, Alderborn G. The effect of moisture content on the compression and bond-formation properties of amorphous lactose particles. Int J Pharm. 1997;146:101–114. doi: 10.1016/S0378-5173(96)04777-1. [DOI] [Google Scholar]
- 30.Sebhatu T, Alderborn G. Relationships between the effective interparticulate contact area and the tensile strength of tablets of amorphous and crystalline lactose of varying particle size. Eur J Pharm Sci. 1999;8:235–242. doi: 10.1016/S0928-0987(99)00025-1. [DOI] [PubMed] [Google Scholar]
- 31.Hiestand EN. Tablet bond. I. A theoretical model. Int J Pharm. 1991;67:217–229. doi: 10.1016/0378-5173(91)90205-3. [DOI] [Google Scholar]
- 32.Hiestand EN, Smith DP. Tablet bond. II. Experimental check of model. Int J Pharm. 1991;67:231–246. doi: 10.1016/0378-5173(91)90206-4. [DOI] [Google Scholar]