Abstract
Silymarin, a mixture of flavonolignans extracted from the seeds of milk thistle, is used clinically as a hepatoprotector to treat liver injuries and chronic hepatitis. However, its therapeutic effect is compromised by its poor oral bioavailability due to the poor solubility and low permeability across intestinal epithelia. The main purpose of this study was to prepare silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices (GMO/P407 LCM) to improve the oral bioavailability of silymarin. GMO/P407 LCMs were prepared by a melting/congealing method. The isotropic phenomenon observed under polarized light microscope confirmed the liquid crystalline structure at the junction of LCM and water. Both differential scanning calorimetry and X-ray diffraction analysis confirmed disappearance of silymarin crystallinity after incorporation into the LCMs. In vitro release of silymarin from LCMs was limited, whereas LCMs were readily degraded by lipase and released silymarin quickly and completely. Pharmacokinetic study in beagle dogs showed significantly increased peak concentration for silymarin GMO/P407 LCM, and, most importantly, a 3.46-fold increase in oral bioavailability as compared with Legalon®, a commercial silymarin formulation.
Key words: glyceryl monooleate, liquid crystalline, oral bioavailability, pharmacokinectics, poloxamer 407, silymarin
INTRODUCTION
Silymarin, extracted from the seeds of milk thistle (Silibum marianum Gaertn.), is a mixture of flavonolignans including silybin, silychristin, isosilybin, silydianin, taxifolin, and various derivatives of these components (1–3). Silymarin as well as silybin, the main active component comprising about 33% of total silymarin weight (4), is used clinically as a hepatoprotector to treat liver injuries and chronic hepatitis. However, the main problem with silymarin is its poor oral bioavailability, which is attributable to its poor solubility (5–7) and low permeability as well as degradation in the gastrointestinal tract (8). Therefore, it appears quite important to improve the solubility and/or the permeability of silymarin to achieve higher oral bioavailability.
In order to improve the oral bioavailability of silymarin, various approaches have been employed, including silymarin/PVP K30 solid dispersion (9), silybin/phosphatidylcholine complex (10), and silymarin self-microemusifying drug delivery systems (11,12). However, the enhancement in oral bioavailability is limited by simply improving the solubility of silymarin (13). Approaches, thus, taken to improve both permeability and solubility seem to be more promising.
Many studies reported that the oral absorption of insoluble drugs was significantly improved after meals (14,15). It happens in the gastrointestinal tract where the digestion of the food fat induces secretion of physiological bile salts and phospholipids, forming lyotropic vehicles such as mixed micelles to solubilize the poorly water-soluble drugs, facilitate their transport across the unstirred water layer (16), and finally enhance their absorption. Lipid-based delivery systems depend on the similar mechanism to enhance the oral bioavailability of poorly water-soluble drugs (17). In our previous proof-of-concept studies, cubic nanoparticles based on glyceryl monooleate/poloxamer 407 show significantly enhanced oral bioavailability of simvastatin, a Biopharmaceutics Classification System (BCS) II drug, and cyclosporine A, a BCS IV drug (18,19). However, the liquid formulations of cubic nanoparticles seem to be unstable upon storage, which mandates development of solid lipid formulations such as lipid crystalline matrices. Glycerate- and phytantril-based liquid crystalline matrices show sustained release and enhanced oral bioavailability for a series of poorly water-soluble drugs (20–23).
In this study, we developed a glyceryl monooleate/poloxamer 407 (GMO/P407) liquid crystalline matrix (LCM) formulation with the aim to enhance the oral bioavailability of silymarin. The matrix system was prepared by a melting/congealing method with glyceryl monooleate/poloxamer 407 ratio of 100/12 (18,19) at which cubic liquid crystalline phases formed upon hydration. The lipid matrix possibly serves as a precursor for cubic nanoparticles (cubosomes) (24). In vitro characterization by differential scanning calorimetry (DSC) and powder X-ray diffractometry was performed to identify the physical state of silymarin in the lipid matrix. Finally, the oral bioavailability of silymarin was evaluated in beagle dogs by monitoring its main component silybin.
MATERIALS AND METHODS
Materials
Silymarin was purchased from Panjinhuacheng Pharmaceuticals (Liaoning, China). Glyceryl monooleate (GMO) was obtained from Danisco Company (Denmark). Poloxamer 407 (P407) was supplied by BASF (Ludwigshafen, Germany). HPLC-grade methanol and 1,4-dioxane were purchased from TEDIA Company Inc. (USA). Lecithin (Lipoid S75, containing about 70% of soy phosphatidylcholine (SPC)) was from Lipoid GmbH company (Ludwigshafen, Germany). Sodium taurocholate (NaTC) was obtained from Shanghai Boyun Biotech Co. Ltd. (Shanghai, China). Porcine pancreatic lipase (30,000 IU/g) was from Shanghai Kayon Biological Technology Co., Ltd. (Shanghai, China). Triz-maleate was purchased from Sigma (USA). All other reagents were of analytical grade and used as received.
Preparation of GMO/P407 Liquid Crystalline Matrices and Cubic Gel Containing Silymarin
The GMO/P407 LCMs were prepared by a melting/congealing method. Briefly, 20 g GMO and 2.4 g P407 were mixed and heated to melt in a 60°C water bath. Then, silymarin was dissolved in acetone and dispersed into the molten mixture. Acetone was removed by rota-evaporation in a 40°C water bath. To solidify the mixture, it was immediately poured into a cylindrical stainless steel mold (ϕ 1 × 1 cm) pre-cooled to −18°C, and stored at −18°C for 4 h to obtain silymarin-containing matrices. The loading of silymarin in the matrices were fixed at 2% (F1), 4% (F2), and 8% (F3), respectively.
To prepare GMO/P407 cubic gel as a reference, silymarin was added to the molten GMO (0.5 g)/P407 (0.06 g) mixture and dissolved with the help of ultra-sonication. Then, 2 mL water was added and the system was mixed by vortex. The mixture was then kept at room temperature for 48 h until equilibrium to obtain the cubic gel (18,19).
Polarized Light Microscope
The silymarin LCM was sliced into thin pieces and put on the microstat which was kept at 37°C, and then a drop of water was added. The interface of the matrix and water was observed under demi- and hol-polarized microscopy (20).
Differential Scanning Calorimetry
Thermal behaviors of the samples (silymarin, GMO, P407, physical mixture, and LCM F1, F2, and F3) were investigated by a DSC 204 Phoenix® calorimeter (NETSCH, Germany). Samples of about 5 mg were sealed in an aluminum pan. The endotherm was recorded in a programmed heating process from 20°C to 300°C at 10°C/min heating speed under a nitrogen purge atmosphere of 20 mL/min.
Powder X-ray Diffractometry
Diffraction intensity of the samples (silymarin, GMO, P407, physical mixture and LCM F1, F2, F3) were measured using a Rigaku D/max-γB diffractometer (Rigaku Corporation, Tokyo, Japan), with monochromatic Cu/Kα radiation (λ = 0.154 nm), voltage 40 kV, current 60 mA, and 2θ over a 2.5−50° range.
In Vitro Release Study
The release study was carried out based on the Chinese Pharmacopoeia (2010 edn.) rotating basket method. Release medium was 900 mL of distilled water (pH 6.5) thermostatically maintained at 37 ± 0.5°C and stirred at 75 rpm. The LCMs were directly put into the basket, whereas the silymarin pure drug was first sealed in hard gelatin capsules and then put into the basket. At time intervals, 5 mL of the release samples were withdrawn and filtered through 0.45 μm Nylon film (Peninsula Trading Ltd., Shanghai, China). The filtrate was assayed for silybin by HPLC. Meanwhile, 5 mL of the blank release medium was supplemented to keep constant volume.
In Vitro Digestion
In vitro digestion was performed over 1 h at 37°C as described by Sek et al. (25) and Han et al. (26) with slight modifications. The experiments were conducted in digestion media consisting of 50 mM Triz-maleate (pH 7.5), 150 mM NaCl, 5 mM CaCl2, 5 mM NaTC, and 1.25 mM SPC (conditions broadly representative of fasted state intestinal conditions). The digestion assembly was consisted of a pH meter and a magnetic stirring apparatus with water bath kettle. Solution of NaOH (0.5 M) was added into the digestion system using micro-burette. At first, the LCMs should be dispersed into coarse dispersion with digestion buffer because the bulk LCMs will form cubic phases and reverse hexagonal phases upon contact with the digestion medium, which will adhere to the electrode and prevent adequate stirring. The LCMs containing 2 g GMO were dispersed in 20 mL digestion buffer under shear provided by a high-speed Ultra-Turrax blender (QilinBeier, Ltd., Jiangsu, China). Then, 2 mL LCM coarse dispersion containing 0.1 g/mL GMO was added into 16 mL digestion medium, and the pH was adjusted to 7.50 ± 0.05. After that, 2 mL digestion buffer with porcine pancreatic lipase (3,000 tributyrin units) was added into the digestion system thermostatically maintained at 37°C. Solution of NaOH (0.5 M) was used to titrate the fatty acid produced by lipase digestion of GMO to maintain the pH at 7.50 ± 0.05 (start or stop the titration when pH went beyond this range). During the whole process, the magnetic stirring apparatus was used to keep the system agitated. After the completion of digestion, the post-digestion mixture was ultra-centrifuged (302,000×g, 30 min, 37°C, CP100MX centrifuge, P55 ST2 rotor, Hitachi, Tokyo, Japan). Either the supernatant phase or the sediment phase was dissolved respectively in 10 mL methanol and the quantity of silymarin in the two phases was determined by HPLC.
Determination of Silybin in Dog Plasma by HPLC
The plasma concentrations of silymarin were determined by a reversed-phase HPLC method based on quantification of its main component silybin (4). The Agilent 1100 series HPLC (USA) was composed of a quaternary pump, a degasser, an autosampler, a column heater, and a tunable ultraviolet detector. Kromasil® C18 column (150 × 4.6 mm, 5 μm) was used to separate silybin at 40°C. The mobile phase was consisted of methanol and 1,4-dioxane/water (1:9). The flow rate was 1.0 mL/min, and the detection wavelength was 288 nm.
Plasma sample (1 mL) was put into a 15-mL centrifuge tube. The internal standard α-naphthol solution (50 μL, 10.6 μg/mL) was added into the plasma sample and mixed thoroughly. Then 5 mL tert-butyl methyl ether was added and mixed by vortex for 15 min. The mixed sample was centrifuged for 10 min (3,000×g). The organic phase was transferred to another centrifuge tube. The residue was extracted by another 5 mL of tert-butyl methyl ether. The organic phase was combined and evaporated under nitrogen at 37°C. The residues were resolved in 100 μL mobile phase. Aliquots (20 μL) were injected for HPLC analysis.
The method was validated by adding various quantities of silybin to blank beagle dog plasma, at concentrations of 62.5, 125, 250, 500, 1,000, 2,000, and 4,000 ng/mL, respectively. These calibrations were subjected to the entire analytical procedure, so as to test the linearity, precision and recovery. Quality control samples were 62.5, 250, and 1,000 ng/mL, respectively.
Pharmacokinetic Study in Beagle Dogs
The animal protocol was overseen and evaluated by the Ethical Review Committee of School of Pharmacy, Fudan University. Six adult male beagle dogs weighing about 10 ± 2 kg were randomly divided into three groups and fasted 24 h before administration but allowed free access to water. The pharmacokinetics of GMO/P407 LCM, cubic gel (sealed into hard gelatin capsules) and Legalon®, a commercial silymarin capsule formulation, were evaluated in beagle dogs in a three-period crossover experiment. F2 was selected as the test LCM formulation due to its moderate drug loading (4%), which guaranteed suitable dosage volume and no concern of phasing out of the incorporated drug during metrical erosion. The three silymarin formulations were orally administered with 6 mL of water at a dose of 14.4 mg/kg based on silybin. Blood samples (3 mL) were collected from the leg vein, into heparinized tubes at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h, and the blood samples were centrifuged at 3,000×g for 10 min to collect the plasma which were stored at −20°C until analysis.
Data Analysis
Pharmacokinetic parameters were obtained by statistical moment analysis using DAS 2.1.1 software package (issued by the Mathematical Pharmacology Advisory Committee of China). Statistical analysis was performed by one-way ANOVA.
RESULTS AND DISCUSSION
Preparation of Silymarin GMO/P407 LCM and Cubic Gel
Silymarin GMO/P407 LCM and cubic gel were prepared successfully with good reproducibility. As the melting points of GMO and P407 are relatively low (30°C and 58°C, respectively), the mixture of GMO and P407 was first kept in a 60°C water bath for about 6 min and allowed to melt completely. However, the heat-to-melt time should not be too long because excessive heating may lead to degradation of the materials. Since silymarin cannot dissolve in the molten lipids quickly, acetone was used to facilitate dissolving. After addition of silymarin into the melting mixture of GMO and P407, the mixture was agitated to mix homogeneously and to prevent silymarin from precipitation. Moreover, in order to prevent recrystallization of silymarin during the congealing process, the molten mixture should be cooled quickly. The final LCM products bear a solid dispersion structure with silymarin being dispersed homogenously throughout the matrix. For the cubic gel preparation, viscous and clear cubic gel containing approximately 2.4% silymarin could form after equilibrating for 48 h, which was similar to our previous reports (18,19).
Polarized Light Microscope
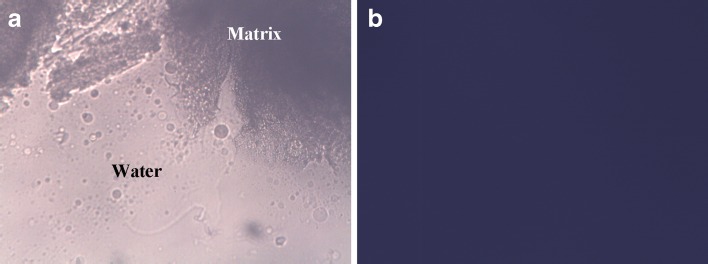
Figure 1 shows the images of demi- and hol-polarized optical microscopy, which showed that the mixture was transferred into a stiff isotropic system at the interface with water. This result indicated that the cubic phase possibly formed upon exposure of the LCM to water. The phenomenon of isotropy is typical for liquid crystalline structures (21), which usually presents as a dark view under hol-polarized optical microscope.
Fig. 1.
The interface of GMO/P407 LCM and water under demi- (a) and hol-polarized (b) light microscope
Differential Scanning Calorimetry
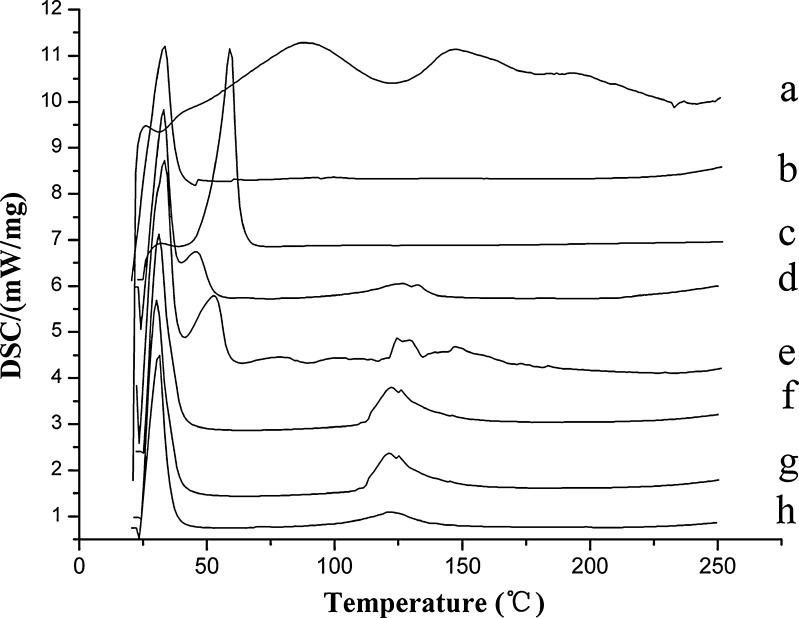
Figure 2 shows the thermo diagrams of the lipids and GMO/P407 LCMs. Being a mixture containing multiple components, silymarin showed a weak endotherm within the range of 50−100°C and 120−160°C. Endotherms of GMO and P407 were at 32°C and 58°C, respectively. The physical mixture showed the typical endotherms of GMO and P407 and weak endotherms of silymarin. However, new endotherms were generated around 115−135°C, which were not directly correlated to neither silymarin nor GMO/P407. At now, we do not have an exact explanation for this phenomenon. However, generation of a new phase may have taken place after melting and mixing of GMO and P407, which possibly contributed to the new endotherm. For GMO/P407 LCM containing different levels of silymarin, there were no thermal peaks that correlated to silymarin except the new GMO/P407 endotherm at 115−135°C. Results indicated that silymarin was homogenously dispersed in the matrices of GMO/P407.
Fig. 2.
DSC thermograms of silymarin (a), GMO (b), P407 (c), mixture of GMO and P407 (d), physical mixture of GMO, P407, and silymarin (e), and GMO/P407 LCMs (F1-f, F2-g, F3-h)
Powder X-ray Diffraction
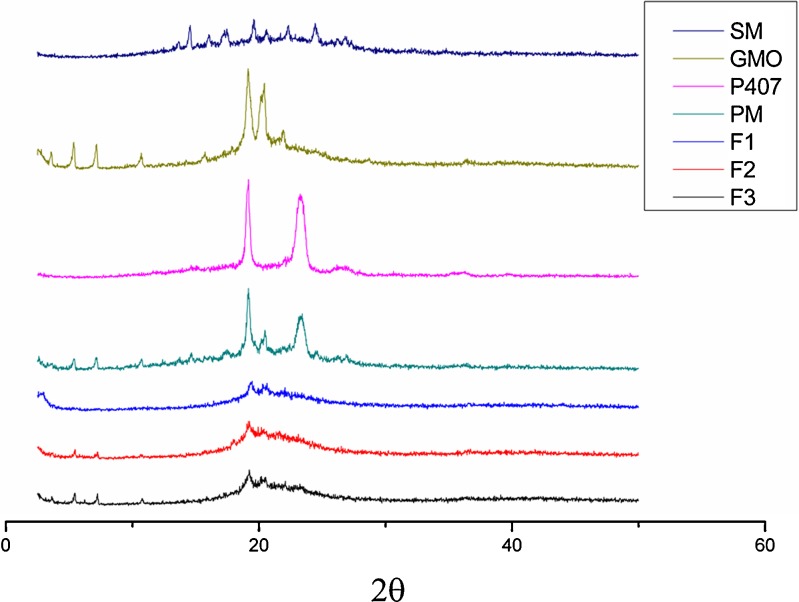
Figure 3 shows the powder X-ray diffraction patterns of the lipids and LCMs. Due to its multiple-component nature, silymarin showed weak characteristics of crystallinity. Both GMO and P407 showed typical peaks of crystallinity. In the PM pattern, silymarin diffraction peaks could be roughly observed, while in the GMO/P407-LCM (F1−F3) patterns, silymarin diffraction peaks disappeared. This phenomenon indicated that silymarin exist in the GMO/P407-LCM in an amorphous state.
Fig. 3.
X-ray diffraction patterns of silymarin (SM), GMO, P407, mixture of GMO and P407, physical mixture of GMO, P407, and silymarin (PM) and GMO/P407 LCMs (F1, F2, and F3)
In Vitro Release Study
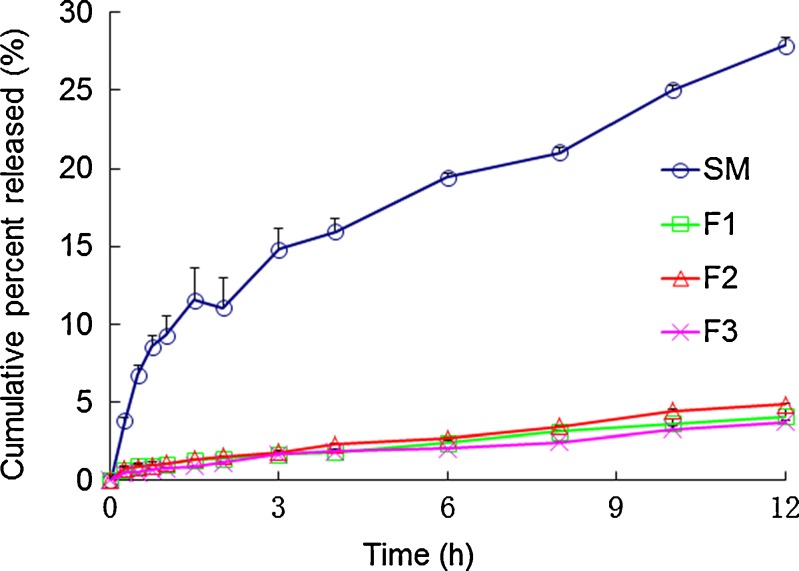
Figure 4 shows the release profiles of silymarin pure drug and the three LCM formulations (F1, F2, and F3). Being sparingly soluble in water, silymarin pure drug showed approximately 9.29% release at 1 h. However, total release reached 27.86% for time duration of 12 h. As for silymarin LCMs, the release was extremely limited with total release of less than 5% at 12 h. The obviously retarded release suggested increased affinity of silymarin with the liquid crystalline matrices. Since GMO in the formulation was assumed to undergo digestion in the gastrointestinal tract, in vitro digestion test should be carried out to simulate in vivo performance of LCM.
Fig. 4.
In vitro release profile of silymarin from GMO/P407 LCM (F1, F2, and F3) and silymarin pure drug. Data are expressed as mean ± SD (n = 3)
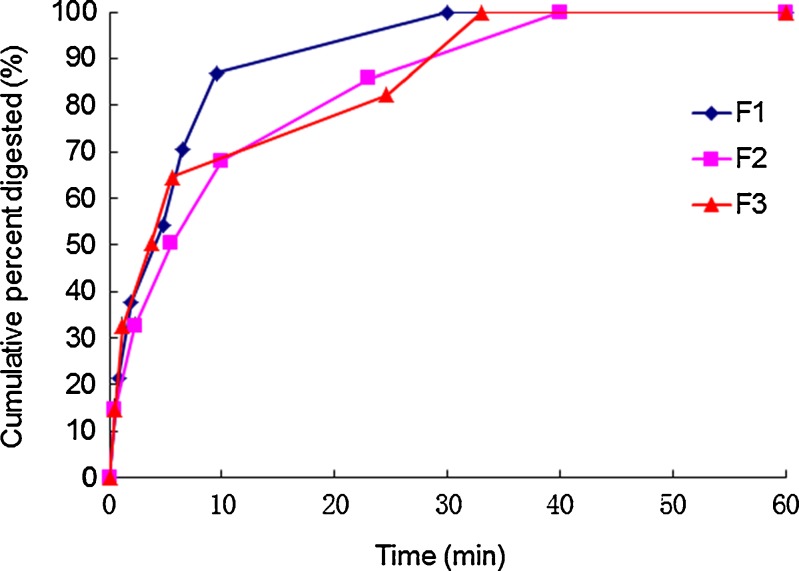
In Vitro Digestion
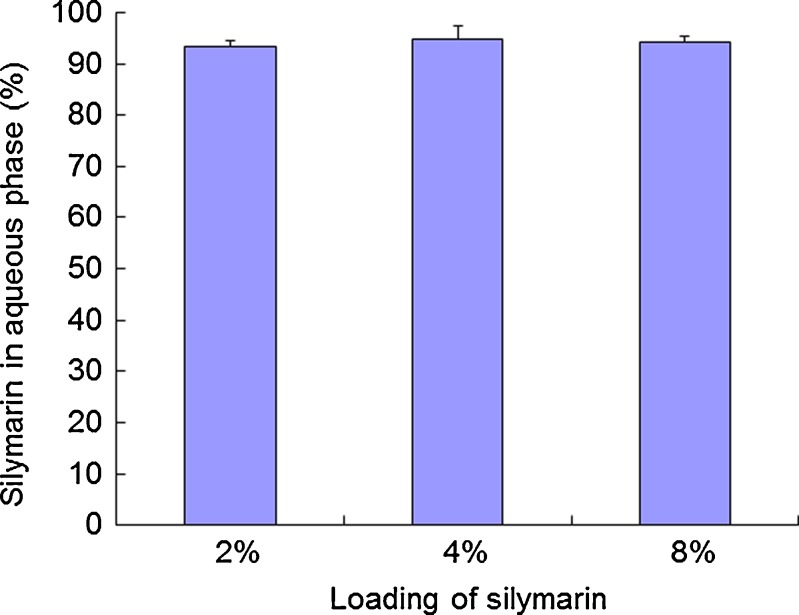
Figure 5 shows the curves of cumulative GMO digestion (%) versus time. The results showed that the GMO was digested rapidly and digestion completed at about 40 min. After centrifugation, the mixture was separated into an aqueous phase and a pellet phase. No oil phase was observed. The proportions of silymarin in aqueous phase for the three formulations (2%, 4%, and 8%) were shown in Fig. 6. The results indicated that most of the drug was recovered in the aqueous phase after digestion. It was concluded that silymarin could be released completely after digestion.
Fig. 5.
In vitro digestion profiles of GMO/P407 LCMs (F1, F2, F3). Data are expressed as mean ± SD (n = 3)
Fig. 6.
Silymarin recovered as detected in aqueous phase (%) after digestion by lipase. Data are expressed as mean ± SD (n = 3)
Determination of Silybin in Dog Plasma by HPLC
Silybin was completely separated from plasma impurities under the analytical conditions described in this study. The two peaks at 21 and 22 min indicated the two isomers of silybin, and the peak areas of the two isomers were calculated as a whole for silybin quantification. Linearity was observed within the range of 62.5 to 4,000 ng/mL (r = 0.9981). The lower limit of quantification for determination of silybin in dog plasma was found to be 40 ng/ml. Accuracy of the determination method is 95.32 ± 4.85% (n = 9). Within-day and between-day precisions were all below 4.0%. Extraction recovery of silybin in dog plasma was 87.64 ± 0.44% (n = 9).
Bioavailability in Beagle Dogs
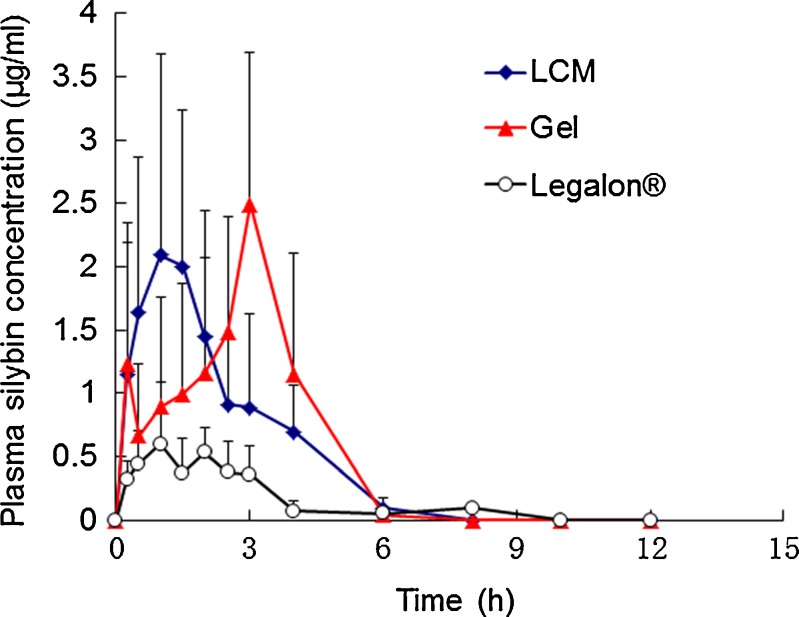
Figure 7 shows the profiles of mean plasma silybin concentration versus time in beagle dogs after oral administration of the three formulations. Table I shows the pharmacokinetic parameters obtained by the statistical moments analysis. The plasma silybin level for Legalon® was relatively low, which was similar to our previous results (13). However, both the cubic gel and the LCM showed significantly enhanced plasma silybin level for time duration of at least 6 h. The Cmax of the GMO/P407 LCM, liquid crystalline gel and Legalon® was 2.27 ± 1.07, 3.412 ± 2.05, and 0.763 ± 0.361 μg/mL, respectively; and tmax was 1.75 ± 0.92, 2.55 ± 1.39, and 1.7 ± 0.67 h, respectively. The relative bioavailability of GMO/P407 LCM and liquid crystalline gel was 345.57% and 367.9%, respectively, with Legalon® as the reference. Moreover, the bioavailability of silymarin after administration of GMO/P407 LCM had no significant difference with liquid crystalline gel (P > 0.05). The most significant difference between GMO/P407 LCM and cubic gel was in the peak time, which can be attributable to the slow hydration rate of the matrix. Although quick digestion rate was observed due to the breaking up procedure in the in vitro digestion test, the actual hydration and erosion rate of the LCM was relatively slow. However, it is difficult to measure the actual erosion rate because the LCMs turn to a semi-solid state, which did not allow accurate measurement of the weight of the matrices. The similar bioavailability of LCMs to the cubic gel suggested similar absorption mechanisms of the two formulations. Taking into account of the liquid crystalline nature of the matrices at the interface with water, it is reasonable to assume a mechanism of transition into cubic phases for the LCMs in the GI tract.
Fig. 7.
The mean plasma silybin concentration vs. time plot in beagle dogs (n = 6) after oral administration of GMO/P407 LCM, liquid crystalline gel, and Legalon®. Data are expressed as mean ± SD (n = 6)
Table I.
The Main Pharmacokinetic Parameters of Silymarin GMO/P407 LCM, Liquid Crystalline Gel and Legalon® in Beagle Dogs (n = 6)
| Formulation | t max (h) | C max (μg/ml) | t 1/2 (h) | MRT0–t (h) | AUC0–t (μg × h/mL) | AUC0–∞ (μg × h/mL) | Relative bioavailability (%)a |
|---|---|---|---|---|---|---|---|
| GMO/P407 LCM | 1.75 ± 0.92** | 2.27 ± 1.07*, ** | 1.13 ± 0.58*, ** | 2.23 ± 0.62 | 5.31 ± 3.59* | 5.46 ± 3.57* | 345.57 |
| Liquid crystalline gel | 2.55 ± 1.39* | 3.41 ± 2.05* | 0.74 ± 0.18* | 2.44 ± 0.76 | 5.65 ± 2.58* | 5.78 ± 2.58* | 367.90 |
| Legalon® | 1.70 ± 0.67 | 0.76 ± 0.36 | 3.69 ± 2.17 | 2.29 ± 1.01 | 1.54 ± 0.57 | 4.023 ± 2.13 |
aCalculated on AUC 0–t with Legalon® as the reference
*P < 0.05 vs. Legalon®, level of significance; **P < 0.05 vs. liquid crystalline gel, level of significance
Although the exact mechanism of enhanced oral bioavailability of a variety of drugs has not been elucidated, it is reported that the lipid-based liquid crystalline matrices may transform into liquid crystalline phases and form lyotropic vehicles such as cubic nanoparticles, vesicles and mixed micelles under the effect of digestion by lipase in the presence of endogenous bile salts and phospholipids, which can pass through the unstirred water layer and facilitate drug absorption (16,17,21). Moreover, the liquid crystalline phases formed upon contact with the physiological fluid are mucoadhesive and can be better retained in the GI tract.
CONCLUSIONS
GMO/P407 LCM containing silymarin can be easily prepared by the melting/congealing method. Both DSC and X-ray diffraction analysis confirmed lack of crystallinity of silymarin in the LCMs. The isotropic phenomenon observed under polarized light microscope confirmed the liquid crystalline structure at the junction of LCM and water. Pharmacokinetic study showed significantly increased peak concentration for silymarin GMO/P407 LCM, and, most importantly, a 3.5-fold increase in silymarin oral bioavailability as compared with Legalon®, a commercial silymarin formulation. The mechanisms of enhanced bioavailability can be attributable to the liquid crystalline phase formed in the GI tract rather than enhanced release because only limited release of silymarin was observed for LCMs in this study.
ACKNOWLEDGMENTS
This work was partly supported by grants from the Ministry of Education of China (200802461092), the National Key Basic Research Program of China (2009CB930300, 2007CB935800), and Shanghai Municipal Commission of Education (10SG05).
Footnotes
Ruyue Lian and Yi Lu contributed equally to this article.
References
- 1.Kvasnicka F, Biba B, Sevcik R, Voldrich M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990:239–245. doi: 10.1016/S0021-9673(02)01971-4. [DOI] [PubMed] [Google Scholar]
- 2.Quaglia MG, Bossù E, Donati E, Mazzanti G, Brandt A. Determination of silymarin in the extract from the dried silybum marianum fruits by high performance liquid chromatography and capillary electrophoresis. J Pharm Biomed Anal. 1999;19:435–442. doi: 10.1016/S0731-7085(98)00231-3. [DOI] [PubMed] [Google Scholar]
- 3.Škottová N, Krecman V, Simánek V. Activities of silymarin and its flavonolignans upon low density lipoprotein oxidizability in vitro. Phytother Res. 1999;13:535–537. doi: 10.1002/(SICI)1099-1573(199909)13:6<535::AID-PTR526>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Lu Y, Chen J, Zhang WT, Wu W. Synchronized and sustained release of multiple components in silymarin from erodible glyceryl monostearate matrix system. Eur J Pharm Biopharm. 2007;66:210–219. doi: 10.1016/j.ejpb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Basaga H, Poli G, Tekkaya C, Aras I. Free radical scavenging and antioxidative properties of “silibin” complexs on microsomal lipid peroxidation. Cell Biochem Funct. 1997;15:27–33. doi: 10.1002/(SICI)1099-0844(199703)15:1<27::AID-CBF714>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus) Fitoterapia. 1995;66:3–42. [Google Scholar]
- 7.Pepping J. Milk thistle: Silybum marianum. Am J Health Syst Pharm. 1999;56:1195–1197. doi: 10.1093/ajhp/56.12.1195. [DOI] [PubMed] [Google Scholar]
- 8.Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharm. 1990;15:333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 9.Sun N, Wei X, Wu B, Chen J, Lu Y, Wu W. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powd Technol. 2008;182:72–80. doi: 10.1016/j.powtec.2007.05.029. [DOI] [Google Scholar]
- 10.Filburn CR, Kettenacker R, Griffin DW. Bioavailability of a silybin–phosphatidylcholine complex in dogs. J Vet Pharmacol Ther. 2007;30:132–138. doi: 10.1111/j.1365-2885.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63:288–294. doi: 10.1016/j.ejpb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Woo JS, Kim TS, Park JH, Chi SC. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch Pharm Res. 2007;30:82–89. doi: 10.1007/BF02977782. [DOI] [PubMed] [Google Scholar]
- 13.Sun N, Zhang X, Lu Y, Wu W. In vitro evaluation and pharmacokinetics in dogs of solid dispersion pellets containing Silybum marianum extract prepared by fluid-bed coating. Plant Med. 2008;74:126–132. doi: 10.1055/s-2008-1034294. [DOI] [PubMed] [Google Scholar]
- 14.Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 15.Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–255. doi: 10.2165/00003088-199937030-00003. [DOI] [PubMed] [Google Scholar]
- 16.Thomson AB, Schoeller C, Keelan M, Smith L, Clandinin MT. Lipid absorption: passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol. 1993;71:531–555. doi: 10.1139/y93-078. [DOI] [PubMed] [Google Scholar]
- 17.Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 18.Lai J, Chen JM, Lu Y, Sun J, Hu FQ, Yin ZN, et al. Glyceryl monooleate/poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS PharmSciTech. 2009;10:960–966. doi: 10.1208/s12249-009-9292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J, Lu Y, Yin Z, Hu F, Wu W. Pharmacokinetics and enhanced oral bioavailability in beagle dogs of cyclosporine A encapsulated in glyceryl monooleate/poloxamer 407 cubic nanoparticles. Int J Nanomedicine. 2010;5:13–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd BJ, Whittaker DV, Khoo SM, Davey G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int J Pharm. 2006;309:218–226. doi: 10.1016/j.ijpharm.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Boyd BJ, Khoo SM, Whittaker DV, Davey G, Porter CJ. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int J Pharm. 2007;340:52–60. doi: 10.1016/j.ijpharm.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TH, Hanley T, Porter CJ, Larson I, Boyd BJ. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water-soluble drugs II. In-vivo evaluation. J Pharm Pharmacol. 2010;62:856–865. doi: 10.1211/jpp.62.06.0006. [DOI] [PubMed] [Google Scholar]
- 23.Shah MH, Paradkar A. Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. Int J Pharm. 2005;294:161–171. doi: 10.1016/j.ijpharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Spicer PT, Small WB, Lynch ML, Burns JL. Dry powder precursors of cubic liquid crystalline nanoparticles (cubosomes) J Nanopart Res. 2002;4:297–311. doi: 10.1023/A:1021184216308. [DOI] [Google Scholar]
- 25.Sek L, Porter CJ, Charman WN. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situ densitometric analysis. J Pharm Biomed Anal. 2001;25:651–661. doi: 10.1016/S0731-7085(00)00528-8. [DOI] [PubMed] [Google Scholar]
- 26.Han SF, Yao TT, Zhang XX, Gan L, Zhu C, Yu HZ, et al. Lipid-based formulations to enhance oral bioavailability of the poorly water-soluble drug anethol trithione: effects of lipid composition and formulation. Int J Pharm. 2009;379:18–24. doi: 10.1016/j.ijpharm.2009.06.001. [DOI] [PubMed] [Google Scholar]