Abstract
Gastrodin is the major bioactive constituent of the traditional Chinese drug “Tianma.” It is used in the treatment of some nervous system diseases and can be transported to the brain via intranasal administration. In the current paper, the development of a novel ion-activated in situ gelling system for the nasal delivery of gastrodin is discussed. An in situ perfusion model was used to determine the absorption-rate constant of gastrodin through rat nasal mucosa. The optimal formulation was determined by measuring the critical cation concentration, anti-dilution capacity, gel expansion coefficient, water-holding capacity, and adhesive capacity. The best formulation consisted of 10% gastrodin, 0.5% deacetylated gellan gum as the gelatinizer, and 0.03% ethylparaben as the preservative. The rheological properties of gastrodin nasal in situ gels were also investigated. The viscosity and elasticity sharply increased at temperatures below 25°C. When physiological concentrations of cations were added into the preparation, the mixture gelled into a semi-solid. The results of an accelerated stability test show that gastrodin nasal in situ gels can be stable for more than 2 years. Mucociliary toxicity was evaluated using the in situ toad palate model and the rat nasal mucociliary method; both models demonstrated no measurable ciliotoxicity. Pharmacodynamic studies suggest that similar acesodyne and sedative effects were induced following intranasal administration of 50 mg/kg gastrodin nasal in situ gels or oral administration of 100 mg/kg gastrodin solution. The in situ gel preparation is a safe and effective nasal delivery system for gastrodin.
KEY WORDS: deacetylated gellan gum, gastrodin, gelling system, intranasal delivery, ion-activated
INTRODUCTION
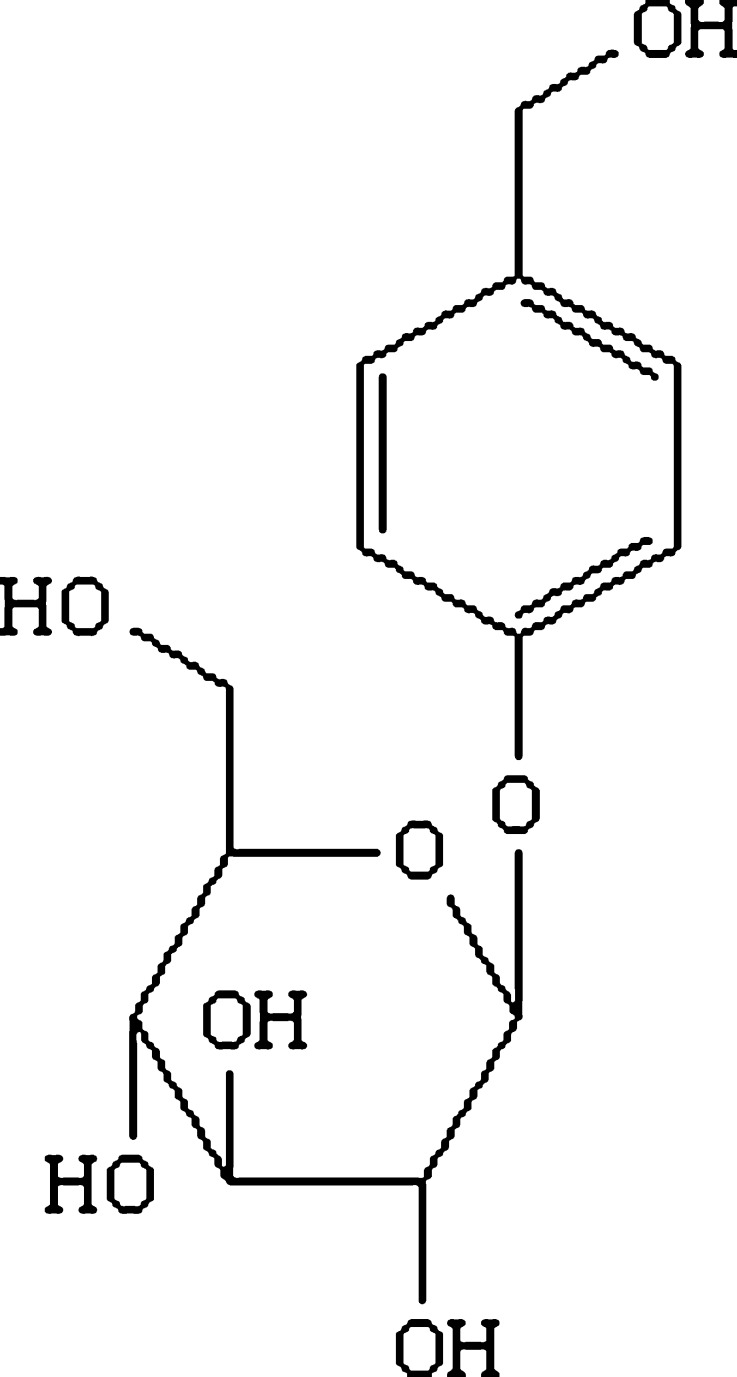
Gastrodin (p-hydroxymethylphenyl-β-d-glucopyranoside; Fig. 1), the main bioactive component of the traditional Chinese drug “Tianma” (Rhizoma Gastrodiae), is used in the treatment of central nervous system diseases such as vertigo, headache, insomnia, neuralgia, neurasthenia, and epilepsy (1–3). Gastrodin has attracted interest because of its low toxicity and therapeutic efficacy. Gastrodin acts on the brain to produce central inhibitory effects (4), but does not easily pass through the blood–brain barrier due to its hydrophilicity. Pharmacokinetic studies demonstrated that gastrodin can be directly transported from the nasal cavity to the brain through the olfactory mucosa (5,6). Therefore, an intranasal administration of gastrodin is a promising alternative to traditional administration.
Fig. 1.
Chemical structure of gastrodin
Most conventional liquid formulations for nasal delivery have low viscosity and thus are prone to running off, greatly reducing mucosal residence time and bioavailability. In situ gelling systems present a novel approach to sustaining mucosal contact and prolonging the release time in the nasal cavity (7). When nasal in situ gels are dripped or sprayed into the nasal cavity as low viscosity solutions, the polymers react to form more viscous gels that tightly adhere to the nasal mucosa, thereby sustaining the release and contact time. Thus, the use of nasal gels results in improved local and systemic bioavailability, reduces dose requirements, and improves patient safety and acceptability (8).
Gellan gum is a linear, anionic polysaccharide secreted by the bacterium Pseudomonas elodea. Deacetylated gellan gum (DGG) is approved in the USA and EU as a gelling, stabilizing, and suspending agent in food products (9). It can form strong clear gels at physiological ion concentrations and has been widely investigated for use as an in situ gelling agent (10–12). The cation-induced gelation of DGG is attributed to the formation of double-helical junction zones followed by the inter-helical association to form a three-dimensional network via binding with cations (13). Human nasal mucus contains sufficient sodium, potassium, and calcium ions. Therefore, a solution-gel phase transition will occur when DGG is administered into the nasal cavity (14).
In the current study, a highly efficient in situ gelling system based on DGG was formulated for the nasal delivery of gastrodin by measuring the critical cation concentration, anti-dilution capacity, gel expansion coefficient, water-holding capacity, and adhesive capacity of different concentrations of DGG. The rheological characteristics, stability, mucociliary toxicity, and pharmacodynamic action of the gastrodin in situ gels were evaluated.
MATERIALS AND METHODS
Materials
Gastrodin (99.7% purity) was synthesized and its purity was determined by Kunming Pharmaceutical Co. Ltd. (Kunming, China). DGG was purchased from Zhejiang Tianwei Biochemistry Products Co. Ltd. (Shaoxin, China). The ion compositions of the artificial nasal fluid (ANF) consisted of 150 ± 32 mM Na+, 41 ± 18 mM K+, and 4 ± 2 mM Ca2+ (15). Water was deionized and then double distilled. Other chemical reagents were of chromatographic or analytical grade and commercially available.
Animals
Healthy male Sprague–Dawley rats (230–270 g), male Kunming strain mice (18–22 g), and male toads (30–40 g) were obtained from the Laboratory Animal Center of Sichuan University (China). Prior to experimentation, the animals were acclimated for at least one week to a 12-h light/dark cycle with free access to standard chow and water. The animals were fasted overnight but supplied with water ad libitum before the experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Sichuan University.
Nasal Perfusion Studies in Rats
In Situ Nasal Perfusion
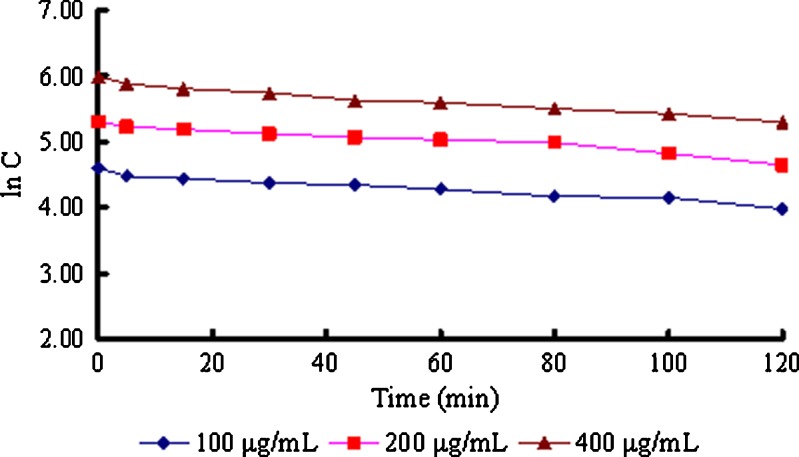
Rats were surgically treated according to a previously described method (16,17). A 10-mL physiological saline containing 100, 200, or 400 μg/mL gastrodin was circulated through the rat nasal cavity at 37 ± 0.5°C for 120 min (n = 3) using a peristaltic pump at a flow rate of 2.0 mL/min. Aliquots (50 μl) were drawn at predetermined time intervals (0, 5, 15, 30, 45, 60, 80, 100, and 120 min), and an equal volume of isotonic saline was added in the meantime. The concentrations of gastrodin in the perfusion fluid (C) were determined using high-performance liquid chromatography (HPLC). The absorption-rate constants (ka) were calculated via the linear regression analysis of lnC versus time (t) (18).
Gastrodin HPLC Determination
All samples were centrifuged at 8,000×g for 15 min and directly injected into the Agilent 1100 series HPLC system (Agilent Technologies, USA) for analysis. The system was equipped with a quaternary pump, vacuum degasser, column thermostat, 20 μL injector loop, UV detector, and HP ChemStation software. The separation was performed on a Diamonsil C18 column (150 × 4.6 mm, i.d., 5.0 μm; Dikma Technologies, China) under the following chromatographic conditions: column temperature, 25°C; sample injection volume, 20 μL; flow rate, 1.0 mL/min; detection wavelength, 221 nm; mobile phase, acetonitrile/water (6.5:93.5) (19). The method was validated over the concentration range of 20–500 μg/mL. Intra- and inter-day precision (RSD%) were all within 5%, and the accuracy ranged from 96% to 103%.
Preparation of the Formulations
A certain amount of DGG (0.2%–1.0%, w/v), gastrodin (10%, w/v), and ethylparaben (0.03%, w/v) were mixed with deionized water, stirred in a water bath at 90°C until all solids were dissolved, and cooled to room temperature. In this formulation, DGG was used as a gelatinizer, and ethylparaben as a preservative.
Critical Cation Concentration for DGG Phase Transition
The critical cation concentration (CCC) for phase transition is an important parameter for ion-activated in situ gels. When in situ gel cation concentrations exceed the CCC, phase transition (solution to gel) will occur instantaneously (20). The CCC of DGG was determined by mixing different concentrations (0.2–1.0%) of DGG solution (1.0 mL) and various amounts of ANF (0.05–0.30 mL) in cillin bottles placed in a water bath at 32°C. After 20 s, the bottles were turned over. If the gels adhered to the bottom instead of flowing or sliding down the side, the formulation showed gel formation and was considered “+”, whereas flowing/sliding formulations were considered “−”. The minimum ANF concentration in the mixture that could induce gel formation was estimated as the CCC.
Dilution Effect of the Nasal Fluid on the Gelation of the In Situ Gels
Nasal fluid can dilute the DGG and affect the gelation of the in situ gel. The nasal cavity contains approximately 100 μL of nasal mucus. Using nasal in situ gel volumes of 100–200 μL, the volume ratios were set from 1:1 to 1:2. Different DGG solution concentrations (0.3–0.6%, 1.0 mL) and various amounts of ANF (0.4–1.0 mL) were mixed in cillin bottles and placed in a water bath at 32°C. After 20 s, the bottles were turned over. If the gels adhered to the bottle wall and did not slide down, the formulation was considered “+”, whereas a flowing or sliding solution was considered “−”.
Effect of the DGG Concentration on the Expansion Coefficient of the In Situ Gels
When the solution transforms into a gel, its volume may increase. The nasal cavity is very small; thus, gel expansion may cause discomfort. Therefore, investigating the expansion coefficient of the in situ gels is necessary. Different DGG solution concentrations (0.4–0.6%, 1.0 mL) were mixed with 0.25 mL ANF in a graduated test tube and placed in a water bath at 32°C with a final total liquid volume, VM, of 1.25 mL. The volume of the gel after transition, VG, was determined by adding 2.0 mL of ANF and recording the total volume V1. Thus, VG = V1 − 2.0, and the expansion coefficient (S%) = (VG − VM)/VM × 100%.
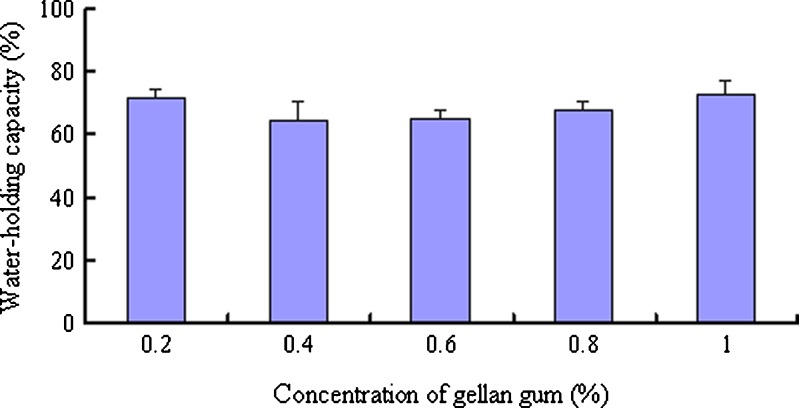
Effect of the DGG Concentration on the In Situ Gel Water-Holding Capacity
Different DGG solution concentrations (0.2%–1.0%, 1.0 mL) were mixed with 0.25 mL of ANF in pre-weighed centrifuge tubes for gel formation. The weight of the gels was measured as W0. The gels were centrifuged at 8,000×g for 10 min, and the seepage water was removed with filter paper. The gel was reweighed to determine the weight of held water (W). The water-holding capacity of the gels = W/W0 × 100% (21).
Effect of the DGG Concentration on the In Situ Gels Adhesive Capacity
The adhesive capacity of the in situ gels was evaluated using a novel in situ toad model. A toad was fixed onto a board, with its mouth opened with hemostats to expose the palate. Approximately 1 mL of ANF was dripped onto the mucosa of the palate to keep it moist. Physiological saline (25 μL) with 0.5% aniline blue was then dripped into the eye socket of the toad palate. The time from the administration of aniline blue until the blue color completely faded from the mucosal surface was recorded as the cilia clearance time (T0). The mucosal surface was then cleaned with ANF. After 10 min, the cilia clearance time of the DGG solution with 0.5% aniline was measured and taken as T1. The relative percentage change in the cilia clearance time (=T1/T0 × 100%) was used as a measure of the adhesive capacity of the in situ gels.
Effect of Cold Storage Time on the In Situ Gels Particle Size
Gastrodin (10%, w/v), DGG (0.5%, w/v), and ethylparaben (0.03%, w/v) were mixed with deionized water, stirred in a water bath at 90°C until all solids were dissolved, and cooled to room temperature. The solution was filtered through a glass funnel, and deionized water was added to adjust the concentration. The liquid was stored at 4°C and sampled at 0, 6, 12, and 24 h to determine the particle size and Zeta potential.
Rheological Properties of the In Situ Gels
The rheology of gastrodin nasal in situ gels was measured using an advanced dynamic rheometer (Gemini 200, BOHLIN Instruments, UK). The in situ gels are a non-Newtonian fluid. The rheological parameters measured include the elastic modulus (G′, in Pa), the viscous modulus (G″, in Pa), and the phase angle (δ, in degrees). The experimental conditions for the tested samples are outlined below.
Sample 1 was the gastrodin nasal in situ gels. The experimental conditions were scanning temperature range, 5–60°C; oscillation frequency, 1 Hz; strain amplitude, 5%; and heating rate, 2°C/min.
Sample 2 was 4-mL gastrodin nasal in situ gel mixed with different amounts of ANF (0, 1, 2, 3, or 4 mL). The experimental conditions were: temperature, 32 ± 0.1°C; frequency range, 0.1–2.0 Hz; and strain amplitude, 5%.
In Situ Gel Stability
The stability of the gastrodin nasal in situ gels was assessed using an acceleration stability test. A quantity of gastrodin in situ gel in cillin bottles was stored in a desiccator containing a saturated solution of sodium chloride, which provided a relative humidity of 75 ± 5%. The desiccator was placed in a hot air oven maintained at 40 ± 2°C, and the samples were withdrawn at 0, 1, 2, 3, and 6 months. The appearance, CCC, pH value, content, and related substance were measured at predetermined time intervals.
Nasal Cilitoxicity of the In Situ Gels
Toad Palate Model
Nasal ciliotoxicity studies were conducted using an in situ toad palate model (14). The upper palate of the toad (n = 6) was exposed and treated with approximately 0.5 mL gastrodin solution (10% gastrodin), blank in situ gel (0.5% DGG), or gastrodin in situ gel for 30 min. The tested sample was removed by washing the palate with physiological saline. A 3 mm × 3 mm area of the palate was dissected and the mucocilia were examined under a microscope at ×400. Physiological saline was used as the negative control. The ciliary movement time (CMT) was recorded, and the relative percentage of CMT (RPC) was calculated as follows: RPC = CMT test/CMT control × 100%.
Rat Model
Twelve SD rats were randomly divided into three groups of four rats each: the saline group, the gastrodin in situ nasal gel group, and the sodium deoxycholate (50 mg/mL) group. After shallow ether anesthesia, the rats were administered one of the three sample solutions at 0.3 mL/kg, three times a day for 7 days. They were sacrificed on the ninth day, and nasal mucosa samples were dissected (22). The tissue sections were rinsed in physiological saline and fixed overnight in 2.5% glutaraldehyde. The sections were then dehydrated through an ethanol series (30–100%), substituted with isoamyl acetate, critical point-dried with CO2, and sputter-coated with gold. The prepared specimens were observed under a scanning electron microscope (JSM-5900LV, Tokyo, Japan).
Pharmacodynamic Actions of the In Situ Gels
Acetic Acid-Induced Writhing Test
All mice were divided randomly into five groups of ten mice each and administered one of the following treatments: 0.5 μL/g intranasal physiological saline (saline group), 100 mg/kg oral gastrodin (oral administration group), and intranasal gastrodin in situ gel at 25, 50, or 100 mg/kg (intranasal administration groups). After 40 min, mice were placed in a large glass cylinder and injected with acetic acid (0.6%, v/v, 10 mL/kg, i.p.). The intensity of nociceptive behavior was quantified by counting the total number of writhes observed between 0 and 10 min after stimulus injection. The writhing response consists of a contraction of the abdominal muscle with a stretching of the hind limbs. The antinociceptive activity of the treatments was expressed as writhing scores over a period of 10 min.
Pentobarbital Sodium-Induced Sleeping Time
All mice were divided randomly into five groups of ten mice each and administered one of the following treatments: 0.5 μL/g intranasal physiological saline (saline group), 100 mg/kg oral gastrodin (oral administration group), and intranasal gastrodin in situ gel at 25, 50, or 100 mg/kg (intranasal administration groups). After 1 h, mice were injected with sodium pentobarbitone at 40 mg/kg. The time between losing and regaining the righting reflex was recorded as the duration of sleep.
Statistical Analysis
Data are presented as mean ± SD, (unless otherwise specified). Significant differences were assessed using unpaired Student’s t tests. A probability value of P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Nasal Mucosa Permeability of Gastrodin
The results indicate that gastrodin can pass through the nasal mucosa via passive diffusion (Fig. 2). The absorption-rate constants (ka) of 100, 200, and 400 μg/mL gastrodin were 4.40 × 10−3, 4.73 × 10−3, and 5.19 × 10−3 min−1, respectively. The average ka value (4.77 × 10−3 min−1) was significantly higher than that of tyrosine (1.1 × 10−3 min−1), ginsenosides (5 × 10−4 min−1) (23), or some other water-soluble drugs, possibly because of the lower molecular weight (286.27) of gastrodin. Hydrophilic gastrodin cannot easily pass through lipid membranes, but a lot of small water channels (two or three times more than in the jejunum), which allow the passage of small molecules and water-soluble drugs like gastrodin, are distributed in the nasal mucosa (24).
Fig. 2.
Absorption kinetic curves of various concentrations of gastrodin in rat nasal cavity (n = 3)
Critical Cation Concentration for DGG Phase Transition
DGG is ion sensitive, and the CCC of DGG was less than 20% (Table I). In mixtures with subcritical cation concentrations, DGG solutions rapidly formed colorless, odorless, and transparent gels. When the DGG concentration exceeded 0.6%, the solution became too viscous for convenient spraying. Thus, the optimal DGG concentration is below 0.6%.
Table I.
Gelation Reaction of DGG with ANF (n = 3)
| Concentration of DGG (%) | Volume of ANF (mL) | |||||
|---|---|---|---|---|---|---|
| 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | |
| 0.2 | − | − | − | − | + | + |
| 0.3 | − | − | − | − | + | + |
| 0.4 | − | − | − | + | + | + |
| 0.5 | − | − | + | + | + | + |
| 0.6 | − | − | + | + | + | + |
| 0.7 | − | − | + | + | + | + |
| 0.8 | − | + | + | + | + | + |
| 0.9 | − | + | + | + | + | + |
| 1.0 | + | + | + | + | + | + |
(+) not slide, (−) slide
Some studies chose a “stirring method” to investigate the phase transition of in situ gels (25,26). In this method, gel formation was evaluated by measuring the time required for controlled stirring of a known torque to stop. However, this method has several problems. First, drug delivery systems generally do not require gels to possess such high viscosity that can stop rotation. Second, controlling the rotation speed is difficult. In the current study, CCC was determined using a novel “timing inversion method” (27). The gel components were mixed in bottles and heated for a short period (20 s). The bottles were then inverted and gel formation was evaluated based on whether the contents stuck to the bottom or slid/flowed down the bottle sides. This simple method can evaluate the adhesion and the transformation speed, the two most important characteristics of in situ gels.
Effect of Nasal Fluid Dilution on the Gelation of the In Situ Gels
When the DGG concentration was less than 0.4%, the in situ gel was too easily diluted by other solutions mimicking mucous volumes and thus would not form a gel in situ. Therefore, the DGG concentration in the in situ gel should not be less than 0.4%; otherwise, ensuring gel formation in situ would be difficult.
In Situ Gel Expansion Coefficient
When the DGG solutions transformed into gels, no obvious volume expansion was observed. The expansion coefficient was only about 5% and was not affected by the DGG concentration. Slight expansion may not cause discomfort to patients when the in situ gel is administered into the nasal cavity.
Effect of the DGG Concentration on the In Situ Gel Water-Holding Capacity
The water-holding capacity is generally expressed as the amount of water the gel structure can hold, or the capacity of the gel to retain this water during storage or when subjected to external force. It is an important parameter for gel characterization. The DGG gels exhibited a water-holding capacity above 60% when subjected to a centrifugal force of 8,000×g for 10 min, which was similar to the results from a previous study (28). The DGG concentration had no significant effect on the water-holding capacity of the in situ gels (Fig. 3).
Fig. 3.
Water-holding capacity of various concentrations of DGG gels (n = 3)
Effect of the DGG Concentration on the In Situ Gel Adhesion
Toad palate mucocilia are similar to human nasal cilia; thus, the toad palate model is commonly used to determine the ciliary clearance of microsphere particles (29). The method was improved by adding a stain to allow for the evaluation of the adhesion capacity of a colorless semi-solid or liquid nasal preparation. The solubility of the dye should be similar to that of the drug; otherwise it will not reflect the clearance of the drug. Gastrodin is easy soluble in water, so water-soluble aniline blue was chosen as the indicator. The adhesion of the 0.5% and 0.6% DGG solutions was similar and significantly stronger than that of 0.4% DGG (Table II). Therefore, the optimal DGG concentration in the in situ gel was 0.5% (m/v).
Table II.
Adhesive Capacity of the DGG Gels Measured Via the Toad Palate Method (n = 5)
| Concentration (%) | T 0 (min) | T 1 (min) | T 1/T 0 (%) |
|---|---|---|---|
| 0.4 | 21 ± 2.1 | 33 ± 2.7 | 157.1 |
| 0.5 | 20 ± 2.3 | 38 ± 2.6 | 190.0 |
| 0.6 | 23 ± 2.6 | 45 ± 3.2 | 195.7 |
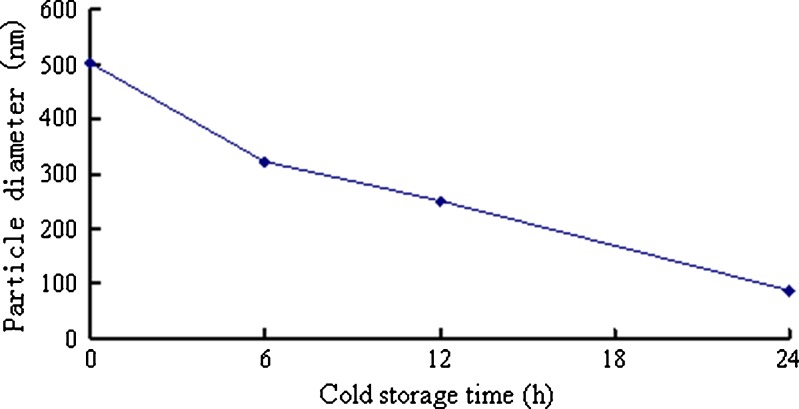
Effect of Cold Storage Time on the In Situ Gel Particle Size
The particle size of the in situ gels was significantly decreased with cold storage time (Fig. 4). Therefore, a 24 h cold storage time is needed to facilitate the dissolution of the polymer. The particle size and Zeta potential of the in situ gels after 24-h cold storage were 87.91 nm and −44.25 mV, respectively. The small particle size and high surface potential indicate that the in situ gelling system was stable.
Fig. 4.
Effect of the cold storage time on in situ gel particle size
Rheological Properties of the In Situ Gels
Methods for the determination of the rheological properties of gels can be divided into static testing and dynamic testing. Static testing is a shear flow method that applies steady stress or strain, whereas dynamic testing applies periodic or oscillatory stress or strain. In the static rheological tests, continuous deformation may change, or even destroy the polymer morphology. The dynamic method usually uses smaller strains so that the testing process has little effect on the structure of the material itself, and instead tests the elastic response.
The elastic modulus describes the characteristic parameters of solid-state properties. It directly reflects the phase transition and strength of the gel. On the other hand, the viscous modulus describes the viscous deformation characteristics. The phase angle (0° < δ <90°) can clearly determine the state of the polymer. The higher the phase angle value, the higher the viscosity, and the fluid exhibits liquid-state characteristics. By contrast, a lower phase angle indicates more obvious elasticity or solid-state characteristics. These parameters can clearly characterize the dynamic and viscoelastic properties of gastrodin nasal in situ gels (30).
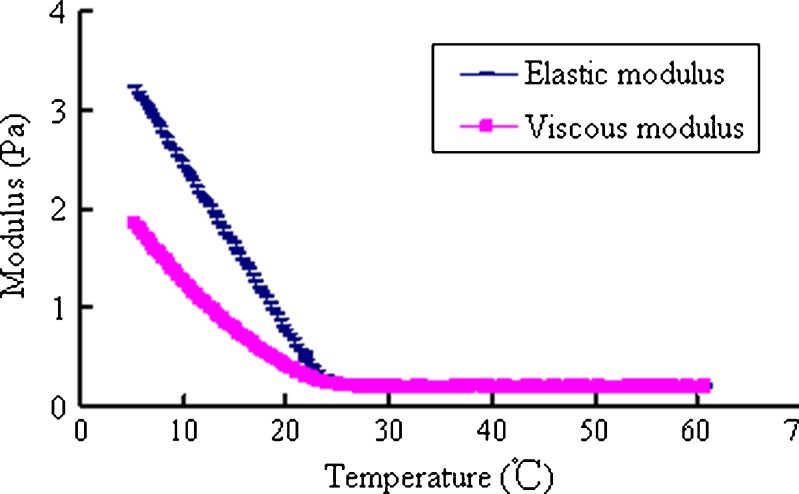
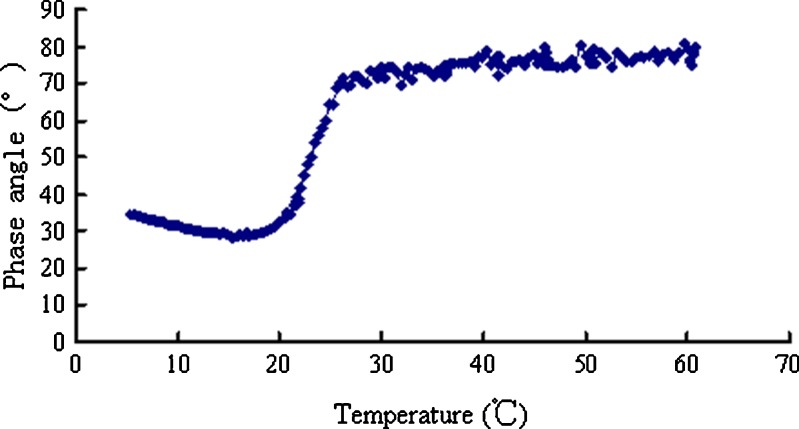
A rheological transition point for gastrodin nasal in situ gels was observed at 25°C (Figs. 5 and 6). When the temperature was below 25°C, the elastic and viscous modulus dramatically increased, indicating significantly increased viscosity and elasticity of the gastrodin in situ gels. Therefore, the design of a spray delivery device should have sufficient driving force for application under low temperature conditions.
Fig. 5.
Elastic and viscous modulus of the in situ gels heated from 5°C to 60°C
Fig. 6.
Phase angles of the in situ gels heated from 5°C to 60°C
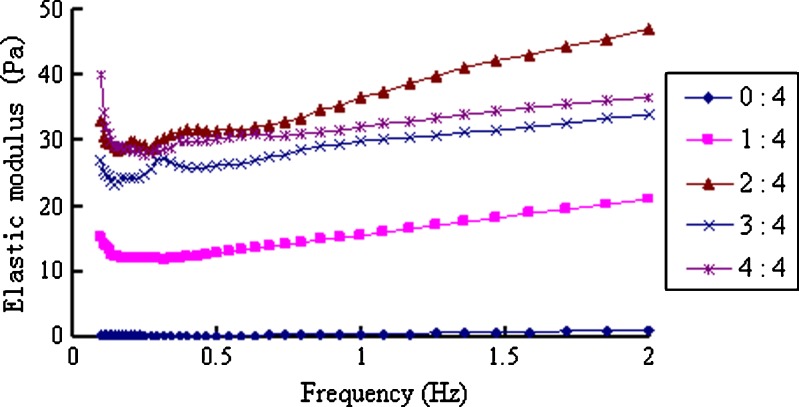
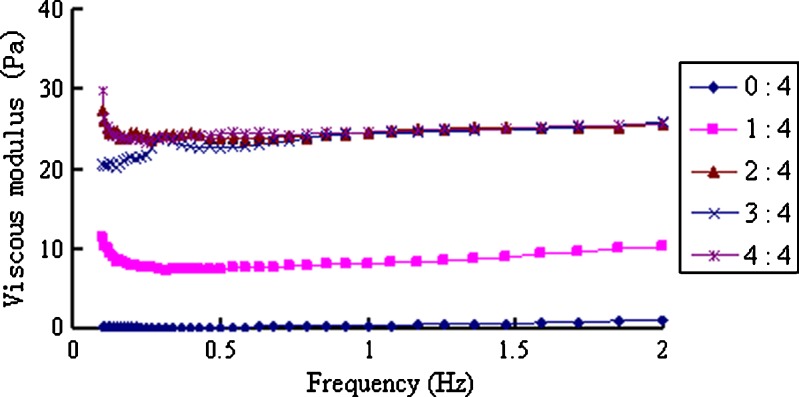
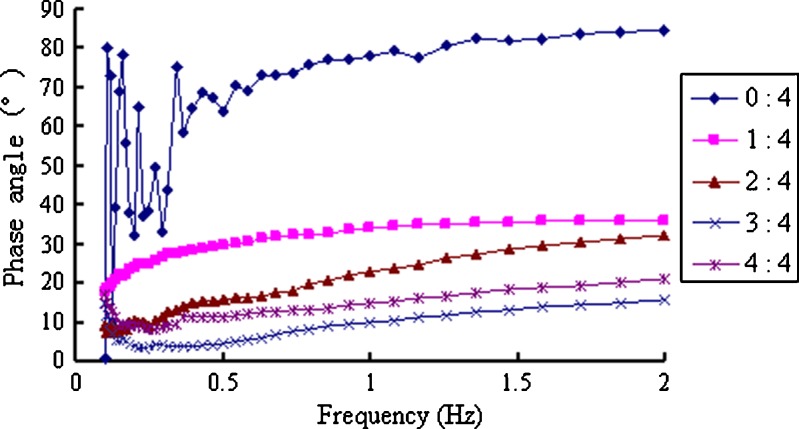
When the in situ gels were mixed with ANF, a gel immediately formed and the elastic and viscous modulus significantly increased, whereas the phase angle decreased to less than 30° (Figs. 7, 8, and 9). When the scanning frequencies exceeded 0.5 Hz, these parameters showed no frequency-dependent solid-state characteristics (30). When the ratio of ANF to the in situ gel increased from 1:4 to 2:4, both the viscosity and elasticity of the formed gel increased accordingly. However, because of the dilution effect, when the ratio increased to 4:4, all parameters slightly decreased.
Fig. 7.
Elastic modulus of the in situ gels mixed with different amounts of ANF at 32°C
Fig. 8.
Viscous modulus of the in situ gels mixed with different amounts of ANF at 32°C
Fig. 9.
Phase angles of the in situ gels mixed with different amounts of ANF at 32°C
In Situ Gel Stability
Based on visual identification, the in situ gel remained in the liquid state for 6 months at 40 ± 2°C without turbidity or gelation. CCC, pH, content, and related substance analysis results also show no changes over 6 months. Therefore, the gastrodin nasal in situ gel is very stable. Based on other gels and other gastrodin preparations in market, the shelf life of gastrodin nasal in situ gel is expected to reach more than 2 years. Exact shelf life should be determined by the long-term stability test.
Nasal Cilitoxicity of the In Situ Gels
A complete evaluation of nasal toxicity must consider irritation of the nasal mucosa, damage to nasal epithelial cells, and effects on nasal ciliary function (motility). These three indices of nasal toxicity generally show a parallel relationship (31). An evaluation of the toxicity on nasal cilia is much more convenient; thus, this index was used to evaluate the general nasal toxicity using the toad palate and rat nasal mucociliary method (32).
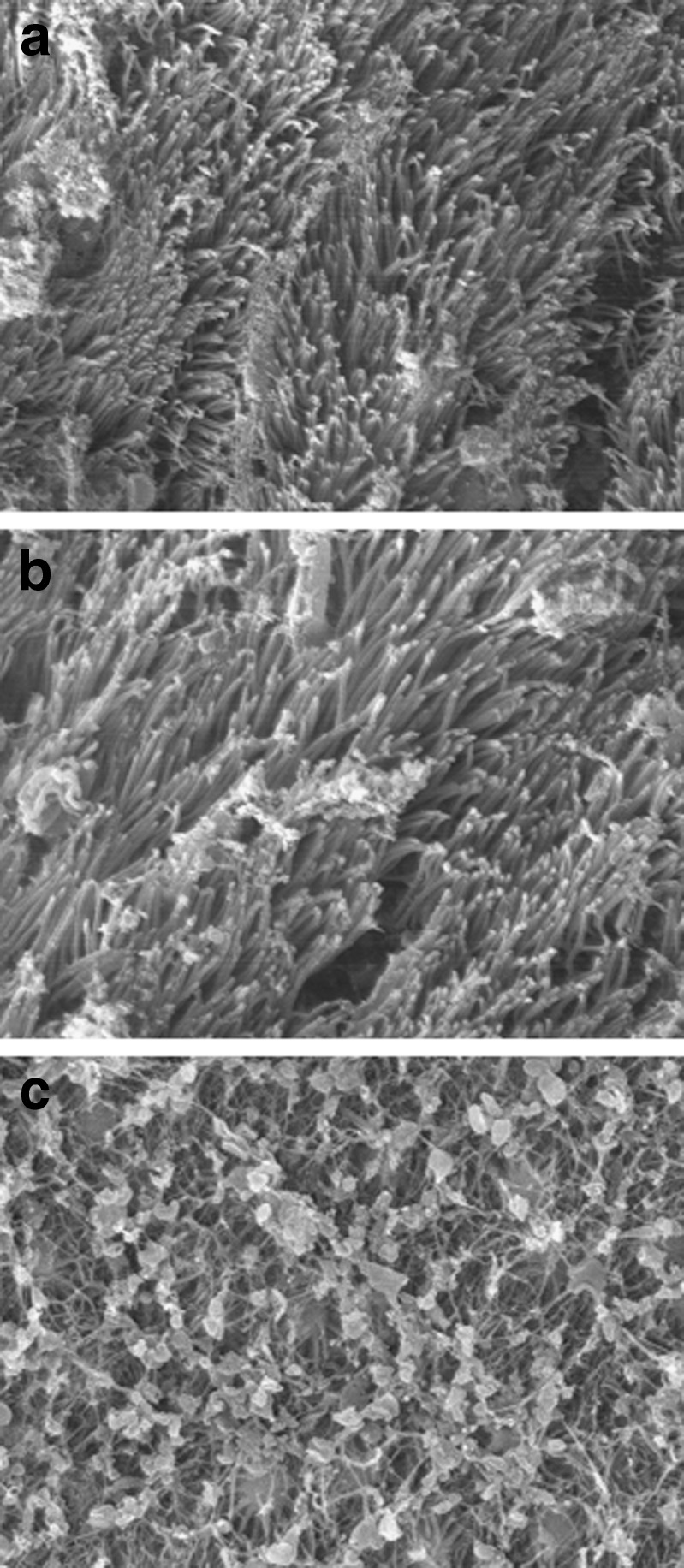
Gastrodin in situ gels showed no obvious cilia toxicity (Table III). Scanning electron microscope images are shown in Fig. 10. In the physiological saline group, cilia were arranged in neat rows and distributed on the surface of rat nasal mucosa. Similar to the physiological saline group, the mucosa exposed to the in situ gels exhibited no cilia loss, lodging or other anomalies. As a positive control group, sodium deoxycholate caused significant nasal cilia toxicity.
Table III.
Effect of Gastrodin and the In Situ Gels on CMT (n = 3)
| Samples | CMT (min) | RPC (%) |
|---|---|---|
| Physiological saline | 1,274 ± 94 | 100 |
| gastrodin solution | 1,174 ± 87* | 92.2 |
| Blank in situ gel | 1,121 ± 73* | 88.0 |
| Gastrodin in situ gel | 1,105 ± 82* | 86.7 |
*P > 0.05, compared with negative control
Fig. 10.
Scanning electron microphotographs (×3,000) of rat nasal mucocilia of rats after intranasal administration of physiological saline a, gastrodin nasal in situ gels b, or sodium deoxycholate c for 7 days
Pharmacodynamic Actions of the In Situ Gels
The results of the acetic acid-induced writhing test are presented in Table IV. Compared with the negative control group, the nasal in situ gel groups showed a significant reduction in the number of writhing episodes induced by acetic acid. Nasal administration of 50 mg/kg gastrodin nasal gel shows an analgesic effect similar to that observed following oral administration of 100 mg/kg gastrodin.
Table IV.
Effect of Gastrodin Nasal In Situ Gels on the Writhing Response Induced by Acetic Acid in Mice (n = 10)
| Groups | Dose (mg kg−1) | Writhing times | Inhibition ratio (%) |
|---|---|---|---|
| Physiological saline | – | 8.2 ± 3.6 | – |
| I.g. gastrodin | 100 | 3.7 ± 1.2** | 54.9 |
| I.n. in situ gel | 25 | 4.7 ± 2.8* | 42.7 |
| 50 | 3.5 ± 1.4** | 57.3 | |
| 100 | 3.1 ± 1.2** | 62.2 |
*P < 0.05; **P < 0.01, compared with negative control
The results of the pentobarbital sodium-induced sleeping time test are shown in Table V. No group showed significant differences in sleep latency. However, the highest nasal dose group showed a significantly longer sleeping time, suggesting that gastrodin has a sedative effect. Furthermore, the nasal preparation also prolonged sleep time relative to the equal-dose oral group (100 mg/kg), indicating that the sedative effect of the nasal in situ gel is stronger than that of the oral solution.
Table V.
Effect of Gastrodin Nasal In Situ Gels on the Hypnotic Effect of Pentobarbital Sodium in Mice (n = 10)
| Groups | Dose (mg kg−1) | Sleep latency (min) | Sleep time (min) |
|---|---|---|---|
| Physiological saline | – | 4.9 ± 1.6 | 37.6 ± 15.3 |
| I.g. gastrodin | 100 | 3.9 ± 1.3 | 83.2 ± 26.8* |
| I.n. in situ gel | 25 | 5.0 ± 1.9 | 53.6 ± 22.2 |
| 50 | 4.1 ± 2.2 | 92.5 ± 30.7* | |
| 100 | 3.6 ± 1.5 | 117.3 ± 27.2*, ** |
*P < 0.01, compared with negative control; **P < 0.05, compared with positive control
CONCLUSIONS
The preparation of gastrodin nasal in situ gels via mixing of gastrodin, DGG, and ethylparaben with deionized water is very simple. The in situ gels possess the best properties, including phase-transition capacity, anti-dilution capacity, water-holding capacity, and adhesive capacity, when the DGG concentration is 0.5% (w/v). The gastrodin in situ gels are stable and show no obvious cilitoxicity. Animal experiments suggest that gastrodin in situ gels can be more effective as an analgesic and sedative compared with the oral solution. Therefore, the in situ gelling system is a promising approach for the intranasal delivery of gastrodin. It is highly effective and the preparation method is suitable for industrial production.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 30902009) and Guangdong Natural Science Foundation (no. 10451008901004959).
REFERENCES
- 1.Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006;8:376–383. doi: 10.1016/j.yebeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Zhao H. Advance in clinical application of gastrodin. Strait Pharm J. 2009;21:29–31. [Google Scholar]
- 3.Zeng X, Zhang Y, Zhang S, Zheng X. A microdialysis study of effects of gastrodin on neurochemical changes in the ischemic/reperfused rat cerebral hippocampus. Biol Pharm Bull. 2007;30:801–804. doi: 10.1248/bpb.30.801. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Tan T, Zhong Y, Wu C. Study of the mechanism of gastrodin and derivatives of gastrodigenin. J West Chin Univ Med Sci. 1991;22:79–82. [PubMed] [Google Scholar]
- 5.Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007;341:20–25. doi: 10.1016/j.ijpharm.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/S0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 7.Watts P, Smith A. PecSys: in situ gelling system for optimised nasal drug delivery. Expert Opin Drug Deliv. 2009;6:543–552. doi: 10.1517/17425240902939135. [DOI] [PubMed] [Google Scholar]
- 8.Nagarwal RC, Pandit JK. Phase transition system: novel oral in-situ gel. Curr Drug Deliv. 2008;5:282–289. doi: 10.2174/156720108785914952. [DOI] [PubMed] [Google Scholar]
- 9.Jansson B, Hagerstrom H, Fransen N, Edsman K, Bjork E. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur J Pharm Biopharm. 2005;59:557–564. doi: 10.1016/j.ejpb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of the Gelrite gellan gum-based ocular delivery system for indomethacin. Acta Pharm. 2003;53:251–261. [PubMed] [Google Scholar]
- 11.Kubo W, Miyazaki S, Attwood D. Oral sustained delivery of paracetamol from in situ-gelling gellan and sodium alginate formulations. Int J Pharm. 2003;258:55–64. doi: 10.1016/S0378-5173(03)00163-7. [DOI] [PubMed] [Google Scholar]
- 12.Narayana RC, Harish NM, Gulzar AM, Prabhakara P, Singh AK, Subrahmanyam EV. Formulation and in vitro evaluation of in situ gels containing secnidazole for vaginitis. Yakugaku Zasshi. 2009;129:569–574. doi: 10.1248/yakushi.129.569. [DOI] [PubMed] [Google Scholar]
- 13.Funami T, Noda S, Nakauma M, Ishihara S, Takahashi R, Al-Assaf S, et al. Molecular structures of gellan gum imaged with atomic force microscopy in relation to the rheological behavior in aqueous systems in the presence or absence of various cations. J Agric Food Chem. 2008;56:8609–8618. doi: 10.1021/jf8007713. [DOI] [PubMed] [Google Scholar]
- 14.Cao SL, Ren XW, Zhang QZ, Chen E, Xu F, Chen J, et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int J Pharm. 2009;365:109–115. doi: 10.1016/j.ijpharm.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Lorin MI, Gaerlan PF, Mandel ID. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med. 1972;80:275–281. [PubMed] [Google Scholar]
- 16.Mei D, Mao S, Sun W, Wang Y, Kissel T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur J Pharm Biopharm. 2008;70:874–881. doi: 10.1016/j.ejpb.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Hirai S, Yashiki T, Matsuzawa T, Mima H. Absorption of drugs from the nasal mucosa of rat. Int J Pharm. 1981;7:317–325. doi: 10.1016/0378-5173(81)90058-2. [DOI] [Google Scholar]
- 18.Lu Y, Chen X, Du S, Wu Q, Yao Z, Zhai Y. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of Geniposide in rats. Arch Pharm Res. 2010;33:691–696. doi: 10.1007/s12272-010-0507-8. [DOI] [PubMed] [Google Scholar]
- 19.Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16:178–184. doi: 10.1080/10611860701794395. [DOI] [PubMed] [Google Scholar]
- 20.Tao T, Zhao Y, Yue P, Dong WX, Chen QH. Preparation of huperzine A nasal in situ gel and evaluation of its brain targeting following intranasal administration. Acta Pharm Sin. 2006;41:1104–1110. [PubMed] [Google Scholar]
- 21.Guinee TP, Feeney EP, Auty MA, Fox PF. Effect of pH and calcium concentration on some textural and functional properties of mozzarella cheese. J Dairy Sci. 2002;85:1655–1669. doi: 10.3168/jds.S0022-0302(02)74238-0. [DOI] [PubMed] [Google Scholar]
- 22.Asai K, Morishita M, Katsuta H, Hosoda S, Shinomiya K, Noro M, et al. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int J Pharm. 2002;246:25–35. doi: 10.1016/S0378-5173(02)00345-9. [DOI] [PubMed] [Google Scholar]
- 23.Jiang XG, Lu X, Cui JB, Qiu L, Xi NZ. Studies on octanol-water partition coefficient and nasal drug absorption. Acta Pharm Sin. 1997;32:458–460. [PubMed] [Google Scholar]
- 24.Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 25.Choi HG, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 26.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83:65–74. doi: 10.1016/S0168-3659(02)00175-X. [DOI] [PubMed] [Google Scholar]
- 27.Chung H, Dong H. Synthesis and characterisation of pluronic grafted chitosan copolymer as a novel injectable biomaterial. Curr Appl Phys. 2005;5:485–488. doi: 10.1016/j.cap.2005.01.015. [DOI] [Google Scholar]
- 28.Mao R, Tang J, Swanson BG. Water holding capacity and microstructure of gellan gels. Carbohydr Polym. 2001;46:365–371. doi: 10.1016/S0144-8617(00)00337-4. [DOI] [Google Scholar]
- 29.Lim ST, Martin GP, Berry DJ, Brown MB. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Control Release. 2000;66:281–292. doi: 10.1016/S0168-3659(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 30.Edsman K, Carlfors J, Harju K. Rheological evaluation and ocular contact time of some carbomer gels for ophthalmic use. Int J Pharm. 1996;137:233–241. doi: 10.1016/0378-5173(96)04525-5. [DOI] [Google Scholar]
- 31.Shusterman D. Toxicology of nasal irritants. Curr Allergy Asthma Rep. 2003;3:258–265. doi: 10.1007/s11882-003-0048-z. [DOI] [PubMed] [Google Scholar]
- 32.Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29:13–38. doi: 10.1016/S0169-409X(97)00059-8. [DOI] [PubMed] [Google Scholar]