Abstract
Cyclodextrins (CDs) are used in oral pharmaceutical formulations, by means of inclusion complexes formation, with the following advantages for the drugs: (1) solubility, dissolution rate, stability, and bioavailability enhancement; (2) to modify the drug release site and/or time profile; and (3) to reduce or prevent gastrointestinal side effects and unpleasant smell or taste, to prevent drug–drug or drug–additive interactions, or even to convert oil and liquid drugs into microcrystalline or amorphous powders. A more recent trend focuses on the use of CDs as nanocarriers, a strategy that aims to design versatile delivery systems that can encapsulate drugs with better physicochemical properties for oral delivery. Thus, the aim of this work was to review the applications of the CDs and their hydrophilic derivatives on the solubility enhancement of poorly water-soluble drugs in order to increase their dissolution rate and get immediate release, as well as their ability to control (to prolong or to delay) the release of drugs from solid dosage forms, either as complexes with the hydrophilic (e.g., as osmotic pumps) and/or hydrophobic CDs. New controlled delivery systems based on nanotechnology carriers (nanoparticles and conjugates) have also been reviewed.
Key words: controlled release, cyclodextrin, inclusion complex, solid dosage forms, solubility
INTRODUCTION
Drugs need to be released from their carriers (formulations and/or delivery systems) in order to produce their therapeutic effects (1), and difficulties on the design of the formulation may arise depending on drugs’ solubility. Currently, many platforms are available for a prolonged or delayed releasing of highly water-soluble drugs (2). However, if drugs are poorly water soluble, their formulation is more challenging (solubilization and/or particle engineering) especially when a controlled-release drug profile is desired. For these drugs, the absorption and bioavailability could be influenced by their intrinsic solubility and dissolution rate in the gastrointestinal (GI) tract. There are many strategies to enhance the solubility of poorly water-soluble drugs in order to formulate them as oral dosage forms. These strategies include salt formation, microenvironmental pH control, solubilization with surfactants (3), complexation with cyclodextrins (CDs) (4), solid dispersions (5), lipid-based formulations (6), and nanoparticle (NP) formulations (7). Besides the solubility, the pharmacokinetic and pharmacodynamic properties of pharmacologically active moieties can be changed by using novel drug delivery systems or by modifying the molecular structure and/or physiological parameters depending on the route of administration. The interaction between CD, drug, and dosage form influences the kinetics of key processes of oral drug delivery, including dissolution and absorption (8,9). Thus, the choice of the best drug delivery system will aim to enhance drug bioavailability and reduce their toxicity. In the complexation processes, the different interactions between chemistry species (molecules, ions, radicals) that do not involve covalent bonds are mostly of the host–guest type. CDs seem to be the most important ones in this domain. Also, the incorporation of CDs into a drug dosage form may affect the activity of basic pharmaceutical ingredients and may modify the properties of the whole drug formulation. Nearly 40 marketed commercial drug products currently benefited from such CD technology (10). The complexation can influence the drug release, but the process does not affect the drug intrinsic ability to permeate lipophilic biomembranes. However, CDs have been used as permeation enhancers in topical formulations (11) and to increase the permeability of water-insoluble drugs by making the drug available at the biological membrane surface (skin, mucosa, or the eye cornea). For water-soluble drugs, CDs increase drug permeability by direct action on membrane and enhance drug absorption and/or bioavailability (12–19). The drug release can be modified by using CDs and their derivatives. Among the different CDs, for example, the hydrophobic CD derivatives such as ethylated and peracylated ones have been employed as slow-release carriers for water-soluble drugs (20). In addition, the use of CDs in biological system often requires amphiphilic properties; thus, several modifications have been performed on CDs with the aim to provide versatile carriers and delivery systems for hydrophilic and hydrophobic drugs (21,22). Many studies have been developed where CDs are used as modified release carriers (e.g., colon-specific delivery systems) (23–26). In the last two decades, synthetic macromolecules with functional groups of pharmacological potential have received considerable attention. CDs are potent candidates for such role through the formation of drug conjugates improving certain drug properties such as solubility and/or bioavailability (27). However, physic and pharmaceutical properties of CD conjugates in which a drug is covalently bonded to the CD may be significantly different from those of the inclusion complexes (28). The use of the CD conjugate-based repeated- and prolonged-release systems is described in various studies (13,29,30).
CYCLODEXTRINS
Parent/Natural
Naturally occurring CDs are obtained through the enzymatic reaction on the starch. CDs are cyclic oligosaccharides built up from glucopyranose units linked by α-1,4 bonds, thus forming a torus-like macro-rings. They are characterized as crystalline, homogeneous, and non-hygroscopic substances. The natural CDs are αCD (Schardinger’s α-dextrin, cyclomaltohexaose, cyclohexaglucan, cyclohexaamylose, αCD, ACD, C6A) comprising six glucopyranose units, βCD (Schardinger’s β-dextrin, cyclomaltoheptaose, cycloheptaglucan, cycloheptaamylose, βCD, BCD, C7A) comprising seven of such units, and γCD (Schardinger’s γ-dextrin, cyclomaltooctaose, cyclooctaglucan, cyclooctaamylose, γCD, GCD, C8A) that comprises eight of such units (Fig. 1) (4,31,32). The most important characteristics of the natural CDs are summarized in Table I (4,31).
Fig. 1.
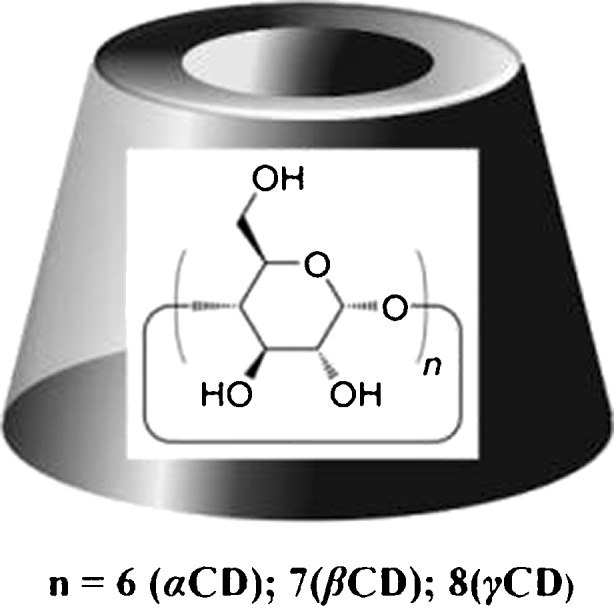
Schematic cyclodextrins structure
Table I.
Characteristics of α, β, and γCD
| α | β | γ | |
|---|---|---|---|
| No. of glucose units | 6 | 7 | 8 |
| Molecular weight | 972 | 1,135 | 1,297 |
| Solubility in water (g/100 ml), room T | 14.5 | 1.85 | 23.2 |
| [R]D25°C | 150.0 ± 0.5 | 162.5 ± 0.5 | 177.4 ± 0.5 |
| Cavity diameter (Å) | 4.7–5.3 | 6.0–6.5 | 7.5–8.3 |
| Height of torus (Å) | 7.9 ± 0.1 | 7.9 ± 0.1 | 7.9 ± 0.1 |
| Diameter of outer periphery (Å) | 14.6 ± 0.4 | 15.4 ± 0.4 | 17.5 ± 0.4 |
| Approximate cavity volume (å3) | 174 | 262 | 427 |
| Approximate cavity volume in 1 mol CD (ml) | 104 | 157 | 256 |
| Approximate cavity volume in 1 g CD (ml) | 0.10 | 0.14 | 0.20 |
| Crystalline forms (from water) | Hexagonal plates | Monoclinic parallelograms | Quadratic prisms |
| Crystal water, wt.% | 10.2 | 13.2–14.5 | 8.13–17.7 |
| Diffusion constant at 40°C | 3.443 | 3.224 | 3.000 |
| Hydrolysis by A. oryzae α-amylase | Negligible | Slow | Rapid |
| V max value, min−1 | 5.8 | 166 | 2,300 |
| Relative permittivity at pH 5.3, 25°C (on incorporating the toluidinyl group of 6-p-toluidynilnaphthalene 2-sulfonate) | 47.5 | 52.0 | 70.0 |
| Relative permittivity at pH 5.3, 25°C (on incorporating the naphthalene group) | –a | 29.5 | 39.5 |
| pK (by potentiometry) at 25°C | 12.332 | 12.202 | 12.081 |
| Partial molar volumes in solution (ml/mol) | 611.4 | 703.8 | 801.2 |
| Adiabatic compressibility in aqueous solutions ml (mol−1 bar−1) × 104 | 7.2 | 0.4 | −5.0 |
aNaphthalene group is too bulky for the αCD cavity
The CDs may modify important properties of the drugs through their inclusion complex formation ability. For a long time, only the parent (or major) CDs (α, β, and γCD) were known and well characterized. Subsequently, other CDs have been discovered. CDs containing less than seven units cannot be formed due to steric hindrance while the homologs with nine or more glucose units are very difficult to purify (33,34).
Derivatives
In recent years, many CD derivatives have been synthesized. These derivatives usually are produced by aminations, esterifications, or etherifications of their primary and secondary hydroxyl groups. These CD derivatives and also CD polymers have been prepared to obtain better properties (i.e., better solubility, stability, and inclusion abilities) than those of the parent CDs (35,36). Depending on the substituent, the CD derivatives may change their hydrophobic cavity volume and also solubility, stability against light or oxygen, and help to control the chemical activity of guest molecules in comparison to their parent CDs. In Table II, various CD derivatives are presented (30,37–39). Other type of CD derivatives—the amphiphilic ones—can be obtained by the introduction of long alkyl or fluoroalkyl chains at primary and/or secondary sides of the CDs. These CDs have shown the ability to form monolayers at the air–water interface and supramolecular structures, such as micelles or bilayer vesicles in water and NPs. In many cases, the CD cavities retain their full capacity to include external guests, showing promise for a possible application in drug delivery (21,40).
Table II.
Cyclodextrins Derivatives and their Release Behavior
| Hydrophilic derivatives | ||
| Methylated β-cyclodextrin | ||
| Methyl-β-cyclodextrin | MeβCD | |
| Randomly methylated-β-cyclodextrin | RMβCD (RAMEB) | |
| Dimethyl-β-cyclodextrin | DMβCD (DIMEB) | |
| Randomly dimethylated-β-cyclodextrin | RDMβCD | |
| Trimethyl-β-cyclodextrin | TMβCD | Immediate release |
| Acetylated dimethyl-β-cyclodextrin | DMAβCD | |
| Hydroxyalkylated β-cyclodextrin | Enhanced dissolution and absorption of poorly water-soluble drugs | |
| 2-Hydroxyethyl-β-cyclodextrin | 2-HEβCD | |
| 2-Hydroxypropyl-β-cyclodextrin | 2-HPβCD | |
| 3-Hydroxypropyl-β-cyclodextrin | 3-HPβCD | |
| Hydroxybutenyl-β-cyclodextrin | HBenβCD | |
| 2,3-Dihydroxypropyl-β-cyclodextrin | 2,3-DHPβCD | |
| Branched β-cyclodextrin | ||
| Glucosyl-β-cyclodextrin | G1βCD | |
| Maltosyl-β-cyclodextrin | G2βCD | |
| Glucuronyl-glucosyl-β-cyclodextrin | GUGβCD | |
| Hydrophobic derivatives | ||
| Alkylated β-cyclodextrin | ||
| 2,6-Di-O-ethyl-β-cyclodextrin | DEβCD | |
| 2,3,6-Tri-O-ethyl-β-cyclodextrin | TEβCD | |
| Acylated β-Cyclodextrin | Prolonged release | |
| 2,3,6-Tri-O-acyl(C2–C18)-β-cyclodextrin | TAβCD | |
| 2,3,6-Tri-O-butanoyl-β-cyclodextrin | TBβCD | Sustained release of water-soluble drugs |
| 2,3,6-Tri-O-valeryl-β-cyclodextrin | TVβCD | |
| 2,3,6-Tri-O-octyl-β-cyclodextrin | TOβCD | |
| Ionizable derivatives | ||
| Anionic β-cyclodextrin | ||
| O-Carboxymethyl-O-ethyl-β-cyclodextrin | CMEβCD | Delayed release (pH-dependent release) |
| β-Cyclodextrin sulfate | βCD sulfate | |
| Sulfobutyl ether group-β-cyclodextrin (d.s.4) | SBE4βCD | Immediate release |
| Sulfobutyl ether group-β-cyclodextrin (d.s.7) | SBE7βCD | |
| Cationic β-cyclodextrin | ||
| Trimethyl-ammonium-β-cyclodextrin | TMAβCD | Prolonged release |
| Drug–CD conjugate | Delayed release (site-specific release) | |
d.s. degree of substitution
Complexation
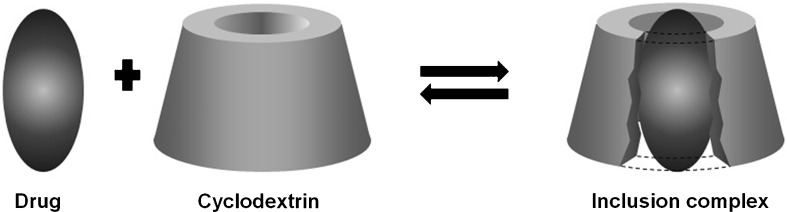
In an aqueous solution, the slightly apolar CD cavity is occupied by water molecules of high enthalpy (polar–apolar interaction). These water molecules can be readily substituted by appropriate “guest molecules” which are less polar than them (Fig. 2). The complexation process is most frequently a 1:1 host/guest ratio, however 2:1, 1:2, 2:2, or even more complicated associations, and higher-order equilibrium exists, almost always simultaneously (4).
Fig. 2.
The interaction of a drug with a cyclodextrin to form an inclusion complex
Applications
The most interesting property of CDs is their ability to form inclusion complexes with a large variety of apolar and hydrophobic molecules. In some cases, only a portion of the molecule is included (4). CDs have been used in pharmaceutical formulations in order to enhance the solubility, dissolution rate, stability, bioavailability, and oral absorption or to modulate biological activity of drugs (41–43). In addition, CDs have been also used to reduce or prevent GI and ocular irritation, to reduce or eliminate unpleasant smells or tastes, to prevent drug–drug or drug–additive interactions, or to convert oil and liquid drugs into microcrystalline or amorphous powders (8,44). Moreover, these substances are also used to modify the drug release from different systems: The hydrophilic CD derivatives such as 2-HPβCD and SBEβCD are used for improving solubility and dissolution rate of poorly water-soluble drugs (45), while hydrophobic CD derivatives such as ethylated and acylated CD are used as slow-release carriers for water-soluble drugs (46). Furthermore, advanced controlled-release profiles can be achieved by a combination of CD derivatives and pharmaceutical additives (47). It is noteworthy that the CDs toxic effects can be minimized by appropriate choice of the CD or its derivative to the administration route (4,31).
RELEASE BEHAVIOR
For better understanding, the drugs release behavior is convenient considering the Biopharmaceutics Classification System that is divided into four classes regarding the expectations on the in vitro–in vivo correlations: class 1—high solubility–high permeability, class 2—low solubility–high permeability, class 3—high solubility–low permeability, and class 4—low solubility–low permeability (48,49).
The relative contribution of the different mechanisms involved in the drug release leads to a change in their dissolution profiles. The influence of CDs derivatives on the drug release place and/or time profile is presented in Table II (24). Some selected studies where the CDs have been used to modify the release of drugs will be presented below.
Immediate Release
All drugs that have poorly water solubility (classes 2 and 4) may improve this property through complexation with hydrophilic CDs or their derivatives and consequently their dissolution rate enhancement. Several examples are shown in Table III. Thus, the inclusion complexes can be used for immediate release applications allowing the drug to dissolve in the GI contents, without delaying or prolonging the dissolution or absorption of drugs (24,50).
Table III.
Examples of the Increase in Solubility by the Use of Cyclodextrins and Their Derivatives
| Cyclodextrin | Drug | Therapeutic group | Other enhanced effect | Observation (ref) |
|---|---|---|---|---|
| HPβCD | Alkannin/shikonin enantiomer | Antioxidant | In vitro permeability, photostability | (113) |
| βCDs and derivatives | Naringenin | Antioxidant | Combined with pH variation (114) | |
| γCD > αCD and βCD | Coenzyme Q10 (CoQ10) | Antioxidant | Higher C max and AUC in adult male and female (115) | |
| βCD < HPβCD | Resveratrol | Antioxidant | Antioxidant activity in free form has little difference with Res in complexed form (116) | |
| HPβCD, βCD | dl-alpha-Tocopheryl acetate | Antioxidant | Water penetration, wettability HPβCD | βCD does not solubilize the drug; drug–HPβCD at 1:2 molar ratio (117) |
| HEβCDs, HPβCDs | Naproxen | NSAID | βCD derivative in the 1:1 molar ratio; similar solubilizing effect (118) | |
| βCD, MeβCD | Ketoprofen ibuprofen naproxen | NSAIDs | MeβCD is the most effective amorphizing agent for ibuprofen and ketoprofen; ibuprofen shows comparable dissolution rates from IB-βCD and IB-MeβCD (119) | |
| MeβCD | Ibuprofen | NSAID | Improved wettability | Complex increased dissolution rate was attributed to its amorphous nature (120) |
| αCD, βCD, γCD | Meloxicam | NSAID | Drug hydrophilicity may be due either to the formation of inclusion complexes or to a highly homogeneous assembly between the CD and the drug in the solid state (121) | |
| SBEβCD, TMAβCD, βCD, MeβCD | Naproxen | NSAID | All SBEβCD products exhibited significantly better dissolution properties than the TMAβCD corresponding ones (38) | |
| βCD | RS(±) Ibuprofen | NSAID | Bioavailability increase and minimizing the drug dose | Dissolution rate coefficient and the dissolved amount of the complex after 75 min was higher than that of unprocessed drug and of its untreated physical mixture (122) |
| SBE7βCD, βCD | Ketoprofen | NSAID | SBE7βCD is more effective solubilizing agent for drug than βCD (123) | |
| βCD MeβCD | Ketoprofen | NSAID | Synergistic effect by combined use of phospholipids and CDs (124) | |
| βCD γCD | Piroxicam | NSAID | Complex was found to be significantly higher than that of the physical mixture (125) | |
| βCD | Ibuprofen indomethacin | NSAIDs | Water amount is not important to obtain complexes if the drug intrinsic solubility is not modified by complex formation (126) | |
| βCD | Meloxicam | NSAID | NaOH was employed to increase βCD solubility to achieve uniform coating (127) | |
| βCD | Ketoconazol | Antifungal | No differences between binary and multicomponent complexes from the same molar ratio (1:2) at pH 5; at pH 6, inclusion complex formed in presence of HCl lead to higher drug solubility (128) | |
| βCD and its amorphous, MeβCD | Econazole | Antifungal | MeβCD complexes exhibited better performance than those with βCD (129) | |
| HPβCD | Itraconazole | Antifungal | (130) | |
| βCD | Oxazepam | Benzodiazepine | (131) | |
| βCD, HPβCD | Bromazepam | Benzodiazepine | (132) | |
| SBEβCDs | Danazol | Steroid | SBEβCDs (with different d.s.) (133) | |
| HPβCD (SBE)7mβCD | Phenytoin | Antiepileptic | (134) | |
| HPβCD, MeβCD, HPγCD | Phenytoin | Antiepileptic | (135) | |
| HPβCD | Bropirimine | Antiepileptic | Bioavailability improved | (136) |
| βCD | Atenolol | Antiepileptic | (137) | |
| HPβCD | Oxcarbazepine | Antiepileptic | Blend of HPβCD with NaCMC (138) | |
| DMβCD | Paclitaxel | Anticancer | The mostly effective was 2.3 mM in a 0.1 M 2,6-DMβCD aqueous solution (139) | |
| βCD, γCD | Artemisinin | Anti-parasites | Drug higher rate and extent of absorption compared to reference preparation using 12 healthy human volunteers (140) | |
| αCD, βCD γCD, HPβCD, SBEβCD, DMβCD, PMβCD, G1βCD, G2βCD | Artemisinin | Anti-parasites | α < γ ≤ βCD G2βCD ≈ G1βCD ≈ PMβCDb ≈ βCD < HPβCD < SBE7βCD < DMβCD most effective CD was DMβCD (141) | |
| βCD | Carvedilol | Beta blocker | (142) | |
| βCD | Warfarin | Anticoagulant | Preparation method and pH influence the βCD ability to include the drug (143) | |
| βCD, HPβCD | Theophylline | Respiratory diseases | HPβCD does not influence drug solubility probably due to the bulky OH groups (144) | |
| HBenβCD | Saquinavir | Antiretroviral in HIV therapy | Enhance the bioavailability | (39) |
| βCD, HPβCD, DMβCD | Tadalafil | Erectile dysfunction | Drug dissolution was markedly enhanced (>75% in the first 5 min) (145) | |
| αCD, βCD, γCD, HPβCD, DMβCD, TMβCD | Azadirachtin | Anti-malarial, anti-tuberculosis, anti-worms, antifungal | High bioactivity low toxicity | Herbal medicine/healthcare products (146) |
| βCD, HPβCD | Rhein | Antiviral, anticancer | (147) | |
| HPβCD | Celecoxib | Anticoagulant | Evaluation of the effect of hydrophilic polymers on the complexation and solubilizing efficiencies of HPβCD (148) | |
| HPβCD SBEβCD | XK469a PPA | Anticancer | Synergistic effect of pH adjustment with cosolvency, micellization, or complexation (149) | |
| HPβCD | Sulfisoxazole | Antibacterial | Solubilization can be improved by addition of the triethanolamine to the complexation medium or by ionization of the drug through pH adjustments (150) | |
| HPβCD | Tanshinone IIA | Antibacterial, antitumor, anti-inflammatory | In vitro permeability | (18) |
2-Phenoxy-propanoic acid
a2-[4-[(7-Cloro-2-quinoxalinyl)oxy]phenoxy]propanoic acid
b2,3,6-Partially methylated-βCD
Modified Release
This term is defined in the European Pharmacopoeia (51) as a modification of the rate or place at which the active substance is released. Modified release products cover a wide range of release models, the principal types of which would include “delayed-release” and “prolonged-release” products.
Prolonged Release
Most of the slow-release preparations have been aimed at achieving zero-order or pH-independent release of drugs to provide a constant blood level for a long period of time; this kind of formulation has many advantages, such as reducing the frequency of dosing, prolonging the drug’s efficacy, and avoiding the toxicity associated with the administration of a simple plain tablet; for this purpose, hydrophobic CDs, such as alkylated and acylated derivatives, are useful as slow-release carriers for water-soluble drugs (classes 1 and 3) (22,24,50,52).
Conventional
It was shown that the freeze-drying method is simple and suitable for obtaining an inclusion complex of salbutamol with βCD and ethylated-βCD (Et-βCD). The different sustained-release behaviors obtained suggest that both the solubility and the dissociation of the complexes are responsible for the dissolution rates of the drug. The results indicate that Et-βCD may be considered as a good carrier for the sustained release of salbutamol (53).
The pharmaceutical application of triacetyl-βCD (TAβCD) as a slow-release carrier for flufenamic acid (FA) was evaluated. An initial high-plasma peak concentration does not occur after the administration of FATAβCD complexes when compared with other excipients. The FATAβCD complex used appears to be appropriate for practical clinical applications that would result in reduced side effects of the drug and prolonged action (20).
The combination of hydrophilic CDs such as HPβCD and hydrophobic CDs such as perbutanoyl-βCD (TBβCD) has been analyzed as controlled-release systems for extremely hydrophilic drugs (captopril, Cap). The binary system of CapHPβCD or CapTBβCD and the ternary system of CapTBβCD/HPβCD were prepared. The release rate of drug from the binary HPβCD system was rather fast, whereas that from the binary TBβCD system was comparatively slower. The retarding effect was dependent on the amount of hydrophobic CD and was attributable to a gel formation of HPβCD in the TBβCD hydrophobic matrix. During this period, a viscous gel layer of the hydrophilic CD gradually forms on the surfaces or inside the tablets, and at a later stage of the release, the drug or the complex may be released through diffusion of the gel layer. This release rate can be controlled by adjusting the relative molar ratio of both components (54).
The controlled-release of metoprolol (Met), a water-soluble β1-selective adrenoreceptor antagonist, by means of a combination of HPβCD and a hydrophobic polymer, ethylcellulose (EC) was reported. The release rate of Met is HPβCD amount dependent, when formulated as complexes in the EC tables, i.e., the release rate decreased when appropriate amounts of HPβCD were added to hydrophobic EC matrix. This rate decrease may be attributable to a gel formation at high concentrations of HPβCD. HPβCD is widely used as a solubilizer and a fast-dissolving carrier for poorly water-soluble drugs. However, the present study showed that it may work as a retarding carrier for the release control of water-soluble drugs such as Met (55).
Fernandes et al. studied the nicardipine release rate using hydrophilic (HPβCD) and hydrophobic (TAβCD) complexes. They observed that required sustained period could be controlled by adjusting the mixing ratio of its complexes, pointing out the importance of the carrier choice. This study showed that both CD inclusion complexes achieved prolonged action, improved bioavailability, and reduced side effects after oral administration in rabbits (25).
The piroxicam (PX) following complexation with HPβCD was used to develop a controlled-release formulation for buccal administration. Matrix tablets containing the PXHPβCD complex and swellable hydrophilic polymers, such as hydroxypropylmethyl cellulose (HPMC) and Carbopol (C940), were prepared. Their release profiles were evaluated and showed the faster PX release (in vitro) when compared to those containing a physical mixture or “free” drug. The PX behavior from the polymer matrices in a constant mode over the passage of time was characterized by prolonged effect. The differences observed in release rates of PX from the all tablets could be attributed to the presence of the polymers and inclusion complexes (56).
The CD was also studied in relation to its relative contribution in the different mechanisms involved in the drug release from tablets produced with a wide range of HPMC proportions. The results showed that the βCD and HPβCD strongly determine the dissolution rate of drugs due to inclusion complexes formation. For some HPMC and drug ratios was observed that low CD concentrations promote drug diffusion due to the decrease on drug interactions with the polymer, while the opposite effect was observed for high CD concentrations (free CDs increase the tortuosity of the diffusional path). CD solubilizing capacity plays an important effect on hydrophobic sulfamethizol release profile, where the release rate was greater for HPMC matrix tablets containing CDs. In contrast, the delay in the hydrophilic diclofenac (Dic) release profile may be attributed to the hindering effect of free CDs on drug diffusivity. The simplex centroid design used to prepare the tablets allowed to obtain the second-order canonical equations, which are particularly useful to predict the release behavior of tablets containing mixtures of HPMC, CD, and lactose (57).
Dic, as poorly soluble-free acid (DicH) or freely water-soluble sodium salt (DicNa), was analyzed following addition of the hydrophilic CD (HPβCD) in erodible hydrophilic poly(ethyleneoxide) matrices. This study, intended for oral drug delivery, has demonstrated that this incorporation affects drug release behavior and transport properties through artificial and biological membranes. Their modulator effect is observed not only on drug dissolution rate but also on environment where drug release occurs (aqueous medium, membrane interface) (58).
The behavior of the vinpocetine (VP), a poorly soluble base-type drug, following multicomponent complexation (MCC) with βCD, SBEβCD, tartaric acid (TA), PVP, and HPMC, on the design of controlled-release hydrophilic HPMC tablets was evaluated. The in vitro release profiles were carried out by a pH gradient method. The addition of βCD and SBEβCD, either in MCC or as physical mixture forms, improved the VP release profiles as a consequence of VP complexation, and the tablet formulations with VPSBEβCD-TA and VPβCD-TA MCC showed some variation on the rate and extent of VP dissolved as a result of different solubility performances of both CDs (59).
Hydrogels of different CDs cross-linked with ethylene glycol diglycidyl ether (EGDE) were prepared. Estradiol presented a higher affinity for βCD, MβCD, and SBβCD than for HPβCD. The affinity for this last CD was greatly enhanced when HPMC (0.25%) was added to the medium and the system was autoclaved. The application of a one-step cross-linking with EGDE, under mild conditions, enabled the formation of MβCD and HPβCD hydrogels. By contrast, the low solubility of βCD and the high anionic character of SBβCD hindered obtaining hydrogels. The estradiol loading was driven by the interaction of the drug with the CD network. The high loading ability of the hydrogels may enable the incorporation of a therapeutic dose of estradiol in a small piece of hydrogel, being the release sustained for several days owing to the affinity of the CD for the drug (60).
In other study with hydrogels consisting of polyvinylpyrrolidone/polyethylene glycol dimethacrylate (PVP/PEG-DMA), the CDs were grafted to the polymer matrix in 4–5% (w/w) in an attempt to retard the release of water-soluble drugs. Vinyl derivatives of α, β, and γCD were produced, added to the prepolymer blend, and cured by UV light. During this curing process, the CD derivatives were covalently incorporated into the hydrogel matrix, which was loaded with ibuprofen by swelling. The release of the model drug from CD modified hydrogels shows that especially covalently bonded βCD can change both the release rate and the release profile of ibuprofen (61).
The interactions between meglumine antimoniate (MA) and βCD induced by heating and freeze-drying were investigated, and their impact on antimony (Sb) absorption, administered by oral route, was evaluated. As a consequence of the slow drug release property of MAβCD nanoassemblies (sustained-release system of MA), following dilution in water, one can expect a change of the drug absorption site in the GI tract, when compared to MA alone or to the heated MAβCD physical mixture (62).
The combination of the metformin hydrochloride (MH) with a hydrophobic CD (TAβCD) and its dispersions in a suitable polymeric matrix (i.e., HPMC, xanthan gum, chitosan, EC, EudragitL100-55, Precirol), varying the relative amounts of MHTAβCD, was effective in adequately modulating the drug release rates. The matrix-tablet formulation containing the 1:1 (w/w) blend of MHTAβCD as co-ground and spray-dried systems fully achieved the prefixed goal. The results showed about 30% released drug after 2 h at gastric pH and overcoming 90% released drug within the subsequent 3 h in jejunal fluid (9).
Floating
The enhancement of the furosemide (FR) bioavailability by prolonging its duration in the stomach via the floating dosage forms with controlled release was evaluated. In this study, the floating dosage forms (bilayer floating tablets) with controlled release were prepared using solid dispersions of FR with βCD in a 1:1 proportion, and both in vivo and in vitro results were studied. The results show that the bioavailability of FR was excessively increased compared to conventional forms and that absorption of FR taken place vastly in stomach and upper part of small intestine (63).
The floating tablets of curcumin βCD (CURβCD) complex were formulated to provide sustained release of drug with an aim to provide an effective therapy with enhanced solubility and bioavailability, targeted action, and better absorption to treat stomach cancer. The formulation CURβCD complex exhibited maximum sustained release of curcumin with excellent floating and swelling properties. Also, in vivo antitumor studies confirmed that the overall rate of tumor incidence and number of tumors/mouse is less in animal group treated with CURβCD complex compared to animals treated with CUR and CURβCD (control II) (64).
The gastroretentive floating effervescent and non-effervescent tablets of silymarin (flavonolignans) were analyzed with purpose to increase the efficacy and stability of the drug in the stomach. The short half-life of the silymarin, poor bioavailability, and faster solubility in acidic medium make it a suitable candidate for gastroretentive drug delivery system. It was concluded that HPMC in combination with crospovidone and microcrystalline cellulose can be promising polymers for effervescent gastroretentive drug delivery system. Swelling studies indicate significant water uptake and contributed to drug release and could be significant in gastric retention. On the other case, non-effervescent floating formulations based on polypropylene foam powder studied for their ability to control drug release over prolonged period of time. The drug release pattern can effectively be adjusted by varying formulation parameters such as concentration of swelling agent and βCD, type of matrix forming polymers, and blend of matrix forming polymers. The developed floating tablets of silymarin may be used for prolonged drug release, thereby improving the bioavailability and patient compliance (65).
Mucoadhesive
The interactions between a cationic polymer hexadimethrine bromide (HDMBr) and an anionic CD, SBEβCD, were investigated, as well as the potential of such a system for mucoadhesive, sustained drug delivery. The results show that interactions between the anionic SBEβCD and the cationic HDMBr can be used to obtain mucoadhesive sustained drug delivery system under certain conditions. Triclosan/SBEβCD/HDMBr complex is better retained on a cation-exchange media than the triclosan/HPβCD/HDMBr complex. The positive charge of HDMBr did not have the expected effect on the buccal mucosa. Thus, it was concluded that although a positive charge is likely to promote mucoadhesion, other polymer attributes, such as molecular weight and viscosity, may have equally beneficial effects (66).
In the continuing challenge to improve the properties of delivery systems, the possibility to modify chitosan by introducing βCD was analyzed, and then the mucoadhesive properties of this synthesized CD polymer were investigated. The use of βCD in combination with chitosan resulted in a polymer which showed substantial mucoadhesive properties, although lower compared to that of chitosan alone. The molecular size of CD–chitosan might lead to a decrease of the interpenetration into the mucus layer with a subsequent decrease in mucoadhesivity. In fact, it is known that, besides molecular weight, mobility and flexibility of polymeric chains play an important role in mucoadhesion. A monosubstituted βCD derivative could be efficiently grafted on chitosan, yielding a CD polymer with promising inclusion and mucoadhesive properties (67).
In another study, a novel thiolated carboxymethyl chitosan-g-β-cyclodextrin (CMC-g-βCD) was synthesized and characterized as mucoadhesive drug carrier. For the drug delivery study, tablets were prepared and the transfection of the hydrophobic model drug, ketoprofen (KP), was investigated as well as the swelling and mucoadhesive properties of the compacts. The swelling may have contributed to the mucoadhesiveness of the thiolated polymers. The KP release results indicate that this system seems to be a very promising vehicle for the administration of controlled release of hydrophobic drugs. The ability of βCD present in the thiolated polymers to form host–guest inclusion complexes with the drug molecules and disulfide cross-linking between the polymer chains could be the main reasons for the slow and steady drug release from thiolated CMC-g-βCD tablets. Perhaps the release of drug may be controlled by swelling followed by a diffusion-controlled mechanism (68).
Other formulations, aimed for buccal delivery, involving binary products containing bupivacaine hydrochloride (BVP HCl), an amide-type local anesthetic, with parent βCD and its soluble epichlorohydrin-βCD polymer (EPI-βCD) were prepared and evaluated as a novel mucoadhesive system. It was possible to properly tailor the drug release rate according to the desired effect, i.e., a rapid onset of anesthetic action for simple dental procedures or a prolonged effect in post surgical procedures or in the treatment of oral mucositis. The inclusion complex with βCD reduced the drug dissolution rate, while the complexation of BVP HCl with EPI-βCD increased the drug dissolution rate (69).
The effect of flufenamic acid (FLU) complexation with HPβCD on its dissolution properties and release rate from buccal film formulations was investigated, with the objective of improving the therapeutic efficacy. Chitosan, a natural cationic polysaccharide consisting in a copolymer of N-acetyl-d-glucosamine and d-glucosamine, was selected as the mucoadhesive polymer for the development of the buccal film formulation. Moreover, it was demonstrated that CD complexation could be a suitable strategy to optimize the drug release feature from the system. In fact, introduction of drug as complex with HPβCD enabled a clear improvement of drug release with respect to the film containing the plain drug, allowing achievement of complete release within 4–5 h, which is considered the usual maximum duration for buccal drug delivery. The main advantage expected from this formulation in comparison with traditional oral therapy is the reduction of drug dose because its direct localization in the inflammation site, with the consequent minimization of potential systemic side effects. Future in vivo studies on human voluntaries are planned to evaluate the actual therapeutic effectiveness of the developed film formulation of FLU, and the effect of different drug loads will be studied (70).
Osmotic Pump
Stella et al. (71–76) developed various studies involving osmotic pump tablets (OPT) and SBEβCD which are described below.
The effect of SBEβCD on the release of poorly water-soluble drugs (testosterone) from therapeutic systems (controlled porosity osmotic pump tablets) showed that CD derivative was capable of increasing the solubility and enhancing the release of the steroid. Moreover, it was proposed that current tablets containing SBEβCD (due to its seven negative charges and seven sodium ions per molecule, in average) may also exhibit osmotically controlled-release properties for drugs (71).
The factors responsible for controlling drug release of the chlorpromazine through the porous membrane were evaluated from release studies. The results suggest that the membrane factors responsible for drug release from OPTs containing cores utilizing SBE7mβCD, as both a solubilizing and osmotic agent, are the amount of micronized lactose and triethyl citrate (TEC), the size of micronized lactose, and the type of plasticizer in the membrane. Moreover, it was also confirmed that the pores in the OPT membranes are formed by leaching micronized lactose into the dissolution medium and by a decreasing plasticizer effect observed with decreasing amounts of TEC. In addition, the change in membrane pores which resulted from all these factors was well indicated by the total membrane surface area per unit weight after the release studies and by the mechanical permeability of the membranes (72).
The osmolality of HP and SBECD solutions over the large concentration range relevant to tablet formulations was measured, and a model capable of describing the osmolality generated in CD solutions as a function of concentration was developed. This study was conducted in water with eight CDs: HPβCD, HPγCD, SBE4mβCD, SBE7mβCD, SBE9mβCD, SBE4mγCD, SBE9mγCD, and SBE12mγCD. Both HPCDs had a total degree of substitution (TDS) of four, and the TDS in the SBECD nomenclature was indicated by the number in subscript, followed by the letter “m” indicating that these materials were composed of mixtures of (SBE)CDs of various degrees of substitution. As expected, it showed that osmolality was directly dependent on the number of particles in solution. This term may be related to the effect of the solute addition on water structure and could be considered as an indicator of the amplitude of deviation from ideality. The unified empirical model applied in this study of the osmotic properties of CDs for use in osmotic pump tablets; it is reasonably for predicted the osmolality of the HP and SBECD solutions solely based on solution concentration and TDS (73).
The role of the SBE7mβCD-to-drug ratio in the OPT core with seven model drugs, including both highly and poorly water-soluble drugs, was studied. SBE7mβCD serves as both a solubility modulator and as an osmotic pumping agent for OPTs, from which the release rate of both water-soluble and poorly water-soluble drugs can be controlled. An appropriate composition ratio was determined for SBE7mβCD to drug (those requiring solubility enhancement) for complete release of drugs over 12 h from the OPTs. For soluble drugs, SBE7mβCD acts primarily as an osmotic and an OPT control agent. Significantly, SBE7mβCD not only enhances the delivery of poorly soluble drugs from OPTs but acts as a controlling excipient for soluble drugs such that the release rate, corrected for tablet surface area, of both poorly soluble and soluble drugs is similar (74).
A controlled porosity osmotic pump pellets (CP-OPP) using SBE7mβCD as a solubilizing and osmotic agent and prednisolone (PDL) as drug was prepared in order to deliver a sparingly water-soluble drug, in a controlled-release fashion. The evaluation of this system includes the factors influencing drug release and the probable mechanisms of drug release. The incorporation of SBE7mβCD significantly improves the solubility and thus the release of PDL through preformed as well as in situ complex formation. The pellet performance can be fabricated to achieve a desired release rate by modifying the molar ratio of SBE7mβCD to PDL, thickness of the microporous coating, and coating composition (e.g., polymer or pore former). In addition, a significant change in the drug release rate with a change in the osmotic pressure difference across the membrane indicates that osmotic pumping may be part of the mechanism of drug release from pellet formulations (75).
The release mechanisms from the CP-OPP containing precomplexed PDL were investigated. The PDL or CD release rate follows zero-order kinetics for up to 30–40% of drug release during the first 1–2 h and nonzero-order kinetics thereafter. The initial zero-order drug release is attributed to the constant driving forces mainly caused by the approximately constant concentration of CD with time. In contrast, the nonzero-order drug release is associated with the combination of more significant diffusion with time as well as the change in concentration of CD resulting in changes in viscosity, diffusivity, and osmotic pressure in the pellet with time (76).
Others
The incorporation of the physical mixtures of SBE7mβCD and a sparingly water-soluble drug into hydrophilic matrices to provide controlled and complete in vitro release, as well as the mechanisms by which SBE7mβCD increases the drug release from HPMC matrix tablets, was investigated. The in situ inclusion complex formation is the main mechanism by which SBE7mβCD improves the drug release rate. Higher medium penetration rate into the SBE7mβCD-containing matrices may also contribute to the improved release rate and is dependent on the ratio of solubility to drug load, on the molar ratio of SBE7mβCD to drug and not the absolute amount of SBE7mβCD (77).
In another work, the HPβCD was used to evaluate its potential to control zolpidem (ZP) release (prolonged-release system) from zolpidem-loaded poly(dl-lactide) or poly(dl-lactide-co-glycolide) microparticles. The release profiles of ZP from these systems showed an initial burst effect, and a subsequent slow ZP release was analyzed (78).
The potential of SBE7βCD together with HPMC or PVP to solubilize and deliver the carbamazepine (CAR) (poorly soluble model drug) from sustained-release (SR) beads was analyzed. SBE7βCD increases the aqueous solubility of CAR as well as HPMC, but not PVP. HPMC acts both alone and synergistically with SBE7βCD. Within a SR bead, SBE7βCD increases the CAR release rate, potentially by enhancing solubility and hence diffusion, while also increasing the osmotic drives through the aqueous ethylcellulose dispersions (Surelease® aqueous pores). The addition of HPMC does not improve the rate of CAR release, but does reduce the ratio of SBE7βCD needed to deliver CAR and therefore has potential advantages over the binary system. Both binary and ternary approaches are considered as suitable techniques to potentially improve the delivery and in vivo bioavailability of poorly soluble drugs, which have previously exhibited slow or incomplete release from SR beads (79).
The γCD derivatives (variation on the alkyl chain length of γCD alkylcarbonates) complexation properties were analyzed, and it was shown that these CDs can improve both the solubility and the release rate of poorly water-soluble drugs. Also it was observed that the combination of various ratios of different alkylcarbonate complexes in a pharmaceutical formulation could enable drug release to be modulated (80).
An innovative particulate system, called “beads” composed of αCD and vegetable oil, safe substances for oral administration, was proposed. The drug used was isotretinoin, a lipophilic molecule, sensitive to heat, oxygen, and light, which was encapsulated in those beads. The results showed that αCD/oil beads were able to efficiently encapsulate and facilitate oral delivery of the drug (81).
The possibility of controlling the release rate of simvastatin (SVA) from SVA/βCD coatings with different pH values was investigated. Results showed that the lower the pH value of the solution, the lower were the release kinetics of SVA, indicating that the release character of SVA could be controlled by adjusting the pH value (82).
In another study, it was assessed whether oral administration of the ethinyl estradiol (EE)βCD clathrate/drospirenone (drsp) formulation affects the bioavailability and pharmacokinetics of EE and drsp. The results showed the relative bioavailability of EE and drsp after the administration of EEβCD clathrate/drsp was comparable with that achieved with EE/drsp or a reference suspension. Therefore, complete in vivo release of EE and drsp was achieved in healthy postmenopausal women following oral administration of EEβCD clathrate/drsp tablets. A βCD clathrate formulation of EE, when combined with drsp, did not influence the relative bioavailability of EE or drsp, or alter the pharmacokinetic profile of these hormones (83).
The potential of βCD to control drug release from poly[methylenebis(acrylamide)aminoethylpiperazine] (HPMA) was investigated, and the in vitro release behavior of anticancer chlorambucil (CLB) from these hyperbranched drug carriers was studied. The βCD introduction into HPMA showed that the initial burst release was slowed down. Furthermore, the release rate of CLB from HPMA-βCDs increased with decreasing βCD content, and the corresponding cumulative amounts of CLB released were between 42.3% and 66.0% for 10 h. This result indicates that the CLB release from the HPMA-βCDs is a function of βCD content, and the presence of βCD in HPMA retarded the CLB release rate. Thus, the controlled-release experiments showed that the presence of βCD can appropriately slow CLB release from HPMA-βCDs and adjust the ratio of CLB released in relation to the total drug load (84).
Highly structured and porous poly(methyl methacrylate) (PMMA) membranes functionalized with HPβCDs, impregnated with ibuprofen using scCO2 in batch mode, were tested as controlled drug delivery devices by in vitro tests into buffer solution at pH 7.4, and the drug release data were modeled using a mathematical theory based on Fick’s second law of diffusion. The introduction of CDs physically entrapped in the PMMA matrix leads to an increase of drug release to the buffer solution at pH 7.4 when compared to the neat PMMA membrane. The supercritical fluid-assisted membrane formation is a greener alternative to produce porous polymeric matrixes containing CDs, with improved characteristics such as enhanced drug loading, controlled drug release ability, which might have promising biomedical applications (85).
Delayed Release
An enteric preparation can be classified as time-controlled release, since the drug is preferentially released in the intestinal tract; hydrophobic excipients having a weak acidic group are preferable because they are less soluble in water at low pH, but soluble in neutral and alkaline regions due to the ionization of the acidic group; under the control of this pH dependence, the delayed-release dosage form, which passes from the stomach into the higher pH environment of the upper small intestine, would experience increased drug release; for this purpose, CMEβCD has been developed to exhibit pH-dependent solubility for use in selective dissolution of the drug–CD complex (24,50). The release property of the CD conjugates can be classified as a delayed release (30).
Colon Specific
The oral administration of PDL—a typical glucocorticoid with significant systemic side effects (higher PDL plasma concentrations)—was compared with its CD conjugate. The results showed that PDLsuc–αCD conjugate (PDL prodrug) is useful as a delayed-release system. Thus, their application is advantageous for colon-specific delivery due to the decrease of the systemic side effect, while maintaining the therapeutic effect (86).
Other authors also have designed CD conjugates for delayed-release systems; the KP, a NSAID, was covalently bound to one of the αCD’s primary hydroxyl groups. The release behavior of the resulting conjugate was investigated, in vitro and using rats. The repeated- and prolonged-release systems based in the CD conjugate also were prepared. The repeated-release system of KP was prepared by combining the CD conjugate with a fast-release fraction such as the KPHPβCD complex. The prolonged-release system of KP was prepared by combining the CD conjugate with a slow-release fraction such as the KP-EC solid dispersion. These CD conjugate-based approaches may be useful for controlled release (30). Formation of prodrugs has improved the delivery properties of the parent drug molecule (87–89).
Activated by the microbial enzymes in the large intestine, 5-aminosalicylic acid (5-ASA) is released from CD-5-ASA at a controlled rate. These results showed the oral administration of 5-ASA gives much higher plasma and urine concentrations of 5-ASA than CD-5-ASA, and the conjugate can lower the plasma concentration of 5-ASA. The lower concentration is attributable to the passage of the conjugate through the stomach and small intestine without significant degradation or absorption, followed by the degradation of the conjugate site-specifically in the cecum and colon (87).
The release behavior of the 5-fluorouracil-1-acetic acid (5-FUA)–βCD conjugate, through ester or amide linkage, was investigated in enzymatic solutions and rat cecal contents. The results have shown that the 5-FUA–βCD ester conjugate may survive passing through stomach and small intestine, and the release of 5-FUA occurs preferentially in cecal and large intestinal tracts of rats after the fermentation of βCD to small oligosaccharides (88).
Others
The in vivo release behavior of anti-inflammatory drug (4-biphenylylacetic acid, BPAA) from its CD ester- and amide-type prodrugs in rat intestines, together with the anti-inflammatory effect after oral administration of the prodrugs, was evaluated. The results suggest that CD/drug prodrugs can survive passage through stomach and small intestine, and drug release is triggered by enzymatic degradation of the CD ring in the colon. The ester prodrugs, particularly the α and γCD forms, are subject to ring opening followed by hydrolysis to the maltose and triose conjugates. The ester bond of the small saccharide conjugates is subsequently hydrolyzed to form BPAA which is absorbed from the rat cecum and colon. On the other hand, the amide prodrugs are hydrolyzed to small saccharide conjugates, but the amide bond resists the hydrolysis and thus resides as the maltose conjugate in these tracts. This type of drug release is essentially classified as delayed release with a fairly long lag time (89).
Physic and pharmaceutical properties of CD conjugates in which a drug is covalently bonded to the CD may be significantly different from those of the inclusion complexes. The preparation of conjugated ibuprofen (IB)– and indomethacin (IN)–βCD as prodrugs and the preparation of linked poly(ethylene glycol)–βCD in conjugation with IB or IN were reported. The hydrolysis of CD linked to poly(ethylene glycol) (Mw = 600) containing IB and IN was studied in aqueous buffer solutions at physiological conditions. In comparison, the hydrolysis of IB in this prodrug/drug compound at the first 5 h is higher than IN (28).
The binding of the bisphosphonate-derivatized βCD conjugate to hydroxyapatite (HA), its complexation with dexamethasone (Dex), and the in vitro drug release profile of the complex immobilized on HA surfaces were investigated. The βCD-based delivery system alendronate-β-cyclodextrin conjugate (ALN-βCD) shows very strong binding to HA, and it formed a molecular inclusion complex with Dex (1:1). In an in vitro release study, the ALN-β-CD/Dex complex bound to HA could gradually release Dex upon repeated extraction with phosphate buffer saline. Potentially, this delivery system may be formulated into a topical formulation with different therapeutic agents and applied in clinical treatment of a variety of diseases in the oral cavity (90).
The capacity of CDs polymers to modulate (to enhance or to retard) the release of a model drug (diflunisal, DF) from HPMC matrices was studied: The incorporation of DFβCD inclusion complex enhanced the release of the drug when compared with its physical mixture. In this study, it was also possible to modify the drug release from HPMC matrices by incorporating βCDPs with different solubilities; soluble polymers promoted drug release while insoluble ones delayed it. This fact can be explained mainly by inclusion of the drug within the CD cavities which is present in the polymeric structure (91).
NANOTECHNOLOGY
More recently, CDs have also been used in nanotechnology as drug delivery and carrier systems (10,46,92). Various studies have been developed in this area involving CDs in nanometric particles in order to design versatile delivery systems that can encapsulate drugs with different physicochemical properties (i.e., hydrophilic and hydrophobic). The most popular formation of the NPs containing CDs consists of the incorporation of previously formed drug–CD inclusion complexes into polymer NPs. Thus, the presence of CDs generally results in an increase in lipophilic or hydrophilic drug loading. Other nanocarriers may result from the design of amphiphilic CDs that self-assemble in the form of stable NPs (21,93) and also from the design of CD conjugates that can transport the drug encapsulated or connected to the block (10,30,46). The CDs may be excellent building blocks for the synthesis of safe and water-soluble polymers for drug delivery. However, due to the multi-functionality of CD molecules, most CD-containing polymers that have been extensively studied are heavily cross-linked. The structures of these polymers are complex and make their characterization difficult (94).
Nanoparticles
Solid lipid nanoparticles containing hydrocortisone and progesterone alone or hydrocortisone and progesterone/βCD or HPβCD complexes were prepared, and the release of both drugs was lower when they were incorporated as inclusion complexes than as free molecules after oral administration (95). Another study for the same route of administration tested two useful techniques for NP preparation using CDs in the design of colloidal carriers. The first possibility consisted on increasing the loading capacity of poly(isobutyl cyanoacrylate) nanospheres prepared by anionic polymerization, employing HPβCD, and the second possibility consisted on the spontaneous formation of either nanocapsules or nanospheres by nanoprecipitation of amphiphilic CD diesters. The new amphiphilic derivatives do not need any polymerization process to form NPs, nanocapsules, or nanospheres presenting a high loading capacity of the lipophilic or even hydrophilic drugs (96). In order to improve the aqueous solubility and bioavailability of ONO-8713, the method of co-grinding using CDs and CD derivatives in an attempt to prepare crystalline NPs was applied. The results show that this method could be applied effectively as a NP preparation method for poorly water-soluble drugs, being the amount of water used, the CD content in the mixture, and the interaction between drug and CD significant factors for the NP formation process (97). Some of the most successful cationic polymers bear CD rings linked together by cationic spacer chains. The novel per(6-guanidino-6-deoxy)CDs display very strong binding toward phosphorylated and very weak binding toward non-phosphorylated guest molecules. Furthermore, they interact with DNA and actually compact it into NPs, a basic requirement for its entry inside cells. It was clearly shown that guanidinylated CDs bind tightly to phosphorylated substrates. Therefore, investigation of the binding behavior toward DNA was considered, as shown recently, that amino CDs can be used in DNA transfection studies (98). Other authors studied the interaction of the isophorone diisocyanate with HPβCD showing that the enhanced molar ratio of isophorone diisocyanate to HPβCD could increase the reaction ratio between the hydroxyl groups of HPβCD and isocyanate groups of isophorone diisocyanate. An increase on the cross-linked polymeric network formation and its cross-link degree was also demonstrated. This fact might reduce the spaces in cross-linked polymeric matrices for the diffusion of drug (nimodipine, NI) and consequently to increase the entrapment efficiencies of nanocapsules decreasing the NI release rate. HPβCD-based nanocapsules showed a slower NI release rate when compared to chitosan-based nanocapsules due probably to the fact that the drug can be included into the HPβCD cavity increasing the molecular interactions between them (99). CDs have also been used in NPs to overpass problems resulting from proteins oral administration. The very low bioavailability of these substances results mainly from their susceptibility to proteolysis and inability to cross biologic membranes. This problem can be solved through CD complexation thus improving the protein therapy by stabilizing them against aggregation, thermal denaturation, and degradation. Proteins hydrophilic characteristics and size may be a problem in the inclusion process. However, inclusion of the hydrophobic side chains into the βCD cavity leads to the formation of non-covalent inclusion complexes, and CDs’ ability to sequester hydrophobic moieties helps in improving the stability of proteins. It was also observed that the NPs displayed good insulin encapsulation efficiency and pH-dependent release profile at acidic/alkaline conditions without changing their biological activity (100). Zhang et al. reported that alginate/chitosan NP system exhibited an effective protection and controlled the release of insulin complexes with cationic βCD polymers (CP-βCDs). An oral insulin formulation of CP-βCD–insulin-loaded alginate/chitosan NPs has shown a significantly higher (40%) cumulative insulin release in simulated intestinal fluid than that without CP-βCDs (18%). This fact can be explained because the insulin was mainly retained in the core of the NPs and well protected against degradation in simulated gastric fluid (101). The preparation, characterization, and evaluation of the NPs formed by the copolymer of methyl vinyl ether and maleic anhydride (Gantrez® AN) and CDs, including βCD, HPβCD, and 6-monodeoxy-6-monoamino-β-cyclodextrin (NHβCD), were also described. The incorporation of CDs in Gantrez® AN NPs increased the bioadhesive capacity of poly(anhydride) NPs. This fact may be related with the high content of hydroxyl groups in the resulting NPs which can increase the possibilities of these carriers to interact with mucosa components by means of hydrogen bonds. In addition, the high bioadhesive capacity for 2-hydroxypropyl-β-cyclodextrin–poly(anhydride) NPs (HPβCD-NP) and NHβCD–poly(anhydride) NPs (NHβCD-NP) could be related with their rough morphology and, thus, a higher specific surface than for smooth NPs. Finally, from in vivo imaging studies, it was observed that the HPβCD-NP remained in the gut not showing any evidence of translocation of distribution to other animals organs (male Wistar rats) (102). The paclitaxel (PTX) from PTXβCD NP and PTXHPβCD NP (poly(anhydride) NPs) shows high relative oral bioavailability (>80%) while for PTXNHβCD NPs the oral bioavailability was found to be 18%. This important difference observed when aminated CD was used can be explained by two reasons: (1) The presence of amino groups would decrease or suppress the inhibitory effect over the P-glycoprotein that has been observed for CDs and (2) the rapid release of an important fraction of PTX (about 70% in less than 30 min) from NPs of PTXNHβCD when dispersed in a stomach simulated fluid. The results corroborate the synergistic effect obtained by the combination of bioadhesive NPs and CDs on the oral bioavailability of PTX. In addition, when the PTX was encapsulated in poly(anhydride) NPs as complex with either HPβCD or βCD, the relative oral bioavailability of this anticancer drug was calculated to be higher than 80% (103). The addition of relatively small amounts of the water-soluble HPγCD to aqueous γCD formulations increases the complexation efficiency (CE) of γCD and reduces the turbidity of γCD solutions. It was hypothesized that the observed effect was somehow related to formation of γCD and HPγCD NPs. Thus, the purpose of this work was to investigate further the solubilizing effects of γCD, HPγCD, and γCD/HPγCD mixtures and their formation of nanosize aggregates. The results show that the mixtures of γCD and HPγCD have synergistic solubilizing effects on hydrocortisone and Dic. Both indomethacin and amphotericin B have very low affinity for γCD and HPγCD as observed by their very low CE value, indicating that only about 2–7% of the CD molecules in the solution are forming complexes with these drug molecules. The parent αCD, βCD, and γCD; their derivatives, such as HPβCD and HPγCD; and complexes self-assemble to form nanoscale aggregates in aqueous solutions. At CD concentration of about 1% (w/v) or lower, the relative mass contribution of these aggregates is less than 0.01%, but it gradually increases with increasing CD concentration up to about 5–10% (w/v) when all increase in dissolved drug/CD complexes is in the form of CD aggregates (104).
Conjugates
A new class of CDs conjugated to polymers through covalent bounds has been synthesized as nontoxic vectors for the delivery of macromolecules. Some examples are described below.
βCD-based linear polymers extremely soluble in aqueous solution and show low toxicity to cultured cells were synthesized and used for the conjugation of camptothecin (CPT). Conjugation increased the CPT solubility by more than 3 orders of magnitude. These polymer–CPT conjugates have been tested in a xenograft mouse model. Initial tumor reduction studies reveal that these conjugates have enhanced efficacy over CPT alone and one of the commercial analogs of CPT (irinotecan). By comparison, the CD-containing polymer conjugates of CPT gave median tumor sizes that ranged from 10% to 12% of the median size of the tumors in the control group (94). CD derivatives conjugated with saccharide(s) were synthesized in order to be drug carrier molecules capable of specific cell recognition and might be useful as targeting drug delivery systems. It was possible to synthesize mono-glucose-branched CDs, which had an appropriate spacer between the βCD and a glucose moiety. These mono-glucose-branched CDs indicated significantly high association constants for doxorubicin (DXR) in the range of 105–106 M−1. The introduction of an appropriate spacer can also enhance the CD’s inclusion ability for drugs having an aromatic ring like DXR (105). Innovative system resulting from the association between a CD polymer (polyβCD) and amphiphilic cationic connectors was developed. The results showed that CDs polymers (polyβCD) complexed with adamantly cationic derivatives (Ada) constitute novel and efficient nonviral vectors for gene delivery (106). Porphyrin–βCD conjugate was used for PTX delivery and porphyrin–γCD conjugate for DXR transport. This study presented a novel versatile supramolecular “Lego system” suitable for the combined cancer therapy, which is based on the effective drug binding as inclusion complexes with porphyrin–CD carriers and on known tumor targeting by porphyrin macrocycles (10). Davis’s group has successfully conjugated low molecular weight polyethylenimine (PEI; Mw 600) with βCD. The resultant PEI600–CD polymeric conjugates (PC) with the molar ratio of 1:1 are capable of mediating efficient gene transfection in cultured neurons and in the central nervous system. Incorporating anticancer drugs onto PEI600–CD conjugates may endow them with new and interesting properties for great applications in combination therapy. A new locally injectable bifunctional delivery system (FU-PEI600–CD, FPC) of an anticancer drug and a gene was developed by combining PEI600–CD (PC) and 5-fluoro-2′-deoxyuridine (FdUrd). The effects of the developed FPC on cancer cells were systematically investigated through a series of experiments including cancer cell viability, invasion, migration, cell cycle, apoptosis, and uptake of FPC. In addition, in vitro and in vivo gene transfection was also conducted. In comparison with an equivalent dose of FdUrd, the antitumor therapeutic effects were largely enhanced by FPC, which still exhibited good gene expression efficiency. Thus, the prepared bifunctional FPC composed of βCD, low molecular weight PEI, and FdUrd showed improved bioavailability, significantly stronger ability to induce apoptosis, and more efficient cellular uptake than equivalent dose of free FdUrd. Such bifunctional FPC with good ability to deliver drugs and transfer genes may have great potential applications for combination therapy of glioma cancer (107).
Other Delivery Systems
The efficacy of carboplatin (Carb) or 5-fluorouracil (5-FU) on MCF-7 and MDA-MB 231 breast cancer cell lines, either alone or in combination, was improved after MeβCD pretreatment. The results obtained suggest that MeβCD pretreatment enhances the susceptibility of breast cancer cells toward low doses of Carb or 5-FU by increasing the intracellular accumulation of these drugs. These data provide a biological basis for the potential therapeutic application of MeβCD in cancer chemotherapy in combination with other conventional cytotoxic drugs (108). The design and in vitro and in vivo evaluations of polypseudorotaxanes of pegylated insulin with CDs as a sustained-release system was reported. The pegylated insulin forms polypseudorotaxanes with α and γCD in a similar manner as poly(ethylene glycol). The polypseudorotaxanes prepared are less soluble in water, and the release rate of the pegylated drug can be controlled by changing the threading and dethreading rates of the polypseudorotaxanes by adjustment of administration conditions such as amount of injection and concentration of CDs in the medium (109). Later a new slow-release system of pegylated lysozyme/CD polypseudorotaxanes was developed. This technology suggests that it may be applicable as a new method of pegylated proteins, peptides, and low molecular weight drugs slow-release systems. For that, PEG (Mw 2,200) chains were introduced into lysozyme molecules and formed polypseudorotaxanes with α and γCD, by inserting one PEG chain in the αCD cavity and two PEG chains in the γCD cavity. The results demonstrated that the pegylated lysozyme/CD polypseudorotaxanes were less soluble in water, and the release rate of the pegylated protein decreased in the order of the pegylated lysozyme > the γCD polypseudorotaxane > the αCD polypseudorotaxane (110). The formation of the complex constituted by genistein (Gen) with the modified amphiphilic CD SC6OH at 1:1 molar ratio in water medium demonstrated that Gen molecules are shown to be not included inside the SC6OH cavity but to interact with the side chains of SC6OH nanoaggregates. The lack of inclusion of the guest molecules inside the host macrocycle was confirmed, in solid phase, by monitoring the significant differences in the spectral features of the FTIR–ATR spectrum of complex. The evidences found in this study shed light on the complexation of drugs in host nanocarriers at solid phase and open the way to detect the supramolecular interactions in complexed species host/drug and host/drug/receptor in targeted drug delivery (111). Quaternization of βCD grafted with chitosan (CD-g-CS) using glycidyl trimethyl ammonium chloride leads to water-soluble CD-g-CS derivative (QCD-g-CS). All QCD-g-CSs showed self-aggregates behavior in water while quaternized native chitosan did not. The surfactant-like or micelle-like structures of QCD-g-CS are firstly presented with the βCD moiety inner core and the quaternary ammonium moiety outer shell. Atomic force microscopy and transmission electron microscopy images revealed that self-aggregates of the QCD-g-CS were spherical shape. It was found that the particle sizes increased with an increasing in degrees of substitution (βCD moiety) into the CS backbone, leading to large aggregation in water. The stronger mucoadhesive strength, the higher degree of quaternization (DQ), zeta-potential, slope value, and the lower critical concentration value were observed. In this study, the DQ played an important role on mucoadhesive property while the cytotoxicity did not correlate with DQ (112).
CONCLUSION
This review highlights the beneficial applications of the CDs and their derivatives on the solubility/dissolution rate and bioavailability enhancement of poorly water-soluble drugs. Their ability for immediate and modified (to prolong or to delay) release of drugs from solid dosage forms, either as complexes or as nanotechnology carriers, is also demonstrated. CDs may also enhance drug absorption across biological barriers. The increased number of CD derivatives/carriers that have been synthesized up to now demonstrates the CD versatility on the oral dosage form formulations. The research accomplished that has contributed for the great success of those formulations may be the base of new achievements on oral controlled release. Although a high number of studies using CDs and their derivatives have been reported, there is plenty of room for new discoveries and applications on controlled release of oral pharmaceutical dosage forms especially at the nanotechnology level and on the delivery of peptides and proteins. CDs may be a useful strategy in life cycle management of medicines.
REFERENCES
- 1.Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem. 2008;62:23–42. doi: 10.1007/s10847-008-9456-y. [DOI] [Google Scholar]
- 2.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132:153–63. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Rupp C, Steckel H, Müller BW. Solubilization of poorly water-soluble drugs by mixed micelles based on hydrogenated phosphatidylcholine. Int J Pharm. 2010;395:272–80. doi: 10.1016/j.ijpharm.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Szejtli S. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–53. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75. doi: 10.1016/j.drudis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Xiong J, Guo J, Huang L, Meng B, Ping Q. The use of lipid-based formulations to increase the oral bioavailability of Panax notoginseng saponins following a single oral gavage to rats. Drug Dev Ind Pharm. 2008;34:65–72. doi: 10.1080/03639040701508292. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Sun C, Hao Y, Liang T, Zheng L, Wang S. Mechanism of dissolution enhancement and bioavailability of poorly water soluble celecoxib by preparing stable amorphous nanoparticles. J Pharm Pharmaceut Sci. 2010;13:589–606. doi: 10.18433/j3530j. [DOI] [PubMed] [Google Scholar]
- 8.Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123:78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Corti G, Cirri M, Maestrelli F, Mennini N, Mura P. Sustained-release matrix tablets of metformin hydrochloride in combination with triacetyl-β-cyclodextrin. Eur J Pharm Biopharm. 2008;68:303–9. doi: 10.1016/j.ejpb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Králová J, Kejík Z, Břísa T, Poučkova, Král A, Martásek P, Král V. Porphyrin–cyclodextrin conjugates as a nanosystem for versatile drug delivery and multimodal cancer therapy. J Med Chem. 2010;53:128–38. doi: 10.1021/jm9007278. [DOI] [PubMed] [Google Scholar]
- 11.Kim EY, Gao ZG, Park JS, Lee H, Han K. rhEGF/HP-β-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2002;233:159–67. doi: 10.1016/S0378-5173(01)00933-4. [DOI] [PubMed] [Google Scholar]
- 12.Haeberlin B, Gengenbacher T, Meinzer A, Fricker G. Cyclodextrins—useful excipients for oral peptide administration? Int J Pharm. 1996;137:103–10. doi: 10.1016/0378-5173(96)04499-7. [DOI] [Google Scholar]
- 13.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–76. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 14.Loftsson T, Järvinen T. Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev. 1999;36:59–79. doi: 10.1016/S0169-409X(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv Drug Deliv Rev. 1999;36:81–99. doi: 10.1016/S0169-409X(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 16.Loftsson T, Másson M, Sigurdsson HH. Cyclodextrins and drug permeability through semi-permeable cellophane membranes. Int J Pharm. 2002;232:35–43. doi: 10.1016/S0378-5173(01)00895-X. [DOI] [PubMed] [Google Scholar]
- 17.Zerrouk N, Corti G, Ancillotti S, Maestrelli F, Cirri M, Mura P. Influence of cyclodextrins and chitosan, separately or in combination, on glyburide solubility and permeability. Eur J Pharm Biopharm. 2006;62:241–6. doi: 10.1016/j.ejpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Ling W, Xuehua J, Weijuan X, Chenrui L. Complexation of tanshinone IIA with 2-hydroxypropyl-β-cyclodextrin: effect on aqueous solubility, dissolution rate, and intestinal absorption behavior in rats. Int J Pharm. 2007;341:58–67. doi: 10.1016/j.ijpharm.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Pescitelli G, Bilia AR, Bergonzi MC, Vincieri FF, Di Bari L. Cyclodextrins as carriers for kavalactones in aqueous media: spectroscopic characterization of (S)-7,8-dihydrokavain and β-cyclodextrin inclusion complex. J Pharm Biomed Anal. 2010;52:479–83. doi: 10.1016/j.jpba.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi K, Masukawa T, Nadai T, Yoshii K, Okada S, Miyajima K. Prolonged release of drug from triacetyl-β-CD complex for oral and rectal administration. J Incl Phenom Macrocycl Chem. 1996;25:181–4. doi: 10.1007/BF01041565. [DOI] [Google Scholar]
- 21.Péroche S, Degobert G, Putauxc J-L, Blanchin M-G, Fessi H, Parrot-Lopez H. Synthesis and characterisation of novel nanospheres made from amphiphilic perfluoroalkylthio-β-cyclodextrins. Eur J Pharm Biopharm. 2005;60:123–31. doi: 10.1016/j.ejpb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Ganapathy HS, Lee MY, Park C, Lim KT. Sustained release applications of a fluoroalkyl ester-functionalized amphiphilic cyclodextrin by inclusion complex formation with water-soluble drugs in supercritical carbon dioxide. J Fluorine Chem. 2008;129:1162–6. doi: 10.1016/j.jfluchem.2008.09.005. [DOI] [Google Scholar]
- 23.Wang Z, Horikawa T, Hirayama F, Uekama K. Design and in-vitro evaluation of a modified-release oral dosage form of nifedipine by hybridization of hydroxypropyl-beta-cyclodextrin and hydroxypropylcellulose. J Pharm Pharmacol. 1993;45:942–6. doi: 10.1111/j.2042-7158.1993.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv Drug Deliv Rev. 1999;36:125–41. doi: 10.1016/S0169-409X(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes CM, Ramos P, Falcão AC, Veiga FJ. Hydrophilic and hydrophobic cyclodextrins in a new sustained release oral formulation of nicardipine: in vitro evaluation and bioavailability studies in rabbits. J Control Release. 2003;88:127–34. doi: 10.1016/S0168-3659(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 26.Woldum HS, Larsen KL, Madsen F. Cyclodextrin controlled release of poorly water-soluble drugs from hydrogels. Drug Deliv. 2008;15:69–80. doi: 10.1080/10717540701829267. [DOI] [PubMed] [Google Scholar]
- 27.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins 3. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–61. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 28.Namazi H, Bahrami S, Entezami AA. Synthesis and controlled release of biocompatible prodrugs of β-cyclodextrin linked with PEG containing ibuprofen or indomethacin. Iran Polym J. 2005;14:921–7. [Google Scholar]
- 29.Duchêne D, Wouessidjewe D, Ponchel G. Cyclodextrins and carrier systems. J Control Release. 1999;62:263–8. doi: 10.1016/S0168-3659(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 30.Kamada M, Hirayama F, Udo K, Yano H, Arima H, Uekama K. Cyclodextrin conjugate-based controlled release system: repeated- and prolonged-releases of ketoprofen after oral administration in rats. J Control Release. 2002;82:407–16. doi: 10.1016/S0168-3659(02)00171-2. [DOI] [PubMed] [Google Scholar]
- 31.Szejtli J. Cyclodextrin technology. Dordrecht: Kluwer Academic; 1988. p. 450. [Google Scholar]
- 32.Wenz G, Han B-H, Müller A. Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev. 2006;106:782–817. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]
- 33.Endo T, Ueda H. Large ring cyclodextrins—recent progress. FABAD J Pharm Sci. 2004;29:27–38. [Google Scholar]
- 34.Taira H, Nagase H, Endo T, Ueda H. Isolation, purification and characterization of large-ring cyclodextrins (CD36 CD39) J Incl Phenom Macrocycl Chem. 2006;56:23–8. doi: 10.1007/s10847-006-9055-8. [DOI] [Google Scholar]
- 35.Szejtli J. The properties and potential uses of cyclodextrin derivatives. J Incl Phenom Mol Recog Chem. 1992;14:25–36. doi: 10.1007/BF01041363. [DOI] [Google Scholar]
- 36.Szente L, Szejtli J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv Drug Deliv Rev. 1999;36:17–28. doi: 10.1016/S0169-409X(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 37.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–57. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mura P, Furlanetto S, Cirri M, Maestrelli F, Corti G, Pinzauti S. Interaction of naproxen with ionic cyclodextrins in aqueous solution and in the solid state. J Pharm Biom Anal. 2005;37:987–94. doi: 10.1016/j.jpba.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Buchanan CM, Buchanan NL, Edgar KJ, Little JL, Ramsey MG, Ruble KM, Wacher VJ, Wempe MF. Pharmacokinetics of saquinavir after intravenous and oral dosing of saquinavir: hydroxybutenyl-β-cyclodextrin formulations. Biomacromolecules. 2008;9:305–13. doi: 10.1021/bm700827h. [DOI] [PubMed] [Google Scholar]
- 40.Roux M, Perly B, Djedaїni-Pilard F. Self-assemblies of amphiphilic cyclodextrins. Eur Biophys J. 2007;36:861–7. doi: 10.1007/s00249-007-0207-6. [DOI] [PubMed] [Google Scholar]
- 41.Frӧmming K-H, Szejtli J. Cyclodextrins in pharmacy. Dordrecht: Kluwer Academic; 1994. [Google Scholar]
- 42.Al-Omar A, Abdou S, De Robertis L, Marsura A, Finance C. Complexation study and anticellular activity enhancement by doxorubicin–cyclodextrin complexes on a multidrug-resistant adenocarcinoma cell line. Bioorg Med Chem Lett. 1999;9:1115–20. doi: 10.1016/S0960-894X(99)00150-X. [DOI] [PubMed] [Google Scholar]
- 43.García-Rodriguez JJ, Torrado J, Bolás F. Improving bioavailability and anthelmintic activity of albendazole by preparing albendazole–cyclodextrin complexes. Parasite. 2001;8:S188–90. doi: 10.1051/parasite/200108s2188. [DOI] [PubMed] [Google Scholar]
- 44.Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem Pharm Bull. 2004;52:900–15. doi: 10.1248/cpb.52.900. [DOI] [PubMed] [Google Scholar]
- 45.Loftsson T, Vogensen SB, Desbos C, Jansook P. Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPS PharmSciTech. 2008;9:425–30. doi: 10.1208/s12249-008-9055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uekama K, Hirayama F, Arima H. Recent aspect of cyclodextrin-based drug delivery system. J Incl Phenom Macrocycl Chem. 2006;56:3–8. doi: 10.1007/s10847-006-9052-y. [DOI] [Google Scholar]
- 47.Dua K, Ramana MV, Sara UV, Himaja M, Agrawal A, Garg V, Pabreja K. Investigation of enhancement of solubility of norfloxacin β-cyclodextrin in presence of acidic solubilizing additives. Curr Drug Deliv. 2007;4:21–5. doi: 10.2174/156720107779314776. [DOI] [PubMed] [Google Scholar]
- 48.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a Biopharmaceutics Classification System. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). August 2000 BP; http://www.fda.gov/cder/guidance/index.htm. Accessed Jun 2010.
- 50.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry SUPAC-MR: Modified release solid oral dosage forms scale-up and postapproval changes: chemistry, manufacturing, and controls; in vitro dissolution testing and in vivo bioequivalence documentation. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), September 1997 CMC 8; http://www.fda.gov/cder/guidance/index.htm. Accessed Jun 2010.
- 51.Council of Europe. Dosage forms. In: European Pharmacopoeia, supplement 6.0, Council of Europe, Strasbourg; 2007. p. 717–53
- 52.Khan MZI. Dissolution testing for sustained or controlled release oral dosage forms and correlation with in vivo data: challenges and opportunities. Int J Pharm. 1996;140:131–43. doi: 10.1016/0378-5173(96)04561-9. [DOI] [Google Scholar]
- 53.Lemesle-Lamache V, Wouessidjewe D, Chéron M, Duchéne D. Study of β-cyclodextrin and ethylated β-cyclodextrin salbutamol complexes, in vitro evaluation of sustained-release behaviour of salbutamol. Int J Pharm. 1996;141:117–24. doi: 10.1016/0378-5173(96)04624-8. [DOI] [Google Scholar]
- 54.Ikeda Y, Kimura K, Hirayama F, Arima H, Uekama K. Controlled release of a water soluble drug, captopril, by a combination of hydrophilic and hydrophobic cyclodextrin derivatives. J Control Release. 2000;66:271–80. doi: 10.1016/S0168-3659(99)00286-2. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda Y, Motoune S, Marumoto A, Sonoda Y, Hirayama F, Arima H, Uekama K. Effect of 2-hydroxypropyl-β-cyclodextrin on release rate of metoprolol from ternary metoprolol/2-hydroxypropyl-β-cyclodextrin/ethylcellulose tablets. J Incl Phenom Macrocycl Chem. 2002;44:141–4. doi: 10.1023/A:1023082226992. [DOI] [Google Scholar]
- 56.Jug M, Bécirevi-Laan M. Influence of hydroxypropyl-β-cyclodextrin complexation on piroxicam release from buccoadhesive tablets. Eur J Pharm Sci. 2004;21:251–60. doi: 10.1016/j.ejps.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Pose-Vilarnovo B, Rodríguez-Tenreiro C, Santos JF Rosa dos, Vázquez-Doval J, Concheiro A, Alvarez-Lorenzo C, Torres-Labandeira JJ. Modulating drug release with cyclodextrins in hydroxypropyl methylcellulose gels and tablets. J Control Release. 2004;94:351–63. doi: 10.1016/j.jconrel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Miro A, Rondinone A, Nappi A, Ungaro F, Quaglia F, La Rotonda MI. Modulation of release rate and barrier transport of Diclofenac incorporated in hydrophilic matrices: role of cyclodextrins and implications in oral drug delivery. Eur J Pharm Biopharm. 2009;72:76–82. doi: 10.1016/j.ejpb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Ribeiro L, Ferreira DC, Veiga FJB. In vitro controlled release of vinpocetine-cyclodextrin-tartaric acid multicomponent complexes from HPMC swellable tablets. J Control Release. 2005;103:325–39. doi: 10.1016/j.jconrel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Tenreiro C, Alvarez-Lorenzo C, Rodriguez-Perez A, Concheiro A, Torres-Labandeira JJ. Estradiol sustained release from high affinity cyclodextrin hydrogels. Eur J Pharm Biopharm. 2007;66:55–62. doi: 10.1016/j.ejpb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen AL, Madsen F, Larsen KL. Cyclodextrin modified hydrogels of PVP/PEG for sustained drug release. Drug Deliv. 2009;16:92–101. doi: 10.1080/10717540802605129. [DOI] [PubMed] [Google Scholar]
- 62.Frézard F, Martins PS, Bahia AP, Le Moyec L, Melo AL, Pimenta AM, Salerno M, Silva JB, Demicheli C. Enhanced oral delivery of antimony from meglumine antimoniate/β-cyclodextrin nanoassemblies. Int J Pharm. 2008;347:102–8. doi: 10.1016/j.ijpharm.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 63.Özdemir N, Ordu S, Özkan Y. Studies of floating dosage forms of furosemide: in vitro and in vivo evaluations of bilayer tablet formulations. Drug Dev Ind Pharm. 2000;26:857–66. doi: 10.1081/DDC-100101309. [DOI] [PubMed] [Google Scholar]
- 64.Shishu, Gupta N, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin—“a technical note”. AAPS PharmSciTech. 2008;9:810–3. doi: 10.1208/s12249-008-9096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garg R, Gupta GD. Preparation and evaluation of gastroretentive floating tablets of silymarin. Chem Pharm Bull. 2009;57:545–9. doi: 10.1248/cpb.57.545. [DOI] [PubMed] [Google Scholar]
- 66.Sigurdsson HH, Knudsen E, Loftsson T, Leeves N, Sigurjonsdotir JF, Másson M. Mucoadhesive sustained drug delivery system based on cationic polymer and anionic cyclodextrin/triclosan complex. J Incl Phenom Macrocycl Chem. 2002;44:169–72. doi: 10.1023/A:1023098730627. [DOI] [Google Scholar]
- 67.Venter JP, Kotzé AF, Auzély-Velty R, Rinaudo M. Synthesis and evaluation of the mucoadhesivity of a CD chitosan derivative. Int J Pharm. 2006;313:36–42. doi: 10.1016/j.ijpharm.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Prabaharan M, Gong S. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr Polym. 2008;73:117–25. doi: 10.1016/j.carbpol.2007.11.005. [DOI] [Google Scholar]
- 69.Jug M, Maestrelli F, Bragagnib M, Mura P. Preparation and solid-state characterization of bupivacaine hydrochloride cyclodextrin complexes aimed for buccal delivery. J Pharm Biom Anal. 2010;52:9–18. doi: 10.1016/j.jpba.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Mura P, Corti G, Cirri M, Maestrelli F, Mennini N, Bragagni M. Development of mucoadhesive films for buccal administration of flufenamic acid: effect of cyclodextrin complexation. J Pharm Sci. 2010;99:3019–29. doi: 10.1002/jps.22068. [DOI] [PubMed] [Google Scholar]
- 71.Okimoto K, Rajewski RA, Stella VJ. Release of testosterone from an osmotic pump tablet utilizing (SBE)7m-β-cyclodextrin as both a solubilizing and an osmotic pump agent. J Control Release. 1999;58:29–38. doi: 10.1016/S0168-3659(98)00142-4. [DOI] [PubMed] [Google Scholar]
- 72.Okimoto K, Ohike A, Ibuki R, Aoki O, Ohnishi N, Rajewski RA, Stella VJ, Irie T, Uekama K. Factors affecting membrane-controlled drug release for an osmotic pump tablet (OPT) utilizing (SBE)7m-β-CD as both a solubilizer and osmotic agent. J Control Release. 1999;60:311–9. doi: 10.1016/S0168-3659(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 73.Zannou EA, Streng WH, Stella VJ. Osmotic properties of sulfobutylether and hydroxypropyl cyclodextrins. Pharm Research. 2001;18:1226–31. doi: 10.1023/A:1010947631380. [DOI] [PubMed] [Google Scholar]
- 74.Okimoto K, Tokunaga Y, Ibuki R, Irie T, Uekama K, Rajewski RA, Stella VJ. Applicability of (SBE)7m-β-CD in controlled-porosity osmotic pump tablets (OPTs) Int J Pharm. 2004;286:81–8. doi: 10.1016/j.ijpharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Sotthivirat S, Haslam JL, Stella VJ. Controlled porosity-osmotic pump pellets of a poorly water-soluble drug using sulfobutylether-β-cyclodextrin, (SBE)7m-β-CD, as a solubilizing and osmotic agent. J Pharm Sci. 2007;96:2364–74. doi: 10.1002/jps.20891. [DOI] [PubMed] [Google Scholar]
- 76.Sotthivirat S, Haslam JL, Lee PI, Rao VM, Stella VJ. Release mechanisms of a sparingly water-soluble drug from controlled porosity-osmotic pump pellets using sulfobutylether-β-cyclodextrin as both a solubilizing and osmotic agent. J Pharm Sci. 2009;98:1992–2000. doi: 10.1002/jps.21567. [DOI] [PubMed] [Google Scholar]
- 77.Rao VM, Haslam JL, Stella VJ. Controlled and complete release of a model poorly water-soluble drug, prednisolone, from hydroxypropyl methylcellulose matrix tablets using (SBE)7m-β-cyclodextrin as a solubilizing agent. J Pharm Sci. 2001;90:807–16. doi: 10.1002/jps.1034. [DOI] [PubMed] [Google Scholar]
- 78.Trapani G, Lopedota A, Boghetich G, Latrofa A, Franco M, Sanna E, Liso G. Encapsulation and release of the hypnotic agent zolpidem from biodegradable polymer microparticles containing hydroxypropyl-β-cyclodextrin. Int J Pharm. 2003;268:47–57. doi: 10.1016/j.ijpharm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 79.Smith JS, Macrae RJ, Snowden MJ. Effect of SBE7-β-cyclodextrin complexation on carbamazepine release from sustained release beads. Eur J Pharm Biopharm. 2005;60:73–80. doi: 10.1016/j.ejpb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Cavalli R, Trotta F, Trotta M, Pastero L, Aquilano D. Effect of alkylcarbonates of γ-cyclodextrins with different chain lengths on drug complexation and release characteristics. Int J Pharm. 2007;339:197–204. doi: 10.1016/j.ijpharm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Trichard L, Fattal E, Besnard M, Bochot A. α-Cyclodextrin/oil beads as a new carrier for improving the oral bioavailability of lipophilic drugs. J Control Release. 2007;122:47–53. doi: 10.1016/j.jconrel.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Yoshinari M, Matsuza K, Hashimoto S, Ishihara K, Inoue T, Oda Y, Ide T, Tanaka T. Controlled release of simvastatin acid using cyclodextrin inclusion system. Dent Mater J. 2007;26:451–6. doi: 10.4012/dmj.26.451. [DOI] [PubMed] [Google Scholar]
- 83.Blode H, Schürmann R, Benda N. Novel ethinyl estradiol-beta-cyclodextrin clathrate formulation does not influence the relative bioavailability of ethinyl estradiol or coadministered drospirenone. Contraception. 2008;77:171–6. doi: 10.1016/j.contraception.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Y, Guo Z, Zhang Y, Huang W, Zhou Y, Yan D. Hyperbranched polyamidoamines containing β-cyclodextrin for controlled release of chlorambucil. Macromol Biosci. 2009;9:1090–7. doi: 10.1002/mabi.200900110. [DOI] [PubMed] [Google Scholar]
- 85.Temtem M, Pompeu D, Jaraquemada G, Cabrita EJ, Casimiro T, Aguiar-Ricardo A. Development of PMMA membranes functionalized with hydroxypropyl-β-cyclodextrins for controlled drug delivery using a supercritical CO2-assisted technology. Int J Pharm. 2009;376:110–5. doi: 10.1016/j.ijpharm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 86.Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended α-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Control Release. 2002;79:103–12. doi: 10.1016/S0168-3659(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 87.Zou M-J, Cheng G, Okamoto H, Hao X-H, An F, Cui F-D, Danjo K. Colon-specific drug delivery systems based on cyclodextrin prodrugs: in vivo evaluation of five aminosalicylic acid from its cyclodextrin conjugates. World J Gastroenterol. 2005;11:7457–60. doi: 10.3748/wjg.v11.i47.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Udo K, Hokonohara K, Motoyama K, Arima H, Hirayama F, Uekama K. 5-Fluorouracil acetic acid/β-cyclodextrin conjugates: drug release behaviour in enzymatic and rat cecal media. Int J Pharm. 2010;388:95–100. doi: 10.1016/j.ijpharm.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 89.Minami K, Hirayama F, Uekama K. Colon-specific drug delivery based on a cyclodextrin prodrug: release behavior of biphenylylacetic acid from its cyclodextrin conjugates in rat intestinal tracts after oral administration. J Pharm Sci. 1998;87:715–20. doi: 10.1021/js9704339. [DOI] [PubMed] [Google Scholar]
- 90.Liu X-M, Lee H-T, Reinhardt RA, Marky LA, Wang D. Novel biomineral-binding cyclodextrins for controlled drug delivery in the oral cavity. J Control Release. 2007;122:54–62. doi: 10.1016/j.jconrel.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 91.Zugasti ME, Zornoza A, Goñi MM, Isasi JR, Vélaz I, Martín C, Sánchez M, Martínez-Ohárriz MC. Influence of soluble and insoluble cyclodextrin polymers on drug release from hydroxypropyl methylcellulose tablets. Drug Dev Ind Pharm. 2009;35:1264–70. doi: 10.1080/03639040902882306. [DOI] [PubMed] [Google Scholar]
- 92.Stancanelli R, Crupi V, De Luca L, Ficarra P, Ficarra R, Gitto R, Guardo M, Iraci N, Majolino D, Tommasini S, Venuti V. Improvement of water solubility of non-competitive AMPA receptor antagonists by complexation with β-cyclodextrin. Bioorg Med Chem. 2008;16:8706–12. doi: 10.1016/j.bmc.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 93.Trapani A, Garcia-Fuentes M, Alonso MJ. Novel drug nanocarriers combining hydrophilic cyclodextrins and chitosan. Nanotechnology. 2008;19:1–10. doi: 10.1088/0957-4484/19/18/185101. [DOI] [PubMed] [Google Scholar]
- 94.Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Synthesis of linear, β-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjug Chem. 2003;14:1007–17. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 95.Cavalli R, Peira E, Caputo O, Gasco MR. Solid lipid nanoparticles as carriers of hydrocortisone and progesterone complexes with β-cyclodextrins. Int J Pharm. 1999;182:59–69. doi: 10.1016/S0378-5173(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 96.Duchêne D, Ponchela G, Wouessidjewen D. Cyclodextrins in targeting: application to nanoparticles. Adv Drug Deliv Rev. 1999;36:29–40. doi: 10.1016/S0169-409X(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 97.Tozuka Y, Wongmekiat A, Sakata K, Moribe K, Oguchi T, Yamamoto K. Co-grinding with cyclodextrin as a nanoparticle preparation method of a poorly water soluble drug. J Incl Phenom Macrocycl Chem. 2004;50:67–71. [Google Scholar]
- 98.Mourtzis N, Eliadou K, Aggelidou C, Sophianopoulou V, Mavridis IM, Yannakopoulou K. Per(6guanidino-6-deoxy)cyclodextrins: synthesis, characterization and binding behaviour toward selected small molecules and DNA. Org Biomol Chem. 2007;5:125–31. doi: 10.1039/b614899a. [DOI] [PubMed] [Google Scholar]
- 99.Du Y-Z, Xu J-G, Wang L, Yuan H, Hu F-Q. Preparation and characteristics of hydroxypropyl-β-cyclodextrin polymeric nanocapsules loading nimodipine. Eur Polym J. 2009;45:1397–402. doi: 10.1016/j.eurpolymj.2009.01.031. [DOI] [Google Scholar]
- 100.Sajeesh S, Sharma CP. Cyclodextrin–insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin delivery. Int J Pharm. 2006;325:147–54. doi: 10.1016/j.ijpharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Zhang N, Li J, Jiang W, Ren C, Li J, Xin J, Li K. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int J Pharm. 2010;393:212–8. doi: 10.1016/j.ijpharm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Agüeros M, Areses P, Campanero MA, Salman H, Quincoces G, Peñuelas I, Irache JM. Bioadhesive properties and biodistribution of cyclodextrin–poly(anhydride) nanoparticles. Eur J Pharm Sci. 2009;37:231–40. doi: 10.1016/j.ejps.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Agüeros M, Zabaleta V, Espuelas S, Campanero MA, Irache JM. Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J Control Release. 2010;145:2–8. doi: 10.1016/j.jconrel.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 104.Jansook P, Kurkov SV, Loftsson T. Cyclodextrins as solubilizers: formation of complex aggregates. J Pharm Sci. 2010;99:719–29. doi: 10.1002/jps.21861. [DOI] [PubMed] [Google Scholar]
- 105.Yamanoi T, Yoshida N, Oda Y, Akaike E, Tsutsumida M, Kobayashi N, Osumi K, Yamamoto K, Fujita K, Takahashi K, Hattori K. Synthesis of mono-glucose branched cyclodextrins with a high inclusion ability for doxorubicin and their efficient glycosylation using Mucor hiemalis endo-β-N-acetylglucosaminidase. Bioorg Med Chem Lett. 2005;15:1009–13. doi: 10.1016/j.bmcl.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 106.Burckbuchler V, Wintgens V, Leborgne C, Lecomte S, Leygue N, Scherman D, Kichler A, Amiel C. Development and characterization of new cyclodextrin polymer-based DNA delivery systems. Bioconjug Chem. 2008;19:2311–20. doi: 10.1021/bc800070f. [DOI] [PubMed] [Google Scholar]
- 107.Lu X, Ping Y, Xu FJ, Li ZH, Wang QQ, Chen JH, Yang WT, Tang GP. Bifunctional conjugates comprising β-cyclodextrin, polyethylenimine, and 5-fluoro-2′-deoxyuridine for drug delivery and gene transfer. Bioconjug Chem. 2010;21:1855–63. doi: 10.1021/bc1002136. [DOI] [PubMed] [Google Scholar]
- 108.Upadhyay AK, Singh S, Chhipa RR, Vijayakumar MV, Ajay AK, Bhat MK. Methyl-β-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-κB and Bcl-2. Toxicol Appl Pharmacol. 2006;216:177–85. doi: 10.1016/j.taap.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Higashi T, Hirayama F, Misumi S, Arima H, Uekama K. Design and evaluation of polypseudorotaxanes of pegylated insulin with cyclodextrins as sustained release system. Biomaterials. 2008;29:3866–71. doi: 10.1016/j.biomaterials.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 110.Higashi T, Hirayama F, Yamashita S, Misumi S, Arima H, Uekama K. Slow-release system of pegylated lysozyme utilizing formation of polypseudorotaxanes with cyclodextrins. Int J Pharm. 2009;374:26–32. doi: 10.1016/j.ijpharm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Cannavà C, Crupi V, Ficarra P, Guardo M, Majolino D, Mazzaglia A, Stancanelli R, Venuti V. Physico-chemical characterization of an amphiphilic cyclodextrin/genistein complex. J Pharm Biomed Anal. 2010;51:1064–8. doi: 10.1016/j.jpba.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 112.Sajomsang W, Gonil P, Ruktanonchai UR, Pimpha N, Sramala I, Nuchuchua O, Saesoo S, Chaleawlert-umpon S, Puttipipatkhachorn S. Self-aggregates formation and mucoadhesive property of water-soluble β-cyclodextrin grafted with chitosan. Int J Biol Macromol. 2011;48:589–95. doi: 10.1016/j.ijbiomac.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 113.Chen C-Y, Chen F-A, Wu A-B, Hsu H-C, Kang J-J, Cheng H-W. Effect of hydroxypropyl-β-cyclodextrin on the solubility, photostability and in-vitro permeability of alkannin/shikonin enantiomers. Int J Pharm. 1996;141:171–8. doi: 10.1016/0378-5173(96)04634-0. [DOI] [Google Scholar]
- 114.Tommasini S, Calabrò ML, Raneri D, Ficarra P, Ficarra R. Combined effect of pH and polysorbates with cyclodextrins on solubilization of naringenin. J Pharm Biom Anal. 2004;36:327–33. doi: 10.1016/j.jpba.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 115.Terao K, Nakata D, Fukumi H, Schmid G, Arima H, Hirayama F, Uekama K. Enhancement of oral bioavailability of coenzyme Q10 by complexation with γ-cyclodextrin in healthy adults. Nutrition Res. 2006;26:503–8. doi: 10.1016/j.nutres.2006.08.004. [DOI] [Google Scholar]
- 116.Lu Z, Cheng B, Hub Y, Zhang Y, Guolin Zou G. Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food Chem. 2009;113:17–20. doi: 10.1016/j.foodchem.2008.04.042. [DOI] [Google Scholar]
- 117.Motoyama K, Nagamoto K, Abd Elazim SO, Hirayama F, Uekama K, Arima H. Potential use of 2-hydroxypropyl-β-cyclodextrin for preparation of orally disintegrating tablets containing dl-alpha-tocopheryl acetate, an oily drug. Chem Pharm Bull. 2009;57:1206–12. doi: 10.1248/cpb.57.1206. [DOI] [PubMed] [Google Scholar]
- 118.Mura P, Bettinetti G, Melani F, Manderioli A. Interaction between naproxen and chemically modified β-cyclodextrins in the liquid and solid state. Eur J Pharm Sci. 1995;3:347–55. doi: 10.1016/0928-0987(95)00025-X. [DOI] [Google Scholar]
- 119.Mura P, Bettinetti GP, Manderioli A, Faucci MT, Bramanti G, Sorrenti M. Interactions of ketoprofen and ibuprofen with β-cyclodextrins in solution and in the solid state. Int J Pharm. 1998;166:189–203. doi: 10.1016/S0378-5173(98)00035-0. [DOI] [Google Scholar]
- 120.Charoenchaitrakool M, Dehghani F, Foster NR. Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin. Int J Pharm. 2002;239:103–12. doi: 10.1016/S0378-5173(02)00078-9. [DOI] [PubMed] [Google Scholar]
- 121.Naidu BN, Chowdary KP, Murthy KV, Satyanarayana V, Hayman AR, Becket G. Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary systems. J Pharm Biom Anal. 2004;35:75–86. doi: 10.1016/j.jpba.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 122.Türk M, Upper G, Steurenthaler M, Hussein Kh, Wahl MA. Complex formation of ibuprofen and β-cyclodextrin by controlled particle deposition (CPD) using SC-CO2. J Supercrit Fluids. 2007;39:435–43. doi: 10.1016/j.supflu.2006.02.009. [DOI] [Google Scholar]
- 123.Fukuda M, Miller DA, Peppas NA, McGinity JW. Influence of sulfobutyl ether β-cyclodextrin (Captisol®) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int J Pharm. 2008;350:188–96. doi: 10.1016/j.ijpharm.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 124.Cirri M, Maestrelli F, Mennini N, Mura P. Physical–chemical characterization of binary and ternary systems of ketoprofen with cyclodextrins and phospholipids. J Pharm Biom Anal. 2009;50:683–9. doi: 10.1016/j.jpba.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 125.Sauceau M, Rodier E, Fages J. Preparation of inclusion complex of piroxicam with cyclodextrin by using supercritical carbon dioxide. J Supercrit Fluids. 2008;47:326–32. doi: 10.1016/j.supflu.2008.07.006. [DOI] [Google Scholar]
- 126.Salústio PJ, Feio G, Figueirinhas JL, Pinto JF, Cabral Marques HM. The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur J Pharm Biopharm. 2009;71:377–86. doi: 10.1016/j.ejpb.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 127.Lu Y, Zhang X, Lai J, Yin Z, Wu W. Physical characterization of meloxicam-β-cyclodextrin inclusion complex pellets prepared by a fluid-bed coating method. Particuology. 2009;7:1–8. doi: 10.1016/j.partic.2008.11.004. [DOI] [Google Scholar]
- 128.Esclusa-Díaz MT, Gayo-Otero M, Pérez-Marcos MB, Vila-Jato JL, Torres-Labandeira JJ. Preparation and evaluation of ketoconazole–β-cyclodextrin multicomponent complexes. Int J Pharm. 1996;142:183–7. doi: 10.1016/0378-5173(96)04666-2. [DOI] [Google Scholar]
- 129.Mura P, Faucci MT, Manderioli A, Bramanti G. Influence of the preparation method on the physicochemical properties of binary systems of econazole with cyclodextrins. Int J Pharm. 1999;193:85–95. doi: 10.1016/S0378-5173(99)00326-9. [DOI] [PubMed] [Google Scholar]
- 130.Lee S-Y, Jung I-I, Kim J-K, Lim G-B, Ryu J-H. Preparation of itraconazole/HP-β-CD inclusion complexes using supercritical aerosol solvent extraction system and their dissolution characteristics. J Supercrit Fluids. 2008;44:400–8. doi: 10.1016/j.supflu.2007.09.006. [DOI] [Google Scholar]
- 131.Moyano JR, Ginés JM, Arias MJ, Rabasco AM. Study of the dissolution characteristics of oxazepam via complexation with β-cyclodextrin. Int J Pharm. 1995;114:95–102. doi: 10.1016/0378-5173(94)00220-Y. [DOI] [Google Scholar]
- 132.Archontaki HA, Vertzoni MV, Athanassiou-Malaki MH. Study on the inclusion complexes of bromazepam with β- and β-hydroxypropyl-cyclodextrins. J Pharm Biom Anal. 2002;28:761–9. doi: 10.1016/S0731-7085(01)00679-3. [DOI] [PubMed] [Google Scholar]
- 133.Jain AC, Adeyeye MC. Hygroscopicity, phase solubility and dissolution of various substituted sulfobutylether β-cyclodextrins (SBE) and danazol–SBE inclusion complexes. Int J Pharm. 2001;212:177–86. doi: 10.1016/S0378-5173(00)00607-4. [DOI] [PubMed] [Google Scholar]
- 134.Savolainen J, Järvinen K, Matilainen L, Järvinen T. Improved dissolution and bioavailability of phenytoin by sulfobutylether-β-cyclodextrin ((SBE)7m-β-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) complexation. Int J Pharm. 1998;165:69–78. doi: 10.1016/S0378-5173(98)00004-0. [DOI] [Google Scholar]
- 135.Latrofa A, Trapani G, Franco M, Serra M, Muggironi M, Fanizzi FP, Cutrignelli A, Liso G. Complexation of phenytoin with some hydrophilic cyclodextrins: effect on aqueous solubility, dissolution rate, and anticonvulsant activity in mice. Eur J Pharm Biopharm. 2001;52:65–73. doi: 10.1016/S0939-6411(01)00144-8. [DOI] [PubMed] [Google Scholar]
- 136.Echezarreta-López M, Torres-Labandeira JJ, Castiñeiras-Seijo L, Santana-Penín L, Vila-Jato JL. Complexation of the interferon inducer, bropirimine, with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2000;9:381–6. doi: 10.1016/S0928-0987(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 137.Ficarra R, Ficarra P, Di Bella MR, Raneri D, Tommasini S, Calabrò ML, Villari A, Coppolino S. Study of the inclusion complex of atenolol with β-cyclodextrins. J Pharm Biom Anal. 2000;23:231–6. doi: 10.1016/S0731-7085(00)00274-0. [DOI] [PubMed] [Google Scholar]
- 138.Patel N, Chotai N, Patel J, Soni T, Desai J, Patel R. Comparison of in vitro dissolution profiles of oxcarbazepine-HP-β-CD tablet formulations with marketed oxcarbazepine tablets. Dissolution Tech. 2008;15:28–34. [Google Scholar]
- 139.Hamada H, Ishihara K, Masuoka N, Mikuni K, Nakajima N. Enhancement of water-solubility and bioactivity of paclitaxel using modified cyclodextrins. J Biosci Bioeng. 2006;102:369–71. doi: 10.1263/jbb.102.369. [DOI] [PubMed] [Google Scholar]
- 140.Wong JW, Yuen KH. Improved oral bioavailability of artemisinin through inclusion complexation with β- and γ-cyclodextrins. Int J Pharm. 2001;227:177–85. doi: 10.1016/S0378-5173(01)00796-7. [DOI] [PubMed] [Google Scholar]
- 141.Usuda M, Endo T, Nagase H, Tomono K, Ueda H. Interaction of antimalarial agent artemisinin with cyclodextrins. Drug Dev Ind Pharm. 2000;26:613–9. doi: 10.1081/DDC-100101276. [DOI] [PubMed] [Google Scholar]
- 142.Wen X, Tan F, Jing Z, Liu Z. Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J Pharm Biom Anal. 2004;34:517–23. doi: 10.1016/S0731-7085(03)00576-4. [DOI] [PubMed] [Google Scholar]
- 143.Zingone G, Rubessa F. Preformulation study of the inclusion complex warfarin–β-cyclodextrin. Int J Pharm. 2005;291:3–10. doi: 10.1016/j.ijpharm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Terekhova IV, Volkova TV, Perlovich GL. Interactions of theophylline with cyclodextrins in water. Mendeleev Commun. 2007;17:244–6. doi: 10.1016/j.mencom.2007.06.022. [DOI] [Google Scholar]
- 145.Badr-Eldin SM, Elkheshen SA, Ghorab MM. Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: Preparation and in-vitro evaluation. Eur J Pharm Biopharm. 2008;70:819–27. doi: 10.1016/j.ejpb.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y, Chen G-S, Chen Y, Lin J. Inclusion complexes of azadirachtin with native and methylated cyclodextrins: solubilization and binding ability. Bioorg Med Chem. 2005;13:4037–42. doi: 10.1016/j.bmc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 147.Petralito S, Zanardi I, Memoli A, Annesini MC, Travagli V. Solubility, spectroscopic properties and photostability of Rhein/cyclodextrin inclusion complex. Spectrochim Acta Part A. 2009;74:1254–9. doi: 10.1016/j.saa.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 148.Chowdary KP, Srinivas SV. Influence of hydrophilic polymers on celecoxib complexation with hydroxypropyl-β-cyclodextrin. AAPS PharmSciTech. 2006;7:E184–9. doi: 10.1208/pt070379. [DOI] [PubMed] [Google Scholar]
- 149.He Y, Tabibi E, Yalkowsky SH. Solubilization of two structurally related anticancer drugs: XK-469 and PPA. J Pharm Sci. 2006;95:97–107. doi: 10.1002/jps.20500. [DOI] [PubMed] [Google Scholar]
- 150.Gladys G, Claudia G, Marcela L. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2003;20:285–93. doi: 10.1016/S0928-0987(03)00202-1. [DOI] [PubMed] [Google Scholar]