Abstract
The first successful development of controlled microwave processing for pharmaceutical formulations is presented and illustrated with a model drug (ibuprofen) and two excipients (stearic acid and polyvinylpyrrolidone). The necessary fine temperature control for formulation with microwave energy has been achieved using a uniquely modified microwave oven with direct temperature measurement and pulse-width modulation power control. In addition to comparing microwave and conventional heating, the effect of the presence of liquid (water) in aiding the mixing of the drug and excipient during formulation was also investigated. Analysis of the prepared formulations using differential scanning calorimetry and dissolution studies suggest that microwave and conventional heating produce similar products when applied to mixtures of ibuprofen and stearic acid. However, the differences were observed for the ibuprofen and polyvinylpyrrolidone formulation in terms of the dissolution kinetics. In all cases, the presence of water did not appear to influence the formulation to any appreciable degree. The application of controllable microwave heating is noteworthy as fine temperature control opens up opportunities for thermally sensitive materials for which microwave methods have not been feasible prior to this work.
KEY WORDS: dielectric, ibuprofen, microwave heating, polyvinylpyrrolidone, stearic acid
INTRODUCTION
Microwave heating arises from the direct interaction of electromagnetic energy with a material. The extent to which a material is heated by microwave energy is dependent on a range of parameters, but of particular importance are its dielectric properties (1). Polar liquids, such as water, are very readily heated, while materials such as silica glass are virtually microwave transparent. For suitable systems, microwave heating can confer several benefits (2) over conventional heating including rapid heating and cooling (because the thermal lag effect produced by the mass of an oven is avoided), reduced temperature gradients across the sample, lower energy usage and, in some cases, enhanced reaction rates.
Microwave heating has become a well-established method for processing and drying chemical products in recent years (3–5). Its suitability for the drying of pharmaceutical products has been thoroughly investigated (6), for example with the application to maintaining long-term drug stability (7). In contrast, this work considers the utilisation of microwave heating for pharmaceutical formulation, an approach which, with the exception of the work of Bergese (8) and Moneghini (9,10) has been the subject of much less investigation.
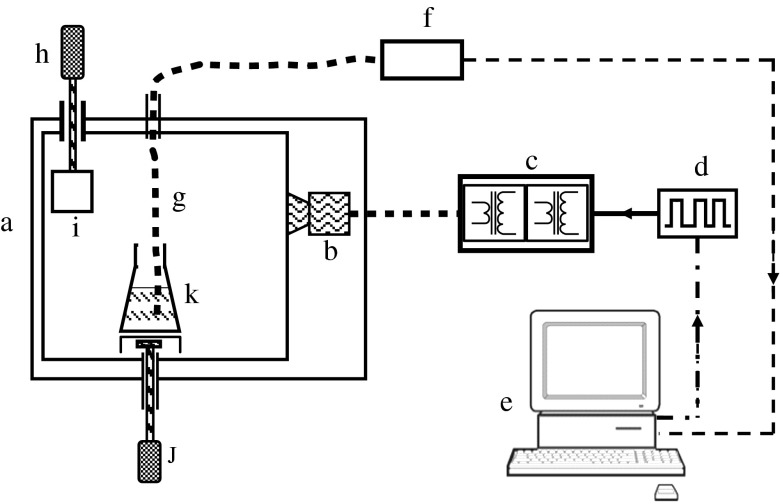
One of the main complexities with the use of microwave heating outside of the context of drying relates to the controllability of the temperature. A constant power level does not guarantee a constant temperature as phase changes, reactions and other processes can alter a material’s dielectric properties considerably. In addition, many materials couple more strongly with microwave energy with increasing temperature, a process that can lead to ‘thermal runaway’ with potentially catastrophic results to the product. In this paper, a modified microwave oven with direct temperature measurement and computerised power control is used to achieve controllable heating (see Fig. 1). Microwaves belong to the portion of the electromagnetic spectrum with frequencies from 300 MHz to 300 GHz (wavelengths from 1 m to 1 mm). Four frequencies have been allocated for microwave heating, the most prevalent being 2.45 GHz. The extent to which a material is heated when subjected to a microwave field is determined crucially by its dielectric properties.
Fig. 1.
Schematic view of the microwave oven used for formulation experiments. a Microwave oven. b Magnetron. c Dual transformer power supply. d Pulse-width modulation unit. e Computer. f Fibre optic control unit. g Fibre optic temperature probe. h DC motor. i Mode stirrer plate. j Magnetic stirrer motor. k Drug and excipient in conical flask
In dielectric materials, bound charges move in response to the electric field component of the microwave energy resulting in polarisation. In the cases where there is either polarisation of electric charge and restricted translational motion, and/or polarisation of molecules and restricted rotational motion, a time lag is produced between the oscillating electric field and the induced polarisation. Dissipation of the energy arising from this lag (dielectric relaxation) results in heating. A number of properties contribute to the dielectric response of materials including ionic conduction and electronic, atomic and dipole (orientation) polarisation. At microwave frequencies, the latter is thought to predominate only in liquids, while for certain materials Maxwell–Wagner polarisation (which results from the accumulation of charge at the interface between different materials) can also be important.
In conventional thermal processing, energy is transferred to the surface of the material from the furnace, or oven, by convection, conduction and radiation. The heat then travels through the material by conduction. In contrast, microwave heating is the result of the direct interaction of microwave energy with a material, via its dielectric properties, rather than via heat transfer. As microwaves can penetrate materials, heat is generated throughout the volume which can produce more uniform heating.
Our work concerns the application of microwave heating to the formulation process for a model drug, namely ibuprofen, with a comparatively low aqueous solubility and melting point (11). To maintain simplicity, only two excipients were considered in this work, namely stearic acid and polyvinylpyrrolidone (PVP). Stearic acid has recently found application as an encapsulation medium as it is non-toxic, inert, cheap and capable of taste masking bitter drugs (12). Under certain conditions, stearic acid forms drug-loaded microspheres, previously exemplified using cefuroxime axetil (13). To form drug-loaded microspheres requires a heating stage, thus justifying this excipient choice (14). In comparison, PVP forms a cross-linked matrix that has been shown to constrain drugs in stable molecular clusters (15). Stearic acid and PVP were chosen in particular for this research as they are both capable of incorporating drugs in their formulated structures yet they have remarkably different melting points, the former being comparatively lower than the latter.
In addition to the influence of the mode of heating, the effect of the use of water on the formulation process was investigated. With no water, or ‘dry melting’, the molten drug would be dispersed into the excipient solely by diffusion. With water (and stirring), or ‘wet melting’, this diffusion process might be expected to be aided through the continuous movement of the drug and excipient and the possible formation of emulsions. The presence of water would also be expected to assist the heating of drugs and/or excipients that couple poorly with microwave energy. However, this approach is only feasible if the drug and excipient have very low solubilities.
The most important impact that a variation in formulation may exhibit is an alteration to the subsequent drug release profile. It has previously been shown that it is possible to control drug release, for example for water insoluble drugs using self-emulsifying systems (16); this is often confirmed using dissolution analysis (17–20). Dissolution experiments are an ideal system for comparing the influence of formulation parameters, such as those under investigation in this work. As a secondary analytical method, differential scanning calorimetry (DSC) provides an insight into the degree of interaction between drug and excipient (21,22). For example, the eutectic phase diagram for stearic acid and ibuprofen has been established, identifying two overlapping peaks for the melting temperatures (23). By combining dissolution and DSC data, it is possible to consider several factors that may influence the character of the resultant formulations, including the influence of these parameters on drug release.
MATERIALS AND METHODS
Materials
Ibuprofen, stearic acid, cross-linked polyvinylpyrrolidone (PVP 40), sodium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Sigma (Dorset, UK). All reagents were ≥99% purity.
Conventional Formulation Methods
Dry Melt Formulation
The samples (10 g) of PVP (or stearic acid) together with ibuprofen were prepared in 9:1, 3:1 and 1:1 mass ratios. The dry powders were then tumble mixed for 5 min to achieve a homogenous mix, transferred to a crucible and placed in an oven for 20 min at 85°C. The crucible was removed from the oven and left to cool to room temperature. All formulations were sieved and those with a diameter between 80 and 250 μm collected for further analysis.
Wet Melt Formulation
The samples (10 g) of PVP (or stearic acid) together with ibuprofen were prepared in 1:1 and 3:1 mass ratios. The dry powders were then tumble mixed for 5 min to achieve a homogenous mix. A sample mass of the powder was added to a beaker containing deionised water to achieve a 10% w/v solution, transferred to a hotplate and adjusted until the water temperature reached 85°C, as monitored by a thermometer. This temperature was maintained for 5 min, and then the beaker was removed from the hotplate and allowed to cool. When the water had reached a temperature of 45°C, the resultant formulation was collected by vacuum filtration and dried overnight in a desiccator over silica gel. All formulations were sieved and those with a diameter between 80 and 250 μm collected for further analysis.
Microwave Formulation Methods
The apparatus used for the microwave heating formulations is shown schematically in Fig. 1. It comprises a modified domestic microwave oven with a separate magnetron power supply that permits rapid switching to allow pulse-width modulation (duty cycle of 1 s) power control. The oven has shielded inlets to allow access for a fibre optic temperature probe (Luxtron) and a magnetic stirrer, while a PC and bespoke software provide user control of the microwave power and data acquisition. A mode stirrer, consisting of a rotating metal plate within the oven, is used to avoid power ‘hot spots’ and to aid uniform heating.
Dry Melt Formulation
The samples (10 g) of PVP or stearic acid together with ibuprofen were prepared in 1:1 and 3:1 mass ratios. The dry powders were then tumble mixed for 5 min to achieve a homogenous mix, transferred to a crucible and placed in the microwave oven. The microwave power was then manually adjusted to allow the mixture to reach a temperature of 85°C (as monitored by the fibre optic probe) within a period of a few minutes. The temperature was maintained for a further 10 min before the microwave power was reduced to allow the sample to cool.
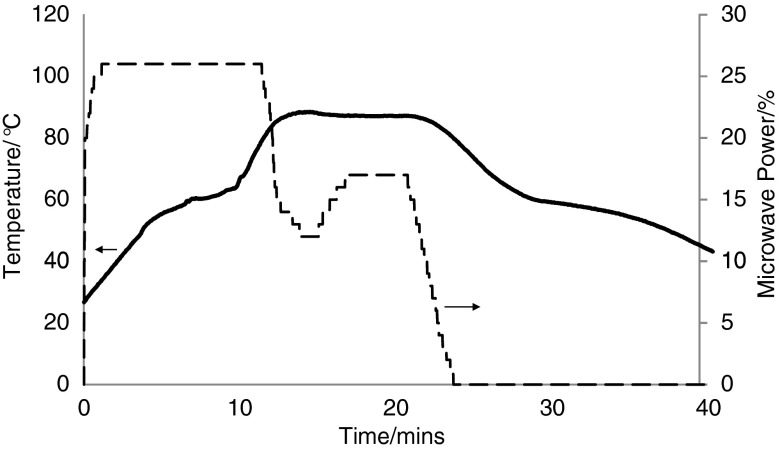
Figure 2 shows a typical result for the microwave dry melt formulation of a 3:1 stearic acid/ibuprofen mixture. The power trace was raised to 25% (200 W) over a period of approximately 90 s to heat the mixture. The sample temperature rises smoothly until it starts to level off as a result of the endothermic effect of enthalpy of the melting of both drug and excipient. The temperature starts to rise again, and the microwave power has to be reduced considerably as the target temperature of 85°C is approached because the liquid form couples more strongly with microwave energy. Further adjustments in the microwave power are required to maintain this temperature constant for 15 min. At the end of this period, the power is reduced to zero and the sample temperature falls smoothly with a plateau around 30 min with the enthalpy of recrystallisation.
Fig. 2.
A typical microwave power (broken line) and temperature (line) plot for the ‘dry melt’ formulation of stearic acid and ibuprofen (3:1) using microwave heating where the power has been adjusted as required to maintain temperature control
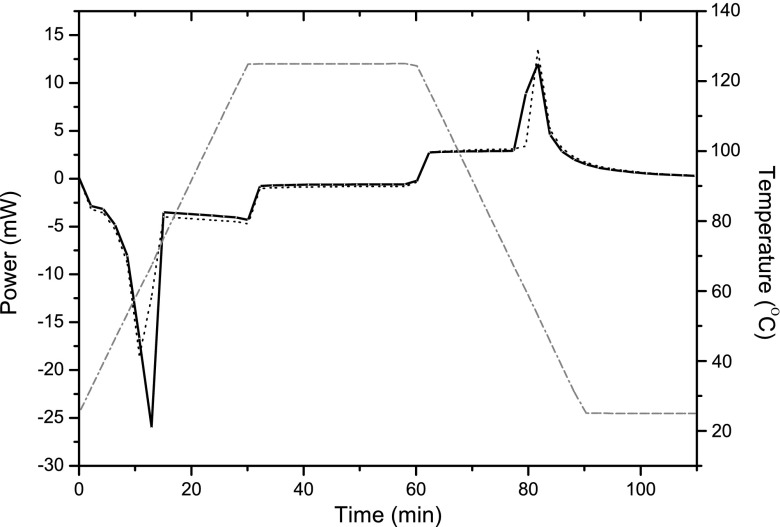
Figure 3 shows the necessity of careful control of the power for microwave formulation with an identical 3:1 stearic acid/ibuprofen mixture heated at a constant 25% (200 W). Initially, the two experiments were similar with the temperature of the mixture rising smoothly until it levelled off with the enthalpy of melting. However, after this period and without power control, the temperature continued to rise and exceeded 115°C when the power was switched off and the sample started to cool. It should also be noted that with no thermal lag, as would occur if the sample had been left in a conventional oven, the temperature of the formulated product fell immediately when the microwave power was switched off.
Fig. 3.
A typical microwave power (broken line) and temperature (line) plot for the ‘dry melt’ formulation of stearic acid and ibuprofen (3:1) using microwave heating where the power has been maintained at a constant 25% (200 W)
Wet Melt Formulation
The samples (10 g) of PVP or stearic acid together with ibuprofen were prepared in 1:1 and 3:1 mass ratios. The dry powders were then tumble mixed for 5 min to achieve a homogenous mix. A sample mass of the powder was added to a beaker containing deionised water to achieve a 10% w/v solution and placed in the microwave oven. The power was manually adjusted until the water temperature reached 85°C, as monitored by the fibre optic probe. This temperature was maintained for 5 min, and the microwave power was then adjusted to allow cooling of the mixture. When the water had reached a temperature of 45°C, the product formulation was collected by vacuum filtration and dried overnight in a desiccator over silica gel. All formulations were sieved and those with a diameter between 80 and 250 μm collected for further analysis.
Drug Release Studies
Dissolution studies were performed using a rotating paddle, fully automated assembly, comprising a dissolution bath (Pharmatest PTW III) and a UV visible spectrophotometer (Cecil 3021, series 3000).
Weighed quantities of drug and excipient formulations, each sample totalling 200 mg, were placed in six separate containers containing 200 mL of aqueous phosphate buffer (0.2 M sodium dihydrogen phosphate and 0.2 M disodium hydrogen phosphate mixed together to produce a solution with pH 8) at 37 ±0.1°C and stirred at 50 rpm. pH 8 buffer was chosen for all experiments to ensure the solubility of the drug was not a concern as it is known to have improved aqueous solubility in basic media. Drug release profiles were established with sink conditions maintained throughout the analysis. All experiments were repeated in triplicate with the percentage drug release expressed with reference to the theoretical maximum absorbance (λmax) at 265 nm. Prior experiments confirmed that at this wavelength, there is no appreciable absorbance peak from either of the excipients used. All formulations were sieved and those with a diameter between 80 and 250 μm collected for further analysis.
Differential Scanning Calorimetry
Calorimetric experiments were performed using a DSC (Mettler Toledo 822), with 5–10 mg of each sample analysed in aluminium pans subjected to a heating and cooling cycle from 25°C to 125°C. All experiments included an isotherm of ten minutes after each stage with a heating rate of 10°C min−1 under nitrogen (flow rate 80 mL min−1).
RESULTS
Influence of Formulation Method—Traditional Heating versus Microwave Heating
Two methods were adopted to produce pharmaceutical formulations; firstly, one that has been established prior to this work and secondly, a novel approach using a modified microwave technique. With the latter, formulation production was quick and simple, requiring less heating time and therefore less total time to formulate. The unique design of the microwave system used in this work facilitated control of the power with accurate real-time temperature measurement. Hence, it was possible to monitor and control the temperature of the formulation process permitting controlled phase selection.
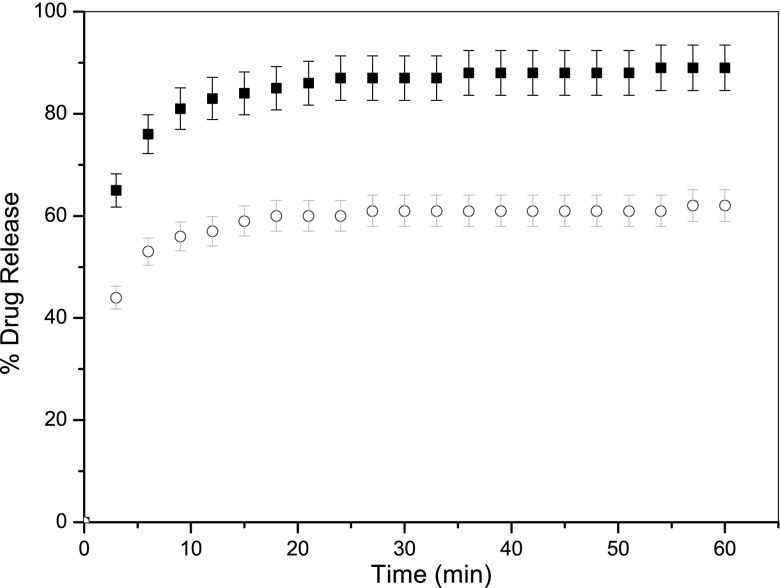
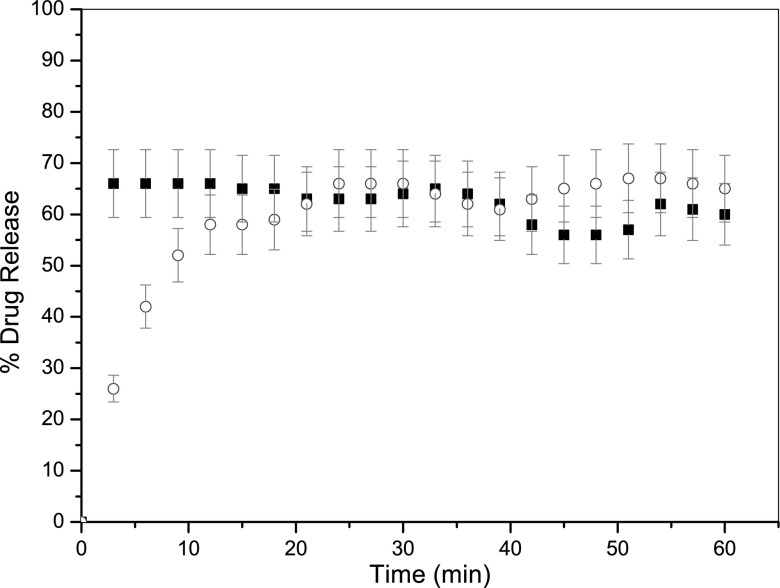
A series of formulations were then produced using both the traditional and controlled heating microwave methods with ibuprofen and two excipients (stearic acid and PVP). For ibuprofen and stearic acid, there were no obvious differences in the physical appearance of the products, the in vitro dissolution profiles (exemplified in Fig. 4) or the DSC profiles (exemplified in Fig. 5). Peaks corresponding to the melting of the drug and excipient in Fig. 5 are directly comparable with the plateau observed in Figs. 2 and 3, further confirming the accuracy and precision of the thermal control achieved. Similar results were observed for formulations containing ibuprofen and stearic acid.
Fig. 4.
Drug release profiles for formulations consisting of ibuprofen and excipient stearic acid ‘dry melt’ formulated using conventional heating (cirlce) and microwave heating (filled square). Each data point represents the mean of 3 results with S.D. (error bars)
Fig. 5.
DSC profiles as a function of temperature (dotted line) for formulations consisting of ibuprofen and excipient stearic acid ‘dry melt’ formulated using conventional heating (line) and microwave heating (broken line)
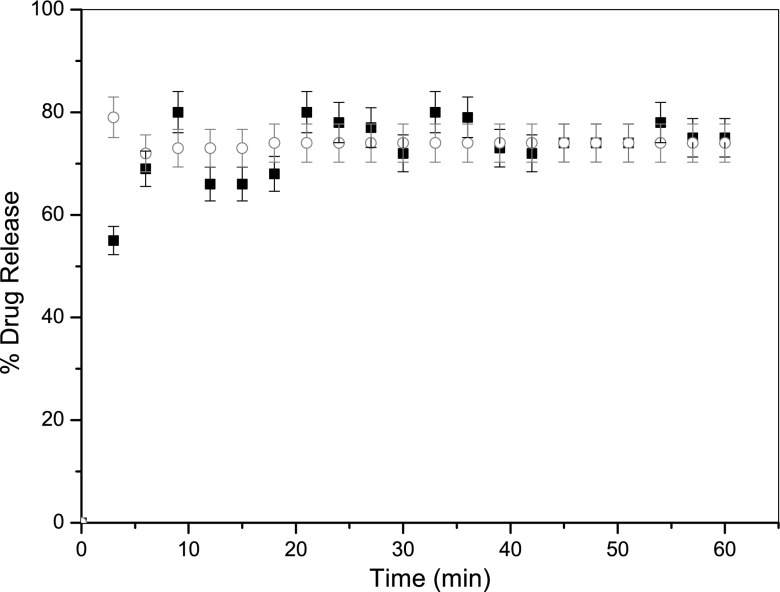
For ibuprofen with PVP the formulation process did appear to influence the rate, and extent of dissolution, as seen in Fig. 6. In this case, using microwave heating enhanced drug release by 27% (±3.1%) compared with conventional heating. An explanation for this difference is the focus of ongoing research as many factors may contribute to this phenomenon.
Fig. 6.
Drug release profiles for formulations consisting of ibuprofen and excipient PVP ‘dry melt’ formulated using conventional heating (circle) and microwave heating (filled square). Each data point represents the mean of 3 results with S.D. (error bars)
Influence of the Presence of Water on the Formulation Process
For ibuprofen and stearic acid formulations, no differences were observed in the drug release profile after 1 h, as seen in Fig. 7, with a final percentage drug release difference of 4% (±3.3%). However, the initial ten minutes of dissolution analysis did demonstrate differences in drug release with the absence of water in the formulation process appearing to retard drug release. This would imply that a lack of water gives rise to a process that encapsulates the drug to a greater extent within the microsphere.
Fig. 7.
Drug release profiles for ibuprofen and excipient stearic acid formulated using microwave heating in the presence (filled square) and absence (circle) of water. Each data point represents the mean of 3 results with S.D. (error bars)
For ibuprofen and PVP formulations, a similar result was obtained in that no differences were observed (after an initial period) in the drug release profiles in both the presence or absence of water, (Fig. 8). Although small differences in the initial rate of drug release are observed, these are much less significant than those seen for stearic acid in Fig. 7.
Fig. 8.
Drug release profiles for ibuprofen and excipient PVP formulated using microwave heating in the presence (filled square) and absence (circle) of water. Each data point represents the mean of 3 results with S.D. (error bars)
Analysis of the formulations using differential scanning calorimetry and IR spectroscopy confirmed that there was no evidence of degradation of ibuprofen as a result of the use of microwave heating. This is of particular concern as it is known that ibuprofen has several potential degradation products which may cause adverse effects upon administration. Such products are classed as impurities, and can form as a result of subjection to raised temperatures or prolonged storage. It is assumed that the extent of drug degradation throughout this experimental study is within the acceptable limits.
DISCUSSION
It can be said that the advantages of using microwave heating in the formulation process are numerous and, with no apparent disadvantages, it seems logical to incorporate this method in future formulation processes. The results presented in this work confirm that it has been possible to design, construct and utilise a new method of heating whereby the temperature of the formulation can be accurately and precisely controlled throughout the process. In addition, the use of microwave heating in this work appears to maintain, and in some cases enhance, drug release which can also be seen to be beneficial and potentially applicable to a wider range of compounds. For example, Fig. 6 compares conventional and microwave heating for ibuprofen with a preferential percentage of drug release over the entire experiment using the latter method. This is not believed to be as a result of a change in drug solubility but more likely to be from a retardation of release as a consequence of the physicochemical properties of the product formulated with conventional heating.
Secondly, it was found that there are no differences in the extent of drug release after one hour for ibuprofen with either excipient in the presence or absence of water. One particularly interesting difference is observed when comparing the initial drug release profiles using microwave heating with stearic acid. With water present in the formulation process, it would appear that there is an initial burst effect as drug is released rapidly, this agrees with published data (14). However, this is not observed when water is absent in the formulation process and further work is currently in progress to investigate this phenomenon further.
The method presented in this work is advantageous for a variety of reasons as the formulation process can be simplified to controlled microwave heating without the need for water to be present as a solvent. This is both environmentally and economically favourable since waste water and formulation time are considerably reduced using the method described in this work.
CONCLUSIONS
With respect to controlling drug release, two separate factors that can be modified during formulation were considered. Firstly, it was confirmed that exchanging conventional heating for a unique form of temperature controlled microwave heating in formulations that require a heating stage has no detrimental effect on the resultant drug release profile for stearic acid and ibuprofen. This is a very encouraging result as formulating using microwave-based methods provides many advantages, both economic and environmental. However, for ibuprofen and PVP, drug release profiles differed depending upon the method of formulation. Why this was seen in this situation will be the basis for some of our future research, for example, we hope to determine the amorphous content of the drug as this would have a pronounced impact on dissolution. Secondly, the presence of water during formulation was investigated and found not to influence subsequent drug release after the initial 15 min of study.
Overall, the modified microwave method presented in this work successfully permitted highly controlled temperature work to be undertaken to formulate pharmaceutical products, thus simplifying the formulation process and refining drug release.
ACKNOWLEDGEMENTS
The authors would like to thank the University of Huddersfield for funding S. Bedford and Dr. G. Midgley for useful discussions.
REFERENCES
- 1.Metaxas AC, Meredith RJ. Industrial microwave heating. 2. London: P. Peregrinus Ltd.; 1988. [Google Scholar]
- 2.National Research Council . Microwave processing of materials. Washington DC, USA: National Academy Press; 1994. [Google Scholar]
- 3.NMAB-473 Publication, Committee on microwave processing of materials: an emerging industrial technology . Microwave processing of materials. Washington DC, USA: National Academy Press; 1994. [Google Scholar]
- 4.Kingston HM, Haswell SJ. Microwave enhanced chemistry. Washington, USA: American Chemical Society; 1997. [Google Scholar]
- 5.Chee SN, Johansen AL, Gu L, Karlsen J, Heng PWS. Microwave drying of granules containing a moisture sensitive drug: a promising alternative to fluid bed and hot air oven drying. Chem Pharm Bull. 2005;53:770–775. doi: 10.1248/cpb.53.770. [DOI] [PubMed] [Google Scholar]
- 6.Loh ZH, Liew CV, Lee CC, Heng PWS. Microwave-assisted drying of pharmaceutical granules and its impact on drug stability. Int J Pharm. 2008;359:53–62. doi: 10.1016/j.ijpharm.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Moneghini M, Zingone G, De Zordi N. Influence of the microwave technology on the physical–chemical properties of solid dispersion with nimesulide. Powder Technol. 2009;195:259–263. doi: 10.1016/j.powtec.2009.06.006. [DOI] [Google Scholar]
- 8.Bergese P, Colombo I, Gervasoni D, Depero LE. Microwave generated nanocomposites for making insoluble drugs soluble. Mat Sci Eng C. 2003;23:791–795. doi: 10.1016/j.msec.2003.09.137. [DOI] [Google Scholar]
- 9.Moneghini M, Bellich B, Baxa P, Princivalle F. Microwave generated solid dispersions containing ibuprofen. Int J Pharm. 2008;361:125–130. doi: 10.1016/j.ijpharm.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Moneghini M, De Zordi N, Grassi M, Zingone G. Sustained release solid dispersions of ibuprofen prepared by microwave irradiation. J Drug Del Sci Tech. 2008;18:327–333. [Google Scholar]
- 11.Dwivedi SK, Sattari S, Jamali F, Mitchell AG. Ibuprofen racemate and enantiomers: phase diagram, solubility and thermodynamic studies. Int J Pharm. 1992;87:95–104. doi: 10.1016/0378-5173(92)90232-Q. [DOI] [Google Scholar]
- 12.Lavasanifar A, Samuel J, Kwon GS. The effect of fatty acid substitution on the in vitro release of amphotericin B from micelles composed of poly(ethylene oxide)-block-poly(N-hexyl stearate-L-aspartamide) J Control Rel. 2002;79:165–172. doi: 10.1016/S0168-3659(01)00537-5. [DOI] [PubMed] [Google Scholar]
- 13.Robson HJ, Craig DQM, Deutsch D. An investigation into the release of cefuroxime axetil from taste-masked stearic acid microspheres Part 2: the effects of buffer composition on drug release. Int J Pharm. 2000;195:137–145. doi: 10.1016/S0378-5173(99)00391-9. [DOI] [PubMed] [Google Scholar]
- 14.Waters LJ, Pavlakis E. In vitro controlled drug release from loaded microspheres—dose regulation through formulation. Can J Pharm Pharm Sci. 2007;10:464–472. doi: 10.18433/j3cc7t. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein MM, Shandryuk GA, Kuptsov SA, Platé NA. Coherence of thermal transitions in poly(N-vinyl pyrrolidone)-poly(ethylene glycol) compatible blends 1. Interrelations among the temperatures of melting, maximum cold crystallization rate and glass transition. Polymer. 2000;41:5327–5338. doi: 10.1016/S0032-3861(99)00802-2. [DOI] [Google Scholar]
- 16.Serratoni M, Newton M, Booth S, Clarke A. Controlled drug release from pellets containing water-insoluble drugs dissolved in a self-emulsifying system. Eur J Pharm Biopharm. 2007;65:94–98. doi: 10.1016/j.ejpb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.O'Hara T, Dunne A, Butler J, Devane J. A review of methods used to compare dissolution profile data. Pharm Sci Tech Today. 1998;1:214–223. doi: 10.1016/S1461-5347(98)00053-4. [DOI] [Google Scholar]
- 18.Albertini B, Cavallari C, Passerini N, Voinovich D, González-Rodríguez ML, Magarotto L, Rodriguez L. Characterization and taste-masking evaluation of acetaminophen granules: comparison between different preparation methods in a high-shear mixer. Eur J Pharm Sci. 2004;21:295–303. doi: 10.1016/j.ejps.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Shi J, Li Y, Chen H, Shen W, Dong X. Storage and release of ibuprofen drug molecules in hollow mesoporous silica spheres with modified pore surface. Micropor Mesopor Mater. 2005;85:75–81. doi: 10.1016/j.micromeso.2005.06.015. [DOI] [Google Scholar]
- 20.Furlanetto S, Cirri M, Maestrelli F, Corti G, Mura P. Study of formulation variables influencing the drug release rate from matrix tablets by experimental design. Eur J Pharm Biopharm. 2006;62:77–84. doi: 10.1016/j.ejpb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Dürig T, Fassihi AR. Identification of stabilising and destabilising effects of excipient-drug interactions in solid dosage form design. Int J Pharm. 1993;9:161–170. doi: 10.1016/0378-5173(93)90136-4. [DOI] [Google Scholar]
- 22.Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Analysis. 2005;37:949–955. doi: 10.1016/j.jpba.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Lerdkanchanaporn S, Dollimore D, Evans SJ. Phase diagram for the mixtures of ibuprofen and stearic acid. Thermochim Acta. 2001;367:1–8. doi: 10.1016/S0040-6031(00)00656-0. [DOI] [Google Scholar]