Abstract
The objective of this study was to investigate the release behaviour of propranolol hydrochloride from psyllium matrices in the presence hydrophilic polymers. The dissolution test was carried out at pH 1.2 and pH 6.8. Binary mixtures of psyllium and hydroxypropyl methylcellulose (HPMC) used showed that an increase in the percentage of HPMC in the binary mixtures caused a significant decrease in the release rate of propranolol. Psyllium–alginate matrices produced lower drug release as compared to when the alginate was the matrix former alone. When sodium carboxy methyl cellulose (NaCMC) was incorporated into the psyllium, the results showed that matrices containing the ratio of psyllium–NaCMC in the 1:1 ratio are able to slow down the drug release significantly as compared to matrices made from only psyllium or NaCMC as retardant agent suggesting that there could be a synergistic effect between psyllium and NaCMC. The double-layered tablets showed that the psyllium and HPMC in the outer shell of an inner formulation of psyllium alone had the greatest effect of protecting the inner core and thus producing the lowest drug release (DE = 38%, MDT = 93 min). A significant decrease in the value of n in Q = ktn from 0.70 to 0.51 as the psyllium content was increased from 50 to 150 mg suggests that the presence of psyllium in HPMC matrices affected the release mechanism. Psyllium powder had the ability in the combination with other hydrophilic polymers to produce controlled release profiles. Care and consideration should as such be taken when formulating hydrophilic matrices in different combinations.
KEY WORDS: drug release, hydroxypropyl methylcellulose, ionic polymers, psyllium husk, synergistic effect
INTRODUCTION
The use of naturally occurring biocompatible polymeric materials has been the focus of research activity in the design of dosage forms for oral controlled release administration (1–6). Polymeric hydrogels are studied for controlled release applications because of their ability in producing drug release close to zero-order kinetics (7–13). Gums from natural sources hydrate and swell on contact with water and have been used for the preparation of single unit dosage forms (14).
Psyllium has been used for the treatment of constipation (15), diarrhoea (16), colon cancer (17) and diabetes (18). Psyllium not only has pharmacological importance, but it also can be used to develop drug delivery devices such as sustained release matrix tablets and hydrogels. Therefore, the double potential of the psyllium hydrogel can be used to prepare novel drug delivery systems (19). The main part of psyllium seed to produce gel is its husk which can be separated through mechanical grinding of its seeds.
Due to the high swelling capability of psyllium when it contacts with water, it has been used in gastroretentive sustained release formulations of ofloxacin (20). Psyllium husk is known to have bioadhesive properties similar to hydroxypropyl methylcellulose (HPMC). In contact with water medium, the mucilage swells and thus it could cause bioadhesion via physical or hydrogen bonds. This bioadhesive property can be beneficial to keep the tablets in the upper GI tract and to increase the gastro-retention time. The swelling property of psyllium was found to be greater than that of HPMC despite taking a longer time to swell (20). The in vitro drug release profile followed Higuchi kinetics and the drug release mechanism was reported as non-Fickian.
Several researchers have modified psyllium husk powder to improve its application in drug delivery systems. Gohel and Amin (21) modified psyllium husk powder with tartaric and succinic acid to develop a suitable sustained release tablet for diltiazem HCl via direct compression. The treated psyllium husk powder showed better gelling and swelling characteristics.
Kaith and coworkers (22) modified psyllium with acrylic acids using KPS-HMTA to optimize the polymer gel. The gel was found to be pH and temperature sensitive and selective towards water absorption from oil–water emulsions.
Singh and coworkers (19) also conducted research to develop psyllium hydrogels by modification using acrylic acid and radiation. They showed that psyllium hydrogels have the capability to be used as double potential drug delivery devices in the colon to provide drug in a controlled and sustained manner. In addition, their investigation on the drug release mechanism from the polymer matrix has led to the conclusion that the drug release occurs via non-Fickian diffusion. In this mechanism, the rate of drug release is dependent on the simultaneous water migration to the matrix and the drug diffusion through the swelling hydrogel.
As discussed above, there are several studies attempting to modify psyllium husk properties for drug delivery purposes, but there is no data reported in the literature about the release behaviour of drug from psyllium matrices in the presence of other hydrophilic polymers. Therefore, in the present study, the effect of HPMC K4M, sodium alginate (Na alginate) and sodium carboxy methyl cellulose (NaCMC) in different concentrations on the drug release from psyllium matrices was investigated. The kinetics of drug release from various matrices was also investigated using power law equation.
MATERIALS AND METHODS
Propranolol hydrochloride (S.I.M.S., Italy), HPMC K4M (Methocel, Colorcon, UK), psyllium seed (Herbi Daru, Iran), NaCMC (low viscosity grade, 4% solution 200 cps at 25°C. Sigma, UK), Na alginate (low viscosity grade, 2% solution 250 cps at 25°C, BDH, UK), lactose monohydrate (Lactose Pharma, Alpavit, Germany) and magnesium state (Merck, Germany) were used.
Separation of Husk from Psyllium Seeds
Psyllium seeds were ground for 5 min to separate husk from the seeds. This grinding enables to get a greater yield (25% w/w). Grinding causes the husk to be fragmented under collision without a significant breakage and size reduction of the non-husk portion. The ground seeds were sieved to separate fine husk particles (usually smaller than 425 μm) from the seeds (usually larger than 1 mm).
Preparation of Tablets
Propranolol hydrochloride matrix tablets were produced by mixing the drug with psyllium for a period of 10 min in a cubic mixer (Erweka, Type UG, Germany). The mixture was then mixed with 0.5% magnesium stearate for an additional 2-min mixing. The mixture was then compressed on an 11-mm punch and die using a manual-tableting machine at a pressure of 150 bar. In the case of the other hydrophilic polymers, the binary mixtures of psyllium–polymer with ratios of 3:1, 1:1 and 1:3 were mixed with drug. Each formulation contained 80 mg propranolol hydrochloride, 200 mg polymer blends, 100 mg lactose as a filler and 0.5% w/w magnesium stearate as a lubricant (Table I).
Table I.
Composition of Matrix Formulations (Binary and Ternary Polymer Mixture)
| Formulation code | Psyllium (mg) | HPMC K4M (mg) | Na Alginate (mg) | NaCMC (mg) |
|---|---|---|---|---|
| F1 | 200 | 0 | 0 | 0 |
| F2 | 150 | 50 | 0 | 0 |
| F3 | 100 | 100 | 0 | 0 |
| F4 | 50 | 150 | 0 | 0 |
| F5 | 0 | 200 | 0 | 0 |
| F6 | 150 | 0 | 50 | 0 |
| F7 | 100 | 0 | 100 | 0 |
| F8 | 50 | 0 | 150 | 0 |
| F9 | 0 | 0 | 200 | 0 |
| F10 | 150 | 0 | 0 | 50 |
| F11 | 100 | 0 | 0 | 100 |
| F12 | 50 | 0 | 0 | 150 |
| F13 | 0 | 0 | 0 | 200 |
| F14 | 50 | 75 | 75 | 0 |
| F15 | 66.6 | 66.6 | 66.6 | 0 |
| F16 | 0 | 100 | 100 | 0 |
| F17 | 100 | 50 | 50 | 0 |
| F18 | 75 | 100 | 25 | 0 |
| F19 | 50 | 100 | 50 | 0 |
All formulations contain 100 mg lactose, 80 mg drug and 0.5% magnesium stearate
In the case of the preparation of tablets from ternary mixtures of polymers (psyllium–alginate–HPMC), all polymers with different ratios and propranolol HCl were mixed for a 10 min in a cube mixer followed by the addition of 0.5% magnesium state for an additional 2 min mixing. The mixture was compressed into tablets as described above. All the compositions for ternary mixtures are listed in Table I.
Preparation of the Double-Layered Tablets
In this type of tablets, the inner formulation was made separately and was inserted into the outer layer formulation as follows. After mixing the inner components (Table II), the mixture was compressed by a 7-mm punch and die, and this matrix was used as a core of tablet or inner layer of tablet. The final weight of the inner tablet was 190 mg. Then the components of outer layer (shell) were mixed for 10 min (Table II), and the half of the outer layer formulation was introduced into the 11 mm die. Then the inner 7-mm tablet was placed inside the die and was filled with the rest of the outer layer (the total amount of outer layer was also 190 mg). Both inner tablet and outer layer were compressed together at a force of 150 bar. The geometric tablets were stored in a glass vial until use.
Table II.
Composition for Double-Layered Tablets
| Formulation code | Inner formulation (Core) | Outer layer (Shell) | ||
|---|---|---|---|---|
| Psyllium (mg) | HPMC K4M (mg) | Psyllium (mg) | HPMC K4M (mg) | |
| F20 | 75 | 0 | 0 | 75 |
| F21 | 75 | 0 | 37.5 | 75 |
| F22 | 75 | 0 | 75 | 75 |
All formulations contain 100 mg lactose, 80 mg drug and 0.5% magnesium stearate. Each layer contains 40 mg drug (80 mg in total)
In Vitro Drug Release
The in vitro dissolution tests were performed on the USP dissolution apparatus 1 (basket method; Erweka, DPT6R, Germany), using 900 ml dissolution medium (pH 1.2 or pH 6.8) prepared according to USP propranolol extended release capsules monograph (USP 26) with a rotation speed of 100 rpm. The amount of propranolol hydrochloride was 80 mg in all formulations. The dissolution tests for all tablets were run for 2 h in a simulated gastric fluid (HCl solution, pH 1.2 without pepsin) at 37°C. After 2 h the baskets containing the matrix tablets were immediately transferred into dissolution vessel containing 900 mL simulated intestinal fluid (phosphate buffer, pH 6.8 without pancreatin) at 37°C for 6 h. As none of the tablets disintegrated during the dissolution run at pH 1.2, therefore, it was easy to transfer the basket holding the whole tablet to another dissolution medium. Samples were collected at suitable time intervals. Five millilitres of aliquot was removed from each dissolution vessel and filtered through a 0.45-μm filter (Millipore Corp., Bedford, MA, USA). The same amount of fresh dissolution fluid was added to replace the amount withdrawn. The samples were then analysed at 288.5 nm by UV/visible spectrophotometer. The mean of 3 determinations was used to calculate the drug release from each of the formulation. The maximum standard deviation for all dissolution data was less than 6%.
Drug Release Kinetics
The dissolution profiles were described by the power law firstly proposed by Peppas and Korsemeyer, and known as Peppas model, where log cumulative percentage of drug release is plotted against the log of time (23,24). In this approach, so-called diffusion model, it is assumed that the drug is initially uniformly distributed through a polymeric matrix. In order to investigate the release kinetics from the studied matrices, the drug release data between 5% and 60% were fitted to Eq. 1 as proposed by Ritger and Peppas (25).
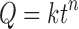 |
1 |
where Q is the percentage of drug released at time t, k is the kinetic constant representing structural and geometric characteristic of the tablet and n is the release exponent indicative of the drug release mechanism (25). For matrix (cylindrical) tablets, an n value equal to or less than 0.45 indicates Fickian mechanism (or case-I) mainly controlled by diffusion. For n ≥ 0.89 (i.e. 0.89 < n <1.00), a super case-II transport takes place, when dissolution process is controlled mainly by erosion and the release rate is independent of time (‘zero-order’ kinetics). Intermediate values (i.e. 0.45 ≤ n ≤ 0.89) represent a non-Fickian or anomalous transport and suggest that erosion (polymer matrix relaxation) and drug diffusion both contribute to the overall drug release mechanisms (25). A very high k value may be an indication of a burst release from the matrix.
To compare the effects of polymer or diluents concentrations on the drug release, the criteria mean dissolution time (MDT) and dissolution efficiency (DE) were used as described in Eqs. 2 and 3 respectively
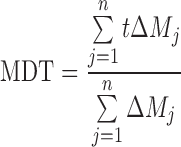 |
2 |
In Eq. 2 (26), j is the sample number, n is the number of dissolution sample times, t is the time at midpoint between t and t−1 (easily calculated with (t + t−1)/2) and ΔMj is the additional amount of drug dissolved between t and t−1. DE is as described below:
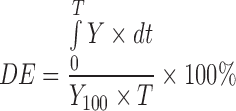 |
3 |
In Eq. 3 (27), Y is the percent drug release as a function of time, T is the total time of drug release and Y100 is 100% drug release.
RESULTS AND DISCUSSION
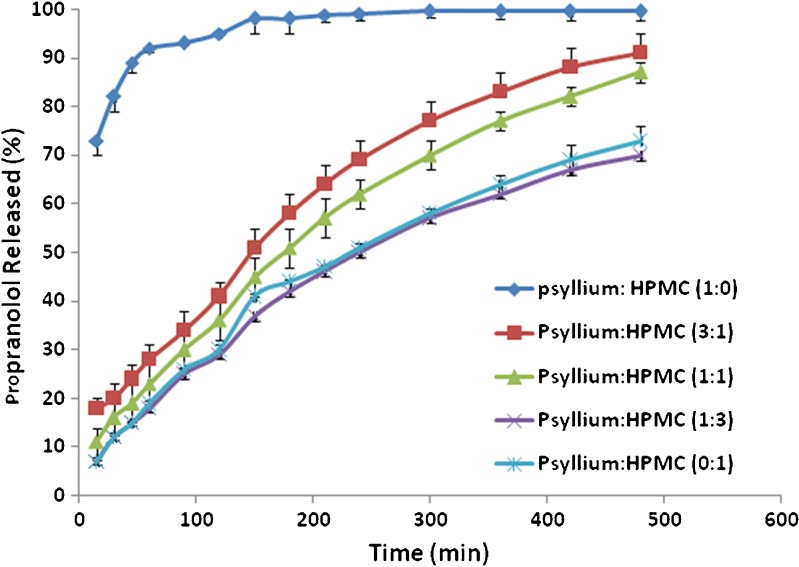
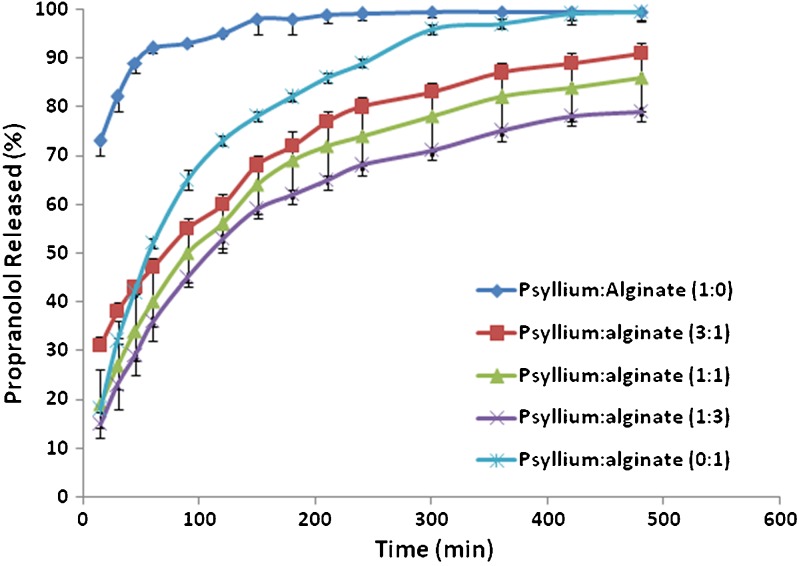
Figure 1 shows the dissolution characteristics of matrices prepared with ground psyllium husk and binary mixtures of psyllium husk and HPMC K4M. In vitro release profiles of propranolol showed that the use of psyllium husk in the absence of HPMC produced relatively fast dissolution. This indicates that psyllium husk is not able to produce extended release matrices containing free water soluble drug per se. This is due to a high swellability of the psyllium husk (psyllium mucilage mainly consists of 75% xylose and 23% arabinose) in contact with water which in turn destroys the integrity of these tablets during the dissolution period after swelling. This is opposite to the results reported by Chavanpatil and coworkers (20). They showed that higher concentrations of psyllium in a formulation had the ability to reduce drug release and maintain its integrity. This could be due to the presence of different excipients/polymers used in these two studies. Chavanpatil et al. (20) used the highest viscosity grade of HPMC (i.e. HPMC K100M) and β-cyclodextrin which they have the capability to make strong gel layer around the matrix compared to HPMC K4M and lactose (highly water soluble filler) used in the present study. Interesting results were observed when binary mixtures of psyllium husk and HPMC were used as a matrix former. The results showed that an increase in the percentage of HPMC in binary mixtures of psyllium–HPMC resulted in a decrease in the release rate of propranolol (Fig. 1). Figure 1 shows that by addition of 50 mg HPMC to formulations containing 150 mg psyllium husk (ratio of psyllium–HPMC is 3:1) remarkable reduction in the release rate was observed. The DE and MDT values changed considerably from 95% and 22 min when psyllium was just the matrix former to a value of 62% and 95 min upon addition of the 50 mg of HPMC in formulation 2 (F2). There was no difference in the drug release between matrices containing 200 mg HPMC and matrices made from binary mixtures of polymers (50 mg psyllium and 150 mg HPMC). These results were confirmed by dissolution efficiencies and mean dissolution times (Table III). The matrices containing 200 mg HPMC and matrices made from binary mixtures of polymers (50 mg psyllium and 150 mg HPMC) had DE values of 47% and 46% and mean dissolution times of 93 min and 98 min respectively, indicating not only their similarity but also their ability in producing lower release rates for these formulations. HPMC is a non-ionic hydrophilic polymer. When the HPMC was replaced by sodium alginate, which is an anionic polymer, in the matrices containing different concentrations of psyllium, different drug release patterns were obtained compared with the binary mixtures of psyllium and HPMC. The results showed that the slowest drug release occurred for the formulation when the ratio of psyllium–alginate was 1:3 (Fig. 2). This ratio had the lowest DE value of 61% and an MDT of 73 min. This release rate was considerably slower than the matrices containing only alginate (DE = 80%, MDT = 79 min). Drug release from the alginate matrices alone was quite fast. One of the most extensive methods studied for the controlling the release of drug molecules is cross-linking. This is a method that allows the incorporating of polymers to produce hydrogels that can prolong drug release. Psyllium as such increases the retardant property of sodium alginate. It can be concluded that neither pure psyllium nor sodium alginate are able to slow down the drug release, whereas their binary mixtures (3:1, 1:1 and 1:3) showed a significant reduction in the drug release (DE values of 72%, 61% and 67%, respectively).
Fig. 1.
The effect of HPMC K4M on drug release from matrices containing psyllium
Table III.
Release Characteristics of the Formulated Tablet Matrices
| Formulation code | DE480 min (%) | MDT (min) | RSQ | n value |
|---|---|---|---|---|
| F1 | 94.93 | 22.07 | – | – |
| F2 | 61.91 | 95.44 | 0.9507 | 0.51 |
| F3 | 56.08 | 95.78 | 0.9909 | 0.64 |
| F4 | 45.41 | 97.93 | 0.9975 | 0.70 |
| F5 | 46.92 | 92.98 | 0.9953 | 0.71 |
| F6 | 72.02 | 67.17 | 0.9974 | 0.32 |
| F7 | 66.63 | 73.26 | 0.9990 | 0.53 |
| F8 | 60.84 | 73.10 | 0.9961 | 0.58 |
| F9 | 80.38 | 78.84 | 0.9941 | 0.72 |
| F10 | 96.22 | 18.15 | – | – |
| F11 | 64.66 | 70.89 | 0.9974 | 0.50 |
| F12 | 91.01 | 40.10 | – | – |
| F13 | 87.09 | 41.48 | – | – |
| F14 | 61.53 | 83.39 | 0.9997 | 0.61 |
| F15 | 55.36 | 89.34 | 0.9986 | 0.63 |
| F16 | 51.67 | 87.73 | 0.9658 | 0.71 |
| F17 | 61.25 | 85.12 | 0.9993 | 0.62 |
| F18 | 69.42 | 79.11 | 0.9965 | 0.47 |
| F19 | 50.81 | 88.00 | 0.9979 | 0.63 |
| F20 | 58.33 | 100.60 | 0.9938 | 0.66 |
| F21 | 64.13 | 92.31 | 0.9945 | 0.61 |
| F22 | 37.91 | 93.31 | 0.9966 | 0.67 |
–Indicates drug release was too quick for n values to be obtained
Fig. 2.
The effect of sodium alginate on drug release from matrices containing psyllium
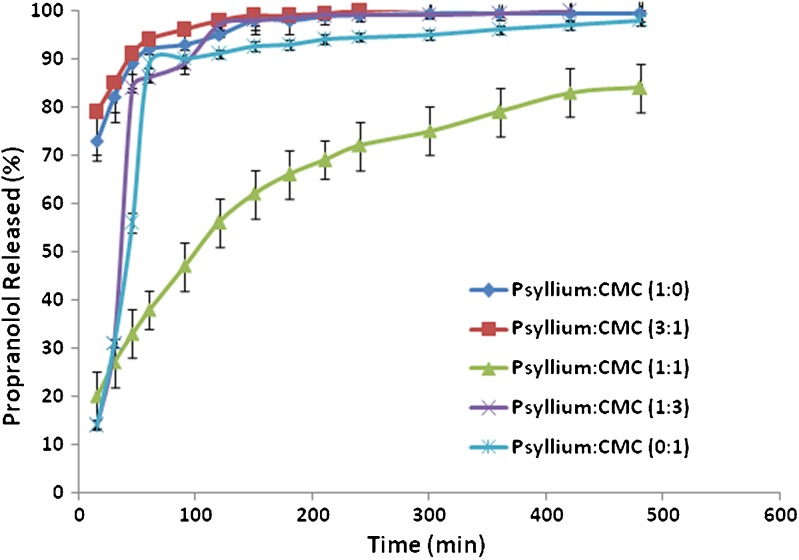
The use of chemically or physically cross-linked polysaccharides, such as carboxy methyl cellulose, is one of the marked thrust on research activities related to the use of natural polysaccharides for the development of the hydrogels as colon specific drug delivery devices (28). The effect of sodium carboxy methyl cellulose (NaCMC) on the release of propranolol from psyllium matrices was interesting (Fig. 3). The drug release from matrices containing either psyllium or NaCMC was faster (the DE value of psyllium alone as a matrix former was 95% and an MDT of 22 min, whereas NaCMC as just the matrix former had a DE value of 87% and an MDT of 41 min). These are all depicted in Table III. Therefore, it is expected that if binary mixtures of psyllium–NaCMC were used the drug release still would be faster. In fact, the results showed that matrices containing the ratio of psyllium–NaCMC 1:1 are able to slow down the drug release significantly (p < 0.05) compared with matrices made from only psyllium or NaCMC as retardant agent (DE = 65%, MDT = 71 min). It is known that for a densely cross-linked gel the release of a solute is more difficult than from a loosely cross-linked network (29). Bajpai and Giri (30) showed that CMC in conjugation with cross-linked polyacrylamide formed a type of highly swelling hydrogel which had the ability to imbibe as much as 60 g water per grammes of dry gel. The crosslink density of a hydrogel is as such intimately related to the degree of swelling and consequently the release behaviour of the hydrogel. The ability of the ratio of psyllium–NaCMC 1:1 to slow down the drug release significantly shows that there should be a synergistic effect between psyllium and NaCMC possibly due to an interaction between polysaccharides (xylose and arabinose) present in psyllium husk with the sodium CMC. It can be concluded that when anionic polymers such as sodium CMC and sodium alginate were used in combination with psyllium, generally binary mixtures showed slower release rate compared to single polymer due to synergistic effect whereas this is not the case when non-ionic polymer such as HPMC was used in binary mixtures with psyllium husk. The possible explanation for this could be the cross link between polysaccharide (xylose and arabinose) contained in psyllium husk and negatively charged polymers. Xylose, particularly arabinose, could be protonated at lower pH (gastric-simulated medium). Therefore, in the case of protonation, there is a possibility of cross-linking between these polysaccharides and ionic polymer such as alginate or carboxy methyl cellulose.
Fig. 3.
The effect of sodium CMC on drug release from matrices containing psyllium
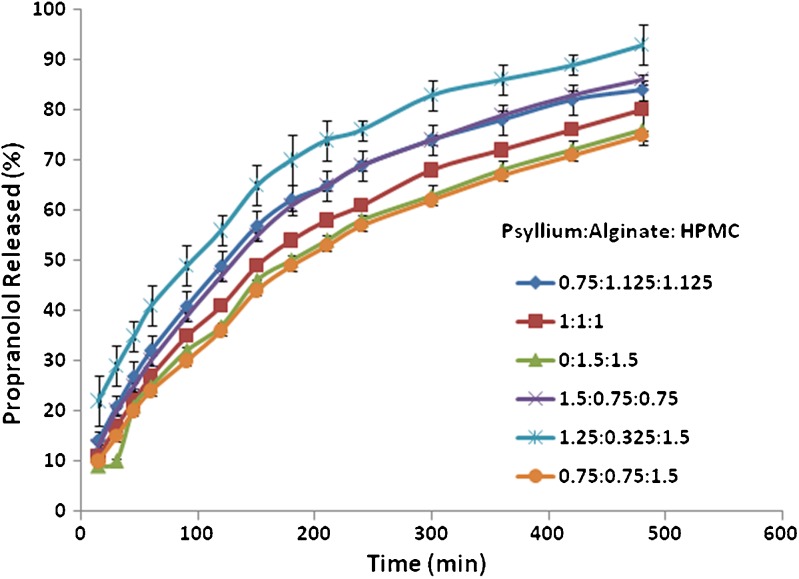
Further investigation was carried out to use ternary mixtures of polymers (psyllium–alginate–HPMC). These ternary polymer mixtures were formulated into tablets using the same model drug propranolol hydrochloride and their dissolution behaviours were investigated. Their dissolution profiles are depicted in Fig. 4. The results showed that by changing the ratios of psyllium–alginate–HPMC, different drug release rates with different release patterns could be obtained. This indicates that by changing the ratio of these polymers the drug release could be modulated to achieve a desirable release profile. The results showed the fastest drug release occurring when alginate was in the smallest concentration as compared to the other two polymers (psyllium–alginate–HPMC 1.25:0.325:1.5), and the slower drug release was observed for the matrices containing more HPMC with psyllium and alginate at the same levels (psyllium–alginate–HPMC 0.75:0.75:1.5) or without psyllium (psyllium–alginate–HPMC 0:1.5:1.5).
Fig. 4.
The release behaviour of propranolol HCI from ternary polymers containing psyllium
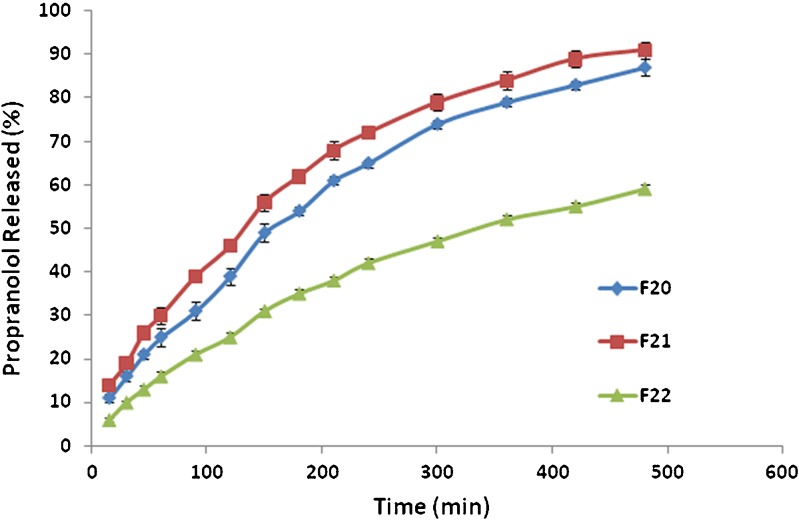
Interesting results were obtained when psyllium was used in a double-layered tablet formulation design (Table II). In these formulations, the core of tablets was made with a smaller tablet size, and then the core was placed within the outer layer formulation and compressed with a bigger punch. The aim of the presence of outer layer in this study was that to see if the amount of HPMC was kept constant in outer layer what would happen if psyllium was incorporated to this layer from 0 to 75 mg. Their release profiles were shown in Fig. 5. The results showed that when psyllium was incorporated in the core formulation and HPMC in outer layer formulation, the release of drug is faster than when mixtures of psyllium–HPMC was included in the outer layer formulation (F22). This indicates that HPMC alone is not able to protect the core, but when psyllium was incorporated to the shell formulation alongside with HPMC, due to synergistic effect between HPMC and psyllium which was discussed before the gel produced around the tablet is strong enough to protect the core. This leads to slow release of the drug from formulation F22. Comparing F21 and F22 showed that when the ratio of psyllium–HPMC in the shell formulation is 1:1 the drug release is slower than when the ratio of psyllium–HPMC is 0.5:1. DE and MDT values for F20, F21 and F22 are 58% and 101 min, 64% and 92 min and 38% and 93 min, respectively. The F22 formulation had the lowest DE of all the formulations.
Fig. 5.
The effect of outer and inner composition on drug release from geometric matrix tablets
DE, mean dissolution time, and mean dissolution rate (MDR) were calculated and the results were reported in Table III. Generally speaking, the statistical analysis of the DE, MDR and MDT from the drug release profiles indicated that a decrease in the drug release profiles occurred for the formulations that had the ability to retard the drug release in their binary mixtures.
The drug releases from psyllium alone and NaCMC alone as matrix formers were too quick to allow ascertaining the kinetics of drug release occurring. Also the binary mixtures of psyllium and NaCMC in the ratios of 3:1 and 1:3 were too quick and as such drug kinetics were not calculated for them as they did not fit the criterion for Peppas. With the exception of formulation F6 where psyllium and Na alginate were in the ration of 3:1 with Fickian diffusion occurring with a value of n as 0.32, all the other formulations had anomalous transport occurring with n values ranging from 0.47 to 0.72.
It is clearly seen that when the psyllium content increased from 50 (F4) to 150 mg (F2), the n value was significantly decreased from 0.70 to 0.51. This indicates that the presence of psyllium in HPMC matrices can affect the release mechanism. Similar values of n of 0.6 (31) and 0.64 (32) were found for propranolol hydrochloride release from hypomellose K4M and hypomellose K15M matrices, respectively. Other values of n obtained for soluble drugs include 0.71 for centperazine release (33) and 0.59 for alperenolol release (31) from matrices containing NaCMC and hypomellose, indicating diffusional-controlled release. Comparing n values of all binary mixtures of psyllium–HPMC and psyllium–Na Alginate showed that in the presence of psyllium the contribution of diffusion is more than the contribution of diffusion when psyllium was removed from HPMC or alginate formulations. In the case of ternary mixtures of polymers (F14 to F19), there was no particular trend for n values as reported in Table I. However, it might be concluded that the contribution of diffusion is very high when the concentration of alginate is very low (F18) showing lower n value of 0.47 as compared to the higher concentrations of alginate where the n values varied between 0.61 and 0.71.
CONCLUSION
The presence of HPMC K4M, sodium alginate and sodium carboxy methyl cellulose (NaCMC) in the different concentrations on the drug release from psyllium matrices had interesting results. HPMC was able to remarkably retard drug release from the psyllium matrices. The Na alginate was also able to reduce/retard drug release from the psyllium matrices as indicated in Table III. The NaCMC however had to be in the right ratio of 1:1 with psyllium to see a significant retardation of the drug release profile. The ternary mixture of the polymers proved very useful in being able to produce different drug release profiles. The results from the double-layered tablets showed that when psyllium was incorporated in the core formulation and HPMC in the outer layer formulation the release is faster than when mixtures of psyllium–HPMC was included in the outer layer formulation in a ratio of 1:1. With anomalous transport being the dominant kinetics of drug release with values ranging from 0.47 to 0.72, it is very evident that that manipulation and combination of polymers in different ratios and viscosity grades should be carefully considered when formulating hydrophilic polymers.
REFERENCES
- 1.Naggar VF, El-Khawas M, Ismail FA, Boraie NA. Pectin, a possible matrix for oral sustained-release preparations of water-soluble drugs. STP Pharma Sci. 1992;2:227–34. [Google Scholar]
- 2.Bonferoni MC, Rossi S, Tamayo M, Pedraz JL, Dominguez-Gil A, Caramella C. On the employment of lcarrageenan in a matrix system. I. Sensitivity to dissolution medium and comparison with Na carboxymethylcellulose and xanthan gum. J Control Release. 1993;26:119–27. doi: 10.1016/0168-3659(93)90111-H. [DOI] [Google Scholar]
- 3.Kristmundsdóttir T, Ingvarsdottir K, Sæmundsdottir G. Chitosan matrix tablets: the influence of excipients on drug release. Drug Dev Ind Pharm. 1995;21:1591–8. doi: 10.3109/03639049509069249. [DOI] [Google Scholar]
- 4.Khullar P, Khar RK, Agarwal SP. Evaluation of guar gum in the preparation of sustained-release matrix tablets. Drug Dev Ind Pharm. 1998;24:1095–9. doi: 10.3109/03639049809089955. [DOI] [PubMed] [Google Scholar]
- 5.Vervoort L, Van den Mooter G, Augustijns P, Kinget R. Inulin hydrogels. I. Dynamic and equilibrium swelling properties. Int J Pharm. 1998;172:127–35. doi: 10.1016/S0378-5173(98)00200-2. [DOI] [Google Scholar]
- 6.Nokhodchi A, Nazemiyeh H, Khodaparast A, Sorkh-Shahan T, Valizadeh H, Ford JL. An in vitro evaluation of fenugreek mucilage as a potential excipient for oral controlled-release matrix tablet. Drug Dev Ind Pharm. 2008;34:323–9. doi: 10.1080/03639040701662594. [DOI] [PubMed] [Google Scholar]
- 7.Colombo P, Conte U, Caramella C, Gazzaniga A, La Manna A. Compressed polymeric mini-matrices for drug release control. J Control Release. 1985;1:283–9. doi: 10.1016/0168-3659(85)90004-5. [DOI] [Google Scholar]
- 8.Colombo P, Bettini R, Massimo G, Catellani PL, Santi P, Peppas NA. Drug diffusion front movement is important in drug release control from swellable matrix tablets. J Pharm Sci. 1995;84:991–7. doi: 10.1002/jps.2600840816. [DOI] [PubMed] [Google Scholar]
- 9.Mockel JE, Lippold BC. Zero-order drug release from hydrocolloid matrices. Pharm Res. 1993;10:1066–70. doi: 10.1023/A:1018931210396. [DOI] [PubMed] [Google Scholar]
- 10.Hussain AS, Johnson RD, Shivanand P, Zoglio MA. Effects of blending a nonionic and an anionic cellulose ether polymer on drug release from hydrophilic matrix capsules. Drug Dev Ind Pharm. 1994;20:2645–57. doi: 10.3109/03639049409042668. [DOI] [Google Scholar]
- 11.Munday DL, Cox PJ. Compressed xanthan and karaya gum matrices: hydration, erosion and drug release mechanism. Int J Pharm. 2000;203:179–92. doi: 10.1016/S0378-5173(00)00444-0. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds TD, Gehrke SH, Hussain AS, Shenouda LS. Polymer erosion and drug release characterisation of hydroxypropylmethylcellulose matrices. J Pharm Sci. 1998;87:1115–23. doi: 10.1021/js980004q. [DOI] [PubMed] [Google Scholar]
- 13.Ughini F, Andreazza IF, Ganter JLMS, Bresolin TMB. Evaluation of xanthan and highly substituted galactomannan from M. scabrella as a sustained release matrix. Int J Pharm. 2004;271:197–205. doi: 10.1016/j.ijpharm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Nakano M, Ogata A. Examination of natural gums as matrices for sustained release of theophylline. Chem Pharm Bull. 1984;32:782–5. doi: 10.1248/cpb.32.782. [DOI] [PubMed] [Google Scholar]
- 15.McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic conspitation. Aliment Pharmacol Ther. 1998;12:491–7. doi: 10.1046/j.1365-2036.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Belknap D, Davidson LJ, Smith CR. The effects of psyllium hydrophilic mucilliod on diarrhea in enterally fed patients. Heart Lung. 1997;26:229–37. doi: 10.1016/S0147-9563(97)90060-1. [DOI] [PubMed] [Google Scholar]
- 17.Juarranz M, Calle-Puron ME, Gonzalez-Navarro A, Regidor-Poyatos E, Soriano T, Martinez-Hernandez D, Rojas VD, Guinee VF. Physical exercise, use of Plantago ovata and aspirin, and reduced risk of colon cancer. Eur J Cancer Prev. 2002;11:465–72. doi: 10.1097/00008469-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinz DJ, Kendall CW, Axelsen M, Augustin LAS, Vuksa V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lippidol. 2000;11:49–56. doi: 10.1097/00041433-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Kumar S, Chauhan N. Radiation crosslinked psyllium and polyacrylic acid based hydrogels for use in colon specific drug delivery. Carbohydr Polym. 2008;73:445–6. [Google Scholar]
- 20.Chavanpatil M, Jain P, Chaudhari S, Shear R, Vavia P. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm. 2006;316:86–92. doi: 10.1016/j.ijpharm.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Gohel M, Patel M, Amin A. Development of modified release diltiazem HCl tablets using composite index to identify optimal formulation. Drug Dev Ind Pharm. 2003;29:565–74. doi: 10.1081/DDC-120018645. [DOI] [PubMed] [Google Scholar]
- 22.Kaith B, Kumar K. In vacuum synthesis of psyllium and acrylic acid based hydrogels for selective water absorption from different oil–water emulsions. Desalination. 2008;229:331–41. doi: 10.1016/j.desal.2007.08.020. [DOI] [Google Scholar]
- 23.Korsemeyer RW, Peppas NA. Macromolecular and modelling aspects of swelling-controlled systems. In: Mansdorf SZ, Roseman TJ, editors. Controlled release delivery systems. New York: Marcel Dekker; 1983. p. 77. [Google Scholar]
- 24.Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-matrices for controlled drug delivery: a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res. 1999;16:1748–56. doi: 10.1023/A:1018914301328. [DOI] [PubMed] [Google Scholar]
- 25.Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 26.Costa FO, Sousa JJS, Pais AACC, Formosinho SJ. Comparison of dissolution profiles of ibuprofen pellets. J Control Rel. 2003;89:199–212. doi: 10.1016/S0168-3659(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 27.Khan KA. Concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 28.Bajpai AK, Mishra A. Ionizable interpenetrating polymer networks of carboxymethyl cellulose and polyacrylic acid: evaluation of water uptake. J Appl Polym Sci. 2004;93(5):2054–65. doi: 10.1002/app.20674. [DOI] [Google Scholar]
- 29.Cohn D, Aronhime M, Azdo B. Poly(urethane)-crosslinked poly(HEMA) hydrogels. J Macromol Sci Pure Appl Chem. 1992;A29(10):841–51. [Google Scholar]
- 30.Bajpai AK, Giri A. Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr Polym. 2003;53:271–2790. doi: 10.1016/S0144-8617(03)00071-7. [DOI] [Google Scholar]
- 31.Ranga Rao KV, Padmalatha Devi P, Buri P. Influence of molecular size and water solubility of the solute on its release from swelling and erosion controlled polymeric matrices. J Control Release. 1990;12:133–41. doi: 10.1016/0168-3659(90)90089-C. [DOI] [Google Scholar]
- 32.Ford JL, Rubinstein MH, McCaul F, Hogan JE, Edgar PJ. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Int J Pharm. 1987;40:223–34. doi: 10.1016/0378-5173(87)90172-4. [DOI] [Google Scholar]
- 33.Baveja SK, Ranga Rao KV. Sustained release tablet formulations of centperazine. Int J Pharm. 1986;31:169–74. doi: 10.1016/0378-5173(86)90228-0. [DOI] [Google Scholar]