Abstract
Microdermabrasion is widely used as a non-invasive cosmetic technique that has recently been adapted to selectively remove stratum corneum to increase skin permeability for transdermal drug delivery. This study measured the kinetics of skin barrier recovery after stratum corneum removal using microdermabrasion in hairless guinea pigs. The skin was abraded at two sites on each animal, one of which was allowed to recover under occlusion while the other remained non-occluded. Histological measurements showed that skin barrier properties to sulforhodamine B largely recovered within 12 h, and the stratum corneum appeared largely reformed within 24 h for both occluded and non-occluded skin. Skin electrical resistance measurements showed significant recovery of the skin barrier within 24 h. We conclude that transdermal drug delivery may occur for up to 12 h after microdermabrasion in guinea pigs; however, humans will probably have a longer recovery time due to expected slower skin healing rates.
Key words: microdermabrasion, skin permeability, skin repair kinetics, stratum corneum barrier function, transdermal drug delivery
INTRODUCTION
The skin is a semi-permeable barrier that protects the body from the external environment and prevents water loss (1–3). The stratum corneum, the upper 10–15 μm, serves as the skin's primary barrier and is composed of non-viable corneocytes that are surrounded by a lipid extracellular matrix. Due to its structure, only low molecular weight (<500 Da) lipophilic molecules can diffuse across intact skin at appreciable rates. Water-soluble molecules and larger molecules have limited diffusion across intact skin. Due to the stratum corneum selectivity, most pharmaceuticals cannot be administered in a transdermal patch formulation.
Several methods, such as chemical enhancers, tape stripping, iontophoresis, electroporation, thermal ablation, and microneedles have been developed to disrupt or remove stratum corneum and increase the skin's permeability to large molecular weight and water-soluble molecules for transdermal drug delivery (1–3). Transdermal drug delivery is an attractive route of administration due to the ease of applying patches and ointments, the lack of drug degradation by the liver's first-pass effect, and the avoidance of hypodermic needles.
Microdermabrasion has been recently introduced as a method to increase skin permeability by selectively removing the stratum corneum. In conventional use, microdermabrasion is a cosmetic procedure that improves the appearance of superficial skin defects such as fine lines, wrinkles, and scars by abrading the stratum corneum with pressurized particles (4–9). The depth of abrasion employed depends on the severity of the patient's skin condition, but in a clinical setting, the stratum corneum is not usually completely removed (6). Although initially designed for cosmetic applications, microdermabrasion has recently been used in several studies for delivery of small hydrophilic molecules, insulin, and vaccines (5,10–13).
Microdermabrasion has been effective, especially for delivery of macromolecules, when the stratum corneum barrier is completely removed. However, the recovery kinetics of the barrier after complete stratum corneum removal has yet to be examined in detail. A previous study was conducted to characterize barrier recovery in humans after microdermabrasion treatment using transepidermal water loss, which showed that the barrier recovered 1 day after treatment (14). However, the degree of stratum corneum removal was not determined in that study. Other studies have examined recovery of the stratum corneum after complete removal with extensive tape stripping. In one such study, tape stripping was performed on pigs, and the stratum corneum was reformed according to histological analysis within 2 weeks (15). The study, however, did not examine any time points between 30 min and 14 days after stripping, so the actual time of recovery is not known. Other studies have examined stratum corneum barrier recovery after tape stripping skin that was affected by diseases that impaired stratum corneum function (16).
While those studies provide useful information, there is a need to measure the rate of stratum corneum recovery after complete removal with microdermabrasion, which is motivated by two competing reasons. On the one hand, it may be desirable to have slow recovery, which would increase the time period during which drugs could be administered to the skin with increased permeability. On the other hand, it may be desirable to have rapid recovery from a safety perspective in order to restore the skin's protective function. The goal of this study is, therefore, to measure the kinetics of skin resealing after complete removal of stratum corneum using microdermabrasion in hairless guinea pigs. We will also examine the effect of occluding the skin after microdermabrasion on the kinetics of stratum corneum recovery.
The general topic of skin healing has been extensively studied and characterized in order to understand the response to injury and pathologies that prevent and delay healing. After injury, the skin typically heals in three phases: inflammation, re-epithelialization, and tissue remodeling (17,18). The inflammation phase occurs within a few minutes after injury and involves activation of neutrophils and the release of cytokines and growth factors that stimulate fibroblast proliferation and attract circulating monocytes to the injury site (17). Re-epithelialization occurs within hours after injury, mediated by proliferation of stem cells in the basement membrane to repair the damaged area and migration of cells from appendages to the injury site (17,19). Animals that have a high density of appendages, such as rodents, exhibit a more rapid healing response to injury (20,21). The final step of healing is tissue remodeling, where collagen is altered to remodel the skin back to its natural form and function. Healing of superficial wounds, such as those caused by microdermabrasion, is expected to undergo this healing process, but at a more rapid rate than deep wounds.
The rate of wound healing is affected by severity of the injury and also by the type of dressing that is used. In a study conducted on humans and pigs, occlusive dressings were shown to accelerate the rate of re-epithelialization and prevent the formation of scabs (22). However, occlusion can also delay the formation of functional stratum corneum after injury (23). Non-occluded wounds tend to form scabs during the healing process (22). Semi-occlusive coverings have been found to promote healing more effectively than occlusive and non-occlusive membranes in tape-stripped skin (24).
Several methods can be used to measure the rate of recovery and functionality of the stratum corneum. One method is histology, which involves sectioning and staining the skin to directly visualize the tissue layers and indirectly assess barrier function. Exposing skin to dyes and then assessing dye penetration in the skin by microscopy or other methods provide a more direct measure of skin barrier function. Finally, electrical resistance can also be used to monitor the skin's integrity non-invasively (25). This study used all three of these methods to investigate the kinetics of skin repair after stratum corneum removal using microdermabrasion.
METHODS AND MATERIALS
Microdermabrasion of Guinea Pigs
Sixteen hairless guinea pigs (Charles River Laboratories, Wilmington, MA) were divided equally into four groups that corresponded to the four skin healing assessment time points: 1 min, 4 h, 12 h, and 24 h after abrasion. Two additional guinea pigs were used for a sham negative control experiment. The animal protocols were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC). Prior to the experiment, the animals were given free access to food and water. The dorsal skin was prepared for abrasion by thoroughly cleaning with alcohol swabs (Becton Dickinson, Franklin Lakes, NJ) and allowing it to air dry. The abrasion area was marked using a rectangular foam adhesive film (Avery Dennison, Painesville, OH) that had a length of 41 mm and a width of 15 mm.
Using a protocol similar to our previous studies (13,26), each guinea pig was anesthetized using isofluorane gas and abraded at two sites on the back on opposite sides of the spinal column that were not in contact with each other. The guinea pigs were abraded using a DermaMed Gold Series microdermabrasion machine (DermaMed USA, Lenni, PA) with the gold tip assembly. The skin was abraded at a suction pressure of −40 kPa and at half of the maximum crystal flow rate by moving the microdermabrasion tip back and forth along the abrasion area for ten passes at a rate of 1 pass/s.
After abrasion, one skin site was occluded with a non-gel electrode (Thought Technology, Montreal, Canada) and Blenderm Tape (3M, St. Paul, MN). The area was further secured with Tegaderm (3M) and a Coban elastic bandage (3M) to prevent the animal from disturbing the site. The other abraded site remained uncovered. An additional site on the back was used for the untreated negative control and was not abraded or occluded. For the sham negative control experiments, microdermabrasion was performed without crystals or pressure for ten passes (1 pass/s) on the two guinea pigs immediately after euthanasia.
Skin Electrical Resistance Measurement
Electrical resistance was used to monitor stratum corneum barrier recovery after microdermabrasion treatment at 1 min, 4 h, 12 h, and 24 h after abrasion. The guinea pigs were lightly sedated with isoflurane, and the baseline skin impedance for every group, except for sham, was measured using a skin resistance measurement device (EIM-105 Prep-Check, modified with a 200 kΩ resistor in parallel; General Devices, Ridgefield, NJ), as previously described (27), at 2 h, 1 h, and 3 min before abrasion. The device operated at 30 Hz to provide a low-frequency electrical impedance measurement, which was interpreted as the electrical resistance (28).
To make measurements, a non-gelled electrode (Thought Technology) was used to measure the resistance of the two abraded sites and the untreated negative control site on each animal. These electrodes were adhered to a thin foam adhesive film mentioned above that isolated the measurement site and prevented the electrode's adhesive from damaging the skin. A gelled reference electrode (Lead-Lok Biomedical Innovations, Sandpoint, ID) was placed on the upper back near the base of the skull to complete the circuit.
The resistance was measured 1 min, 4 h, 12 h, and 24 h after abrasion. At each time point, resistance was measured on all animals not yet euthanized. Although measurements were made on all animals, the electrical resistance data presented below include only data from the animals that were euthanized at the 24-h time point so that the same number of measurements were averaged at each time point from the same group of animals (i.e., n = 4). The value of the skin's specific electrical resistance (i.e., resistivity) was determined by subtracting the meter's inline 200 kΩ resistor from the measured value and multiplying by the electrode contact area (0.79 cm2).
All statistical tests were performed by Minitab software (Minitab, State College, PA). A P value of less than 0.05 was considered significant. A two-way ANOVA and two-sample t tests were used for statistical evaluation.
Skin Staining and Histology
All animals were sacrificed using pentobarbital at the designated endpoints of 1 min, 4 h, 12 h, or 24 h after abrasion. After euthanasia, 100 μL of 10−2 M sulforhodamine B (Invitrogen, Carlsbad, CA) in phosphate-buffered saline was applied to the abrasion sites and negative control sites using a cotton swab. Sulforhodamine is a hydrophilic molecule that will not significantly penetrate intact skin, but will stain abraded skin. After 15 min, the sulforhodamine solution was removed by gently cleaning the skin with deionized water and Kimwipes (Kimberly-Clark, Neenah, WI), and the skin was excised for histological analysis.
All tissue samples were embedded in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA) and snap frozen in dry ice for histological analysis. The skin was sectioned using a Leica 3050S cryostat (Leica Microsystems, Wetzlar, Germany) at a thickness of 10 μm. The sulforhodamine fluorescence was imaged before the slides were stained with routine hematoxylin and eosin (H&E). All slides were imaged using a Nikon 600E microscope (Nikon, Tokyo, Japan) and Qcapture software (Q Imaging, Pleasanton, CA).
RESULTS
Histological Analysis
Negative Control Experiments
We first carried out two different negative control experiments. The first negative control was untreated skin to show normal skin histology and barrier properties. The second negative control was a “sham” exposure to microdermabrasion, in which the microdermabrasion device was passed across the skin ten times (1 pass/s), but with no crystal flow or suction pressure. After treatment, sulforhodamine was applied to the skin to assess skin barrier properties.
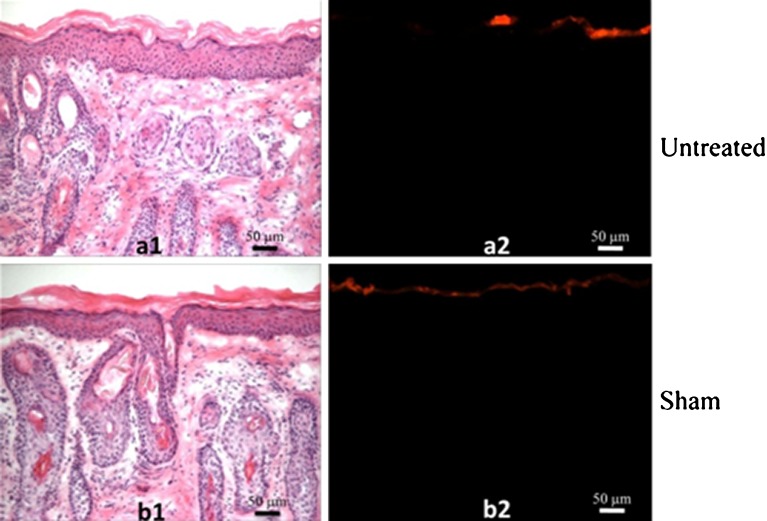
Representative histological sections from these negative controls are shown in Fig. 1. The H&E-stained sections are presented on the left to show skin structure, and fluorescence imaging that shows sulforhodamine penetration to assess skin barrier function is shown on the right. In the untreated skin stained with H&E, the pink-stained stratum corneum appears on top, the blue-stained nuclei of the viable epidermis are seen below, and the pink-stained dermis is deeper still (Fig. 1a1). As shown by fluorescence imaging, the red-fluorescent sulforhodamine did not penetrate into the skin, indicating an intact stratum corneum barrier (Fig. 1a2). In the sham-abraded skin, the treated area retained an intact stratum corneum (Fig. 1b1) that did not exhibit sulforhodamine penetration (Fig. 1b2). This indicates that contact with the microdermabrasion probe scraping across the skin surface (i.e., with the machine turned off) did not have significant effects on the stratum corneum.
Fig. 1.
Histology of hairless guinea pig skin after negative control treatments. a Untreated skin and b skin exposed to “sham” microdermabrasion were imaged. Each pair of images shows the same skin section imaged with different staining using different optics: (1) after routine H&E staining to show skin microanatomy by brightfield microscopy and (2) showing staining with red-fluorescent sulforhodamine as a measure of skin barrier function by fluorescence microscopy. Skin was excised from hairless guinea pigs in vivo. The H&E-stained images show pink-stained stratum corneum on top, blue-stained nuclei of the viable epidermis below, and pink-stained dermis at the bottom. The sulforhodamine-stained images show dye present only on the skin surface, indicating an intact stratum corneum barrier. The sham site received the same treatment as microdermabraded sites involving passing the microdermabrasion tip across the skin ten times (1 pass/s), but with no crystal flow or suction pressure. These images are representative of untreated skin samples from 16 different animals and sham-treated skin samples from two different animals
Occluded Skin
We next assessed the effect of microdermabrasion on stratum corneum integrity and the kinetics of repair with the skin under occlusion. After the skin was treated with microdermabrasion, mild erythema was observed, which resolved within 24 h (data not shown).
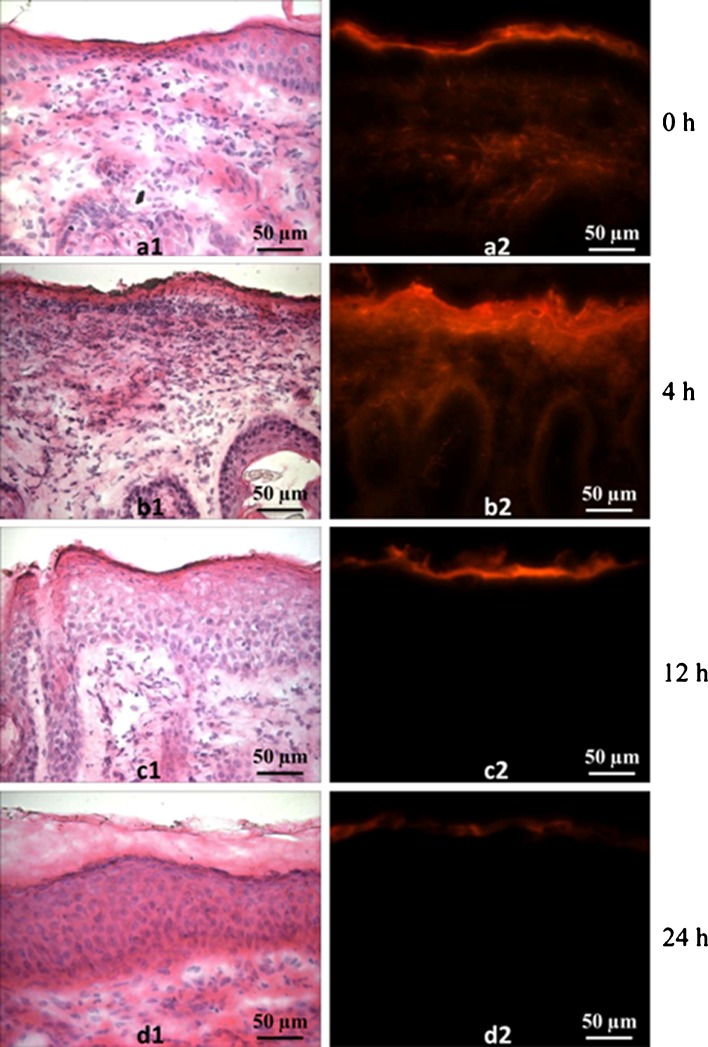
Histological sections of skin at various time points after microdermabrasion are shown in Fig. 2. Immediately after treatment, H&E staining shows that the pink-stained stratum corneum was removed, but the dense layer of cells with characteristic blue-stained nuclei remained, indicating the presence of the viable epidermis (Fig. 2a1). The corresponding fluorescence image shows penetration of red-fluorescent sulforhodamine deep into the skin, indicating that the skin's barrier properties have been compromised, which is consistent with removal of stratum corneum (Fig. 2a2).
Fig. 2.
Histology of occluded hairless guinea pig skin excised at different times after microdermabrasion: a 1 min, b 4 h, c 12 h, and d 24 h. Each pair of images shows the same skin section imaged with different staining using different optics: (1) after routine H&E staining by brightfield microscopy and (2) showing staining with red-fluorescent sulforhodamine by fluorescence microscopy. These images are representative of skin samples from four different animals at each time point
Four hours after microdermabrasion, the stratum corneum had not yet recovered (Fig. 2b1), and sulforhodamine again penetrated deeply into the skin (Fig. 2b2). These skin samples also exhibited signs of inflammation, as indicated by the increased number of blue-stained cells, which are believed to be inflammatory cells. The characteristic dermal–epidermal junction was also difficult to see.
Twelve hours after microdermabrasion, the stratum corneum appears to have at least partially recovered, as indicated by the return of pink-stained tissue atop the viable epidermis (Fig. 2c1), and the skin's barrier properties returned as well, as shown by the inability of sulforhodamine to penetrate into the skin (Fig. 2c2). After 24 h, skin histology looked similar to untreated skin (Fig. 2d2), and sulforhodamine was again not able to penetrate into skin significantly (Fig. 2d2).
Non-occluded Skin
Previous studies have shown that skin occlusion affects wound healing (23,24,29). We therefore observed the kinetics of stratum corneum barrier recovery in microdermabraded skin without occlusion. Similar to occluded skin, mild erythema was initially observed, which resolved within 24 h (data not shown).
Figure 3 shows representative histological sections of skin at various time points after microdermabrasion that was allowed to heal without an occlusive covering. Similar to the occluded samples, the images show stratum corneum removal and loss of barrier function immediately after microdermabrasion (Fig. 3a1); continued absence of the stratum corneum barrier, accompanied by apparent inflammation after 4 h (Fig. 3b1); at least partial restoration of stratum corneum and return of barrier properties after 12 h (Fig. 3c1); and appearance of normal skin histology and intact barrier function after 24 h (Fig. 3d1).
Fig. 3.
Histology of non-occluded hairless guinea pig skin excised at different times after microdermabrasion: a 1 min, b 4 h, c 12 h, and d 24 h. Each pair of images shows the same skin section imaged with different staining using different optics: (1) after routine H&E staining by brightfield microscopy and (2) showing staining with red-fluorescent sulforhodamine by fluorescence microscopy. These images are representative of skin samples from four different animals at each time point
Electrical Resistance Measurements
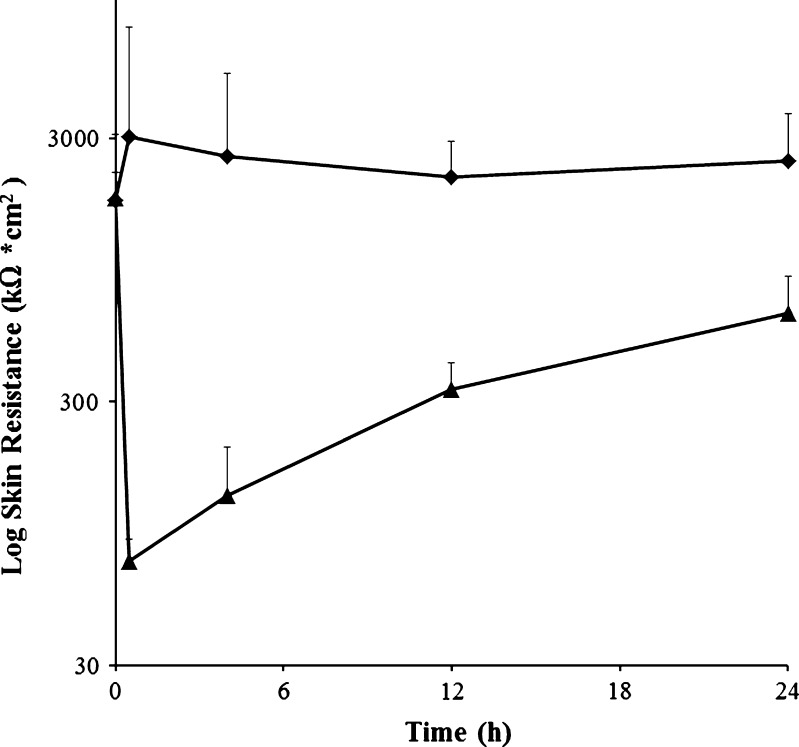
To supplement the histological analysis, we further studied the kinetics of skin barrier recovery by measuring skin electrical resistance over the course of the study, as shown in Fig. 4. Previous studies have shown that skin electrical resistance correlates inversely with skin permeability to molecules (28,30,31). The untreated negative control skin sites had an average initial specific resistance of 1,750 ± 1,340 kΩ cm2, which did not significantly change over the course of the 24-h study (ANOVA, P > 0.05).
Fig. 4.
Skin electrical resistance as a function of time after microdermabrasion measured in hairless guinea pigs in vivo: untreated negative control (diamonds) and microdermabraded skin (triangles). Each point is an average of four replicates with positive error bars representing the standard deviation. All data are for non-occluded skin. We measured skin resistance of occluded skin too (data not shown), but the resistance remained low at all time points, which we attribute to an artifact of the electrical resistance measurement method. Occlusion causes the skin to become highly hydrated, which reduces skin resistance and thereby masks the expected recovery of skin barrier properties
The pre-treatment skin resistance values for microdermabraded skin were not significantly different from the untreated negative control (Student's t test, P > 0.05). After treatment, the microdermabraded skin had a significantly smaller electrical resistance compared to the untreated negative control (ANOVA, P < 0.05), consistent with the removal of stratum corneum. Over time, the resistance of the microdermabraded skin increased (ANOVA, P < 0.05), and at the 24-h time point, its resistance was not significantly different from the untreated negative control (Student's t test, P > 0.05), indicating recovery of skin barrier properties.
DISCUSSION
This study measured the kinetics of skin repair after stratum corneum removal using microdermabrasion. The rate of recovery was studied using histological imaging, dye penetration, and skin electrical resistance measurements. Immediately after microdermabrasion, all three analytical methods indicated substantially reduced skin barrier function corresponding to removal of the stratum corneum, while leaving the viable epidermis largely intact. After 4 h, skin barrier function remained substantially impaired, and histological imaging shows the loss of a clear dermal–epidermal barrier and the increased presence of nucleated cells in the dermis, believed to be inflammatory cells. By 12 h, skin barrier properties largely recovered, and stratum corneum could be seen histologically. By 24 h, the skin appeared fully recovered, at least by the measures used in this study. Additional analysis will be needed to assess the kinetics and degree of skin recovery in more detail. According to histological imaging, skin occlusion had no apparent effect on the skin repair process.
This study employed guinea pig skin, which is widely accepted as a model for human skin barrier properties (2). Guinea pig skin, nonetheless, has significant differences from human skin that may be important to its healing after injury. For this reason, we expect that the very rapid skin repair seen in this study after microdermabrasion may be slower in humans. Previous studies have shown that the skin of rodents, including guinea pigs, heals more quickly than human skin (20,32,33). This is believed to be due, as least in part, to the higher density of hair follicles in rodent skin. During the process of healing, cells from hair follicles migrate to the site of injury to repair the wound (21). For example, it has been shown that rodents can heal centimeter-size skin wounds within 10 days, which cannot be accomplished by a human without medical intervention (20).
Several previous studies have investigated the recovery of stratum corneum after disruption with microdermabrasion, chemical treatment, and microneedles. After microdermabrasion without occlusion, human skin has been shown to heal within between 1 and 2 days, as measured by transepidermal water loss measurements, although no information was given in these studies about the degree of stratum corneum removal (14,34). In this study, stratum corneum recovered within just 12–24 h. This rapid recovery was probably facilitated by the choice of animal model and the small area of stratum corneum removal. Barrier recovery after exposure to chemical peels and microneedles was reported after 3 days (14,35).
Wounds created in human skin by puncture with microneedles were shown to heal within 1–2 h without occlusion and up to 1–2 days with occlusion, as measured by skin electrical resistance (36). This dramatic effect of occlusion on skin repair rate seen in humans after microneedle puncture was not seen in our study in guinea pigs after microdermabrasion. In addition to differences between animal skin and human skin, microneedles make multiple narrow punctures (i.e., without removing tissue) that penetrate into the superficial dermis, whereas microdermabrasion removes a larger area of tissue, but only with a depth into the viable epidermis. Other studies showed that microneedle puncture in hairless guinea pigs increased skin permeability for at least 2 days (37), which was extended up to 1 week with the addition of diclofenac to delay repair (38), as measured by transepidermal water loss.
Applications of microdermabrasion for transdermal drug delivery seek to increase skin permeability for a period of time. Because diffusion of drugs into permeabilized skin is a slow process and because many transdermal patch applications would benefit from drug delivery over an extended time, it would be desirable for microdermabrasion to increase skin permeability for many hours or even days. This study, in guinea pigs, indicates that delivery for up to 12 h is possible. In human skin, this delivery period may be longer, as discussed above.
From a safety standpoint, rapid skin resealing should lower the risk of infections. Thus, the timescale of skin repair needs to be optimized to ensure increased skin permeability long enough to accomplish the drug delivery application, but short enough to increase safety. Additional studies will be needed to identify conditions that achieve this optimization, which will vary depending on the drug, indication, and other specifics of the application.
CONCLUSIONS
This study measured skin repair kinetics after stratum corneum removal by microdermabrasion using three different methods: histological imaging, dye penetration, and skin electrical resistance measurements. Microdermabrasion was shown to remove stratum corneum while retaining viable epidermis, which corresponded to increased skin permeability to sulforhodamine and reduced skin electrical resistance. After 4 h, skin remained permeable and exhibited altered skin microanatomy. After 12 h, the skin permeability barrier returned, and skin histology showed at least partial reformation of stratum corneum. By 24 h, skin microanatomy appeared to be largely repaired, including an intact stratum corneum with barrier function. Skin occluded or left non-occluded after microdermabrasion showed similar repair kinetics. Examination of the broader literature suggests that the kinetics of repair in human skin may be slower. We conclude that microdermabrasion offers a method to increase skin permeability by removing stratum corneum and that the skin is repaired in guinea pigs within 12–24 h after microdermabrasion.
Acknowledgments
We thank Dr. Laura O'Farrell and the Georgia Tech Physiological Research Laboratory staff for assisting with the animal studies and Donna Bondy for administrative support. This work was supported in part by the National Institutes of Health.
References
- 1.Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- 2.Bronaugh RL, Maibach HI, et al. Percutaneous absorption: drugs, cosmetics, mechanisms, methodology. 4. New York: Marcel Dekker; 2005. [Google Scholar]
- 3.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhalla M, Thami G. Microdermabrasion: reappraisal and brief review of literature. Dermatol Surg. 2006;32:809–814. doi: 10.1111/j.1524-4725.2006.32165.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. Dermatol Surg. 2006;32(8):1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- 6.Shim EK, Barnette D, Hughes K, Greenway HT. Microdermabrasion: a clinical and histopathologic study. Dermatol Surg. 2001;27(6):524–530. doi: 10.1046/j.1524-4725.2001.01001.x. [DOI] [PubMed] [Google Scholar]
- 7.Spencer JM, Kurtz ES. Approaches to document the efficacy and safety of microdermabrasion procedure. Dermatol Surg. 2006;32(11):1353–1357. doi: 10.1111/j.1524-4725.2006.32305.x. [DOI] [PubMed] [Google Scholar]
- 8.Karimipour DJKS, Johnson TM, Orringer JS, Hamilton T, Hammerberg C, Voorhees JJ, Fisher G. Microdermabrasion with and without aluminum oxide crystal abrasion: a comparative molecular analysis of dermal remodeling. J Am Acad of Derm. 2006;54(3):405–410. doi: 10.1016/j.jaad.2005.11.1084. [DOI] [PubMed] [Google Scholar]
- 9.Rajan P, Grimes PE. Skin barrier changes induced by aluminum oxide and sodium chloride microdermabrasion. Dermatol Surg. 2002;28(5):390–393. doi: 10.1046/j.1524-4725.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- 10.Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium : YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151(1):132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- 11.Gill HS, Andrews SN, Sakthivel SK, Fedanov A, Williams IR, Garber DA, et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur J Pharm Sci. 2009;38(2):95–103. doi: 10.1016/j.ejps.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WR, Shen SC, Wang KH, Hu CH, Fang JY. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol. 2003;121(5):1118–1125. doi: 10.1046/j.1523-1747.2003.12537.x. [DOI] [PubMed] [Google Scholar]
- 13.Andrews S, Lee JW, Choi SO, Prausnitz MR. Transdermal insulin delivery using microdermabrasion. Pharm Res. 2011;28:2110–2118. doi: 10.1007/s11095-011-0435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JY, Kang HA, Kim MY, Park YM, Kim HO. Damage and recovery of skin barrier function after glycolic acid chemical peeling and crystal microdermabrasion. Dermatol Surg. 2004;30(3):390–394. doi: 10.1046/j.1076-0512.2003.30107.x. [DOI] [PubMed] [Google Scholar]
- 15.Berrutti LE, Singer AJ, McClain SA. Histopathologic effects of cutaneous tape stripping in pigs. Acad Emerg Med. 2000;7(12):1349–1353. doi: 10.1111/j.1553-2712.2000.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Zhen YX, Tagami H. Normal recovery of the stratum corneum barrier function following damage induced by tape stripping in patients with atopic dermatitis. Br J Dermatol. 1997;136(6):966–967. doi: 10.1111/j.1365-2133.1997.tb03946.x. [DOI] [PubMed] [Google Scholar]
- 17.Hantash BM, Zhao LM, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- 18.Rizzi SC, Upton Z, Bott K, Dargaville TR. Recent advances in dermal wound healing: biomedical device approaches. Expert Rev Med Devic. 2010;7(1):143–154. doi: 10.1586/erd.09.57. [DOI] [PubMed] [Google Scholar]
- 19.Dunkin CSJ, Pleat JM, Gillespie PH, Tyler MPH, Roberts AHN, McGrouther DA. Scarring occurs at a critical depth of skin injury: precise measurement in a graduated dermal scratch in human volunteers. Plast Reconstr Surg. 2007;119(6):1722–1732. doi: 10.1097/01.prs.0000258829.07399.f0. [DOI] [PubMed] [Google Scholar]
- 20.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121(2):219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 21.Jahoda CAB, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358(9291):1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- 22.Hinman CD, Maibach H, Winter GD. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200(490):377. doi: 10.1038/200377a0. [DOI] [PubMed] [Google Scholar]
- 23.Silverman RA, Lender J, Elmets CA. Effects of occlusive and semiocclusive dressings on the return of barrier function to transepidermal water loss in standarized human wounds. J Am Acad Dermatol. 1989;20(5):755–760. doi: 10.1016/S0190-9622(89)70086-4. [DOI] [PubMed] [Google Scholar]
- 24.Visscher M, Hoath SB, Conroy E, Wickett RR. Effect of semipermeable membranes on skin barrier repair following tape stripping. Arch Dermatol Res. 2001;293(10):491–499. doi: 10.1007/PL00007463. [DOI] [PubMed] [Google Scholar]
- 25.Davies DJ, Ward RJ, Heylings JR. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro. 2004;18:351–358. doi: 10.1016/j.tiv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Andrews SN, Zarnitsyn V, Bondy B, Prausnitz MR. Optimization of microdermabrasion for controlled removal of stratum corneum. Int J Pharm. 2011;407:95–104. doi: 10.1016/j.ijpharm.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta J, Prausnitz MR. Recovery of skin barrier properties after sonication in human subjects. Ultrasound Med Biol. 2009;35(8):1405–1408. doi: 10.1016/j.ultrasmedbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prausnitz MR. The effects of electric current applied to skin: a review for transdermal drug delivery. Adv Drug Deliver Rev. 1996;18(3):395–425. doi: 10.1016/0169-409X(95)00081-H. [DOI] [Google Scholar]
- 29.Zhai HB, Maibach HI. Effect of occlusion and semi-occlusion on experimental skin wound healing: a reevaluation. Wounds-a Compendium Clin Res Pract. 2007;19(10):270–276. [PubMed] [Google Scholar]
- 30.Burnette RR, DeNuzzio JD. In: Mechanisms of transdermal drug delivery. Potts RO, Guy RH, editors. New York: Marcel Dekker; 1997. [Google Scholar]
- 31.Karande P, Jain A, Mitragotri S. Relationships between skin's electrical impedance and permeability in the presence of chemical enhancers. J Cont Release. 2006;110(2):307–313. doi: 10.1016/j.jconrel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8(2):83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 33.Wong V, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical Approaches to Create Murine Models of Human Wound Healing. J Biomed Biotechnol 2011 (in press) [DOI] [PMC free article] [PubMed]
- 34.Kim HS, Lim SH, Song JY, Kim MY, Lee JH, Park JG, et al. Skin barrier function recovery after diamond microdermabrasion. J Dermatol. 2009;36(10):529–533. doi: 10.1111/j.1346-8138.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- 35.Milewski M, Brogden NK, Stinchcomb AL. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opinion on Drug Deliv. 2010;7(5):617–629. doi: 10.1517/17425241003663228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154:148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banks SL, Pinninti RR, Gill HS, Paudel KS, Crooks PA, Brogden NK, et al. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs. J Pharm Sci. 2010;99(7):3072–3080. doi: 10.1002/jps.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks SL, Paudel KS, Brogden NK, Loftin CD, Stinchcomb AL. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm Res. 2011;28(5):1211–1219. doi: 10.1007/s11095-011-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]