Abstract
The objective of the present investigation was to study the ability of sulfobutyl ether7-β-cyclodextrin to form an inclusion complex with carbamazepine, an anti-epileptic drug with poor water solubility. The formation of the complex was carried out using the industrially feasible spray-drying method. The inclusion complex and physical mixtures were characterized by various techniques such as differential scanning calorimetry (DSC), infrared (IR), nuclear magnetic resonance (NMR), X-ray diffraction (XRD), and molecular modeling. The DSC, IR, and NMR studies confirmed the formation of an inclusion complex between carbamazepine and sulfobutyl ether7 β-cyclodextrin whereas XRD studies indicated an amorphous nature of the inclusion complex. Molecular modeling studies disclosed different modes of interaction between carbamazepine and sulfobutyl ether7 β-cyclodextrin with good correlation with experimental observations. The inclusion complex exhibited significantly higher in vitro dissolution profile as compared with pure carbamazepine powder. The in vivo anti-epileptic activity of carbamazepine/sulfobutyl ether7 β-cyclodextrin complex was evaluated in pentylenetetrazole-induced convulsions model. The carbamazepine/sulfobutyl ether7 β-cyclodextrin complex showed significantly higher anti-epileptic activity (p <0.01) as compared with that of carbamazepine suspension on oral administration.
Key words: anti-epileptic activity, carbamazepine, poor water solubility, spray drying, sulfobutyl ether7 β-cyclodextrin
INTRODUCTION
Carbamazepine (CBZ), 5H-dibenzazepine-5-carboxamide, is a sodium channel blocker recommended for the treatment of epilepsy and trigeminal neuralgia for over 40 years (1). CBZ has become a best-selling anticonvulsant due to its favorable therapeutic profile. However, due to poor aqueous solubility of CBZ (∼113 μg/mL) (2), time required to attain peak plasma concentrations of CBZ after oral administration varies from 4 to 24 h (3). Moreover, CBZ also has low oral bioavailability (<50%) (3). The variable and delayed absorption and low oral bioavailability of CBZ are due to its slow dissolution rate in the gastrointestinal tract. Thus, bioavailability (and in turn therapeutic efficacy) of CBZ is considered to be dependent on the dissolution rate. In conditions like epilepsy, where a rapid action is desirable, it is essential to have an anti-epileptic agent that gives quick onset of action in order to achieve immediate relief from convulsions (4). Also, improved bioavailability of CBZ could result in dose reduction and thus could possibly minimize the formation of chemically reactive metabolites like 2-hydroxyiminostilbene in vivo that are postulated to be responsible for the CBZ-mediated idiosyncratic reactions (5). In view of this, it is important to design suitable approach to improve the dissolution rate of CBZ in order to improve its onset of action and therapeutic efficacy.
Researchers have established the potential of various approaches such as solid dispersions of CBZ with PEG 6000 (5) and PVP (2) and formation of inclusion complexes with β-cyclodextrin and hydroxypropyl β-cyclodextrin (6) for improving the dissolution rate and/or oral bioavailability of CBZ. However, until today, the potential of sulfobutyl ether7 β-cyclodextrin (SBE7 β-CD) in improving the dissolution rate and therapeutic efficacy of CBZ has not been established.
SBE7 β-CD is a highly water-soluble derivative of β-cyclodextrin that is commercially available as Captisol® (7,8). The water solubility of SBE7 β-CD (∼70 g/100 ml at 25°C) is significantly higher than the parent β-cyclodextrin (1.85 g/100 ml at 25°C). Furthermore, SBE7 β-CD does not exhibit the nephrotoxicity associated with β-cyclodextrin (8,9). It has been demonstrated by Tötterman et al. that SBE7 β-CD does not show any cytotoxic effects on intestinal epithelial Caco-2 cells (8,10). Thus, SBE7 β-CD can be considered to be very safe for oral administration. SBE7 β-CD inclusion complexes of some hydrophobic drugs such as chlorpromazine, valdecoxib, and thalidomide have already been evaluated, and SBE7 β-CD inclusion complexes were able to improve the oral bioavailability and/or therapeutic efficacy of these drugs (11–13).
Smith et al. have described the use of SBE7 β-CD for improving water solubility of CBZ (14). However, only equilibrium constants of CBZ/SBE7 β-CD were evaluated and the CBZ/SBE7 β-CD complex was mainly employed for fabrication of sustained release beads. Furthermore, a detailed characterization of the CBZ/SBE7 β-CD interaction and in vivo proof of therapeutic efficacy had not been established.
In the present investigation, formation of an inclusion complex between CBZ and SBE7 β-CD by the industrially feasible spray-drying method was studied. The CBZ/SBE7 β-CD complex was characterized by various methods. Furthermore, we have also studied the interaction of CBZ and SBE7 β-CD by molecular dynamics (MD) simulations and have analyzed the factors responsible for the binding of CBZ with SBE7 β-CD. Finally, the superior in vivo efficacy of the CBZ/SBE7 β-CD complex compared with the plain drug has also been demonstrated.
MATERIALS AND METHODS
Materials
Chemicals
Carbamazepine (Novartis Labs, Mumbai, India) and lactose (Signette Chemicals, Mumbai, India) were gift samples. Sulfobutyl ether7 β-cyclodextrin (Captisol®; CyDex Inc., USA) Hydrochloric acid (AR grade, Qualigens Chemicals, Mumbai, India) and ethanol (S.D. Fine Chemicals, Mumbai, India) were used without further purification. Double distilled water was prepared freshly whenever required.
Phase Solubility Studies
A phase solubility study was carried out to investigate the effect of SBE7 β-CD on the solubility of CBZ, using the method reported by Higuchi and Connors (15). Plain double distilled water (without SBE7 β-CD) and aqueous solutions of SBE7 β-CD (average molecular weight, 2,163 and degree of substitution, 7) of different concentrations (23.1, 46.2, 69.3, and 92.4 mM) were added to excess amounts of CBZ and were shaken at 30°C for 60 h. After equilibrium, the solutions were filtered using Whatmann no. 1 filter paper, centrifuged, and diluted suitably to determine the concentration of CBZ spectrophotometrically at 285 nm. A graph of concentration (in mM) of CBZ was plotted against the concentration (in mM) of SBE7 β-CD. The stability constant for the complex was determined from the graph using the following equation,
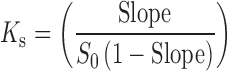 |
1 |
where slope was obtained from the graph and S0 was the equilibrium solubility of CBZ in water.
Preparation of CBZ/SBE7 β-CD Inclusion Complex and Physical Mixture
The inclusion complex of CBZ and SBE7 β-CD was prepared in a molar ratio of 1:1. Briefly, predetermined amount of CBZ and SBE7 β-CD was dissolved in a 50% (v/v) ethanol solution. The total concentration of CBZ and SBE7 β-CD in the solution was 35.71 mg/mL. The solution was spray dried, according to the method reported by Figueiras et al., with some modifications (16), in a JISL spray dryer (Mumbai, India) under the following operational conditions: inlet temperature, 95°C; outlet temperature, 57°C; flow rate, 1.7 mL/min; aspirator, 30%; and aspiration pressure, 2 bar . The yield of the spray-drying process was measured as the weight percentage of the powder obtained in the final operation compared with the amount of solids (CBZ plus SBE7 β-CD) present in the sprayed solution. The physical mixture (PM) was prepared by geometric mixing of CBZ and SBE7 β-CD without applying pressure. All dispersions were prepared in triplicates and stored over anhydrous calcium chloride in a dessicator.
Characterization of CBZ/SBE7 β-CD Inclusion Complex and PM
Assay for CBZ Content
The CBZ content in the inclusion complex and PM was determined spectrophotometrically at 285 nm.
Differential Scanning Calorimetry Studies
Samples each of CBZ, SBE7 β-CD, SDCDC inclusion complex and PM, in the weight range of 3–5 mg, were scanned at a rate of 10°C/min on a Shimadzu DT-40 Thermal Analyzer between 30°C and 330°C under an inert atmosphere of nitrogen.
Infrared Spectroscopic Studies
The infrared (IR) spectra of CBZ, SBE7 β-CD, CBZ/SBE7 β-CD complex, and PM were recorded on a Jasco FT/IR 5,300 spectrophotometer with pellets made from potassium bromide.
X-ray Diffraction Studies
Powder X-ray diffraction (XRD) patterns of CBZ, SBE7 β-CD, CBZ/SBE7 β-CD complex, and PM were recorded on a Philips P Analytical X’Pert powder X-ray diffractometer (Philips P) using Ni-filtered, Cu Kα radiation, a voltage of 40 kV and 25 mA current. The scanning rate was 1°/min over the diffraction angle range (2θ) of 10–40°.
Nuclear Magnetic Resonance Studies
The 1H-NMR spectra of CBZ, CBZ/SBE7 β-CD complex, PM of SBE7 β-CD and CBZ, and pure SBE7 β-CD were taken in D2O and that of pure SBE7 β-CD and pure CBZ powder in a mixture of CD3OD/H2O (1:1) on a Bruker Ultra shield 500 MHz FT nuclear magnetic resonance (NMR) instrument at 298 K. The spectra were processed with software Topspin 2.0.
Scanning Electron Microscopy Studies
Surface characteristics of the spray-dried complex were evaluated by scanning electron microscopy (SEM). The samples were coated with gold sputter using a JEOL JEC-550 Twin coater (JEOL Japan) and examined by JEOL-JSM 5,400 SEM (JEOL Japan).
Molecular Modeling Studies
Computational Details
The computational studies were carried out on an Intel Xeon processor-based computing cluster with the Rocks Cluster Suite 5.4. Preparation of the structure, simulations and analysis were carried out with Maestro v 9.1, (Schrödinger LLC, New York, NY, 2010). The docking studies were carried out with Fast Rigid Exhaustive Docking (acronym FRED, v 2.2.5, OpenEye Scientific Software, Santa Fe, USA) while the MD simulations was performed using Desmond (v 2.4, DE Shaw Research, NY, USA).
Structure Preparation
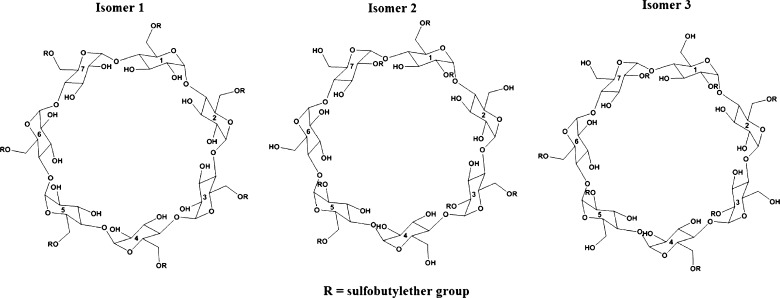
The 3D structures of β-cyclodextrin (BCD) and carbamazepine were retrieved from the Protein Data Bank and PubChem. Since no 3D structure for sulfobutyl ether7 β-cyclodextrin (SBE7 β-CD) was available, the structure was built starting from BCD with the Builder module in Maestro. The SBE7 β-CD used in the experimental studies had seven sulfobutyl ether groups per cyclodextrin molecule and the substitution on the OH groups is random (actual configuration is unknown). For molecular modeling studies, three different isomeric forms of the SBE7 β-CD were constructed, such that there was sufficient representation of the hindrance induced by the sulfobutyl ether groups. In isomer 1, all the sulfobutyl ether groups were grafted to the primary hydroxyl groups (6-OH on the glucose subunit) of BCD whereas in isomers 2 and 3, three sulfobutyl ether groups were grafted to the primary hydroxyl groups and four to the secondary hydroxyl groups (2-OH on the glucose subunit) of BCD. A 2D representation of all the three SBE7 β-CD isomers has been shown in Fig. 1. The structures were assigned atom types and partial charges based on the OPLS 2005 forcefield in the Schrödinger Suite.
Fig. 1.
2D depiction of the different isomeric forms of sulfobutyl ether7 β-cyclodextrin constructed for computational studies
Docking Studies
After preparing the equilibrium structures, the three isomers SBE7 β-CD were docked with FRED-RECEPTOR (v 2.2.5). Prior to docking carbamazepine, a box of the active site had to be defined for the ligand or guest molecule in SBE7 β-CD. The active site was described by two contours, referred to as the inner and outer contour. The outer contour had a larger extent and the ligand was docked in the volume of outer contour. On the other hand, the inner contour was smaller in volume and ensured that the center of at least one heavy atom of the guest touched the inner contour during docking. Docking was subsequently carried out by sequential rotational and translational motions of the rigid guest structure in the active site. The docking solutions were ranked based on the intrinsic scoring function Chemgauss3. From the set of docking solutions, the best solution was subjected to a 5-ns MD simulation to completely relax the complexes.
MD Simulations
Each CBZ/SBE7 β-CD complex was solvated with TIP3P waters (17) such that a water shell 5 Å in thickness was formed around the complex. The solvated complex was then simulated with the “NPT relax protocol” in Desmond. The protocol involved an initial minimization of the solvent with restrains on the solute, which was followed by short MD simulations of 12–24 ps in sequential NVT and NPT ensembles with the Langevin thermostat and barostat (18). The temperature was maintained by coupling to an external bath maintained at 300 K based on the Langevin algorithm (18). Similarly, the pressure was isotropically restrained to 1 bar with the Langevin barostat (18). High-frequency vibrations were removed by the application of the SHAKE algorithm (19) which constrains all bonds to their equilibrium values. Initial velocities were generated randomly from a Maxwell distribution at 300 K in accordance with the masses assigned to the atoms. After completion of the relaxation protocol, an unrestrained 5-ns MD simulation was carried out on the solvated complex, avoiding introduction of any structural artifacts. During the simulation, the geometries and the corresponding energies of the complex were sampled after every 5 ps. After the trajectories were obtained from the MD simulations, the structural stability of the complexes was analyzed based on the root-mean-square deviation (RMSD) computed using the starting structure as a reference. On completion of the simulation, the binding energies and intermolecular hydrogen bonds were computed with the Simulation Event Analysis module in Desmond.
Preparation of CBZ/SBE7 β-CD Inclusion Complex Using Lactose as a Bulking Agent
In order to increase the yield of the CBZ/SBE7 β-CD complex, the utility of lactose, a bulking agent was evaluated. Briefly, predetermined amounts of CBZ, SBE7 β-CD, and lactose were dissolved in a 50% (v/v) ethanol solution. The total concentration of CBZ and SBE7 β-CD in the solution was 35.71 mg/mL and that of lactose was 71.42 mg/mL. The solution was spray dried in a similar manner as described for the CBZ/SBE7 β-CD complex. The yield of the spray-dried complex was calculated.
In Vitro Dissolution Profile of Various CBZ Formulations
The in vitro dissolution profile of various CBZ formulations was evaluated in pH 1.2 buffer (900 mL) using USP Type 2 apparatus maintained at 37 ± 0.5°C and operated at the speed of 75 ± 2 rpm. Pure CBZ, CBZ-SBEBCD inclusion complex with lactose and PM (all equivalent to 25 mg of CBZ) were weighed accurately and dispersed in the dissolution medium. During the study, 1 mL of aliquots were withdrawn at predetermined time intervals (15, 30, 45, 60, 90, 120, and 180 min) from the dissolution medium and were replaced with fresh medium. The amount of CBZ released in the dissolution medium was determined by measuring the absorbance of the suitably diluted aliquot at 285 nm.
Evaluation of In Vivo Anti-epileptic Activity
Anti-epileptic activity of CBZ/SBE7 β-CD inclusion complex was evaluated and compared with a CBZ suspension. The study was carried out on Swiss albino mice (18–22 g). Pentylenetetrazole (PTZ) was used to induce seizures (20). Animal care and handling throughout the experimental procedure were in accordance with the CPCSEA guidelines. The experimental protocol was approved by the Animal Ethics Committee of Bombay College of Pharmacy. Animals were randomly divided into following groups (n = 6 per group)
Control (no treatment)
CBZ suspension
CBZ/SBE7 β-CD complex
Groups 2 and 3 were orally administered CBZ equivalent to 30 mg/kg. After 45 min, PTZ was injected intraperitonially at a dose of 85 mg/kg to all animals including the control group to induce seizures. The severity of seizures after administration of PTZ was evaluated on basis of various behavioral changes.
Comparison among the groups was mainly done on basis of the time required for the onset of major seizures with loss of righting ability and final death of the animal. The results were statistically evaluated by ANOVA followed by Dunnet’s test (GraphPad InStat Demo Version). Differences were considered statistically significant at p < 0.05. Percent mortality for each group was also calculated and compared.
RESULTS AND DISCUSSION
Phase Solubility Studies
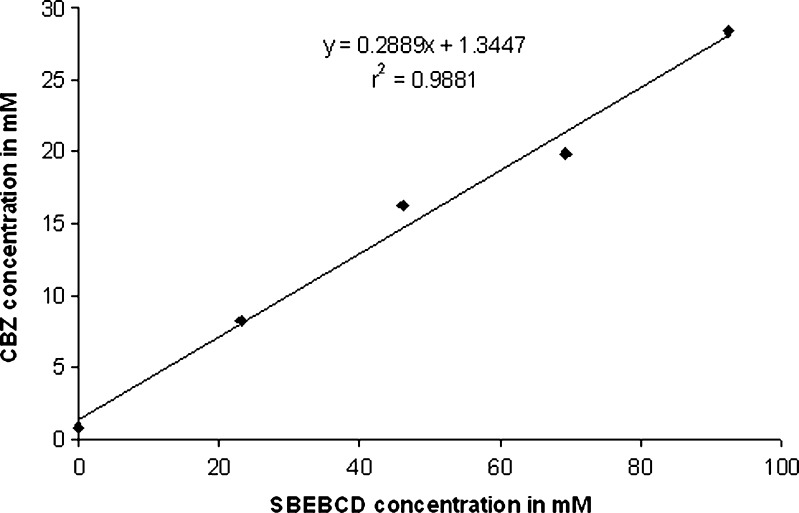
The phase solubility plot for CBZ and SBE7 β-CD has been shown in Fig. 2. It is evident that solubility of CBZ increased linearly with increase in concentration of SBE7 β-CD. Correlation coefficient, “r” was found to be 0.9940. The phase solubility diagram (Fig. 2) can be classified as Type AL according to Higuchi and Connors. As the slope of the line was less than unity, it can be assumed that the increase in solubility was due to formation of a 1:1 inclusion complex. The apparent stability constant (Ks) is found to be 498.04 M−1. It has been reported that cyclodextrin/drug complexes with the values of stability constant in the range of 200 to 5,000 M−1 yield improvement in oral bioavailability and/or therapeutic efficacy (21,22). In view of this, the CBZ/SBE7 β-CD complex is expected to show higher therapeutic efficacy or oral bioavailability of CBZ. It was observed that SBE7 β-CD yielded a 35-fold increase in the aqueous solubility of CBZ (from 0.8158 to 28.369 mM/L at 92.4 mM or 20% (w/v) solution of SBE7 β-CD).
Fig. 2.
Phase solubility studies for carbamazepine (CBZ) in presence of sulfobutyl ether7 β-cyclodextrin (SBE 7 β-CD)
Characterization of CBZ/SBE7 β-CD Complex and PM
Differential Scanning Calorimetry Studies
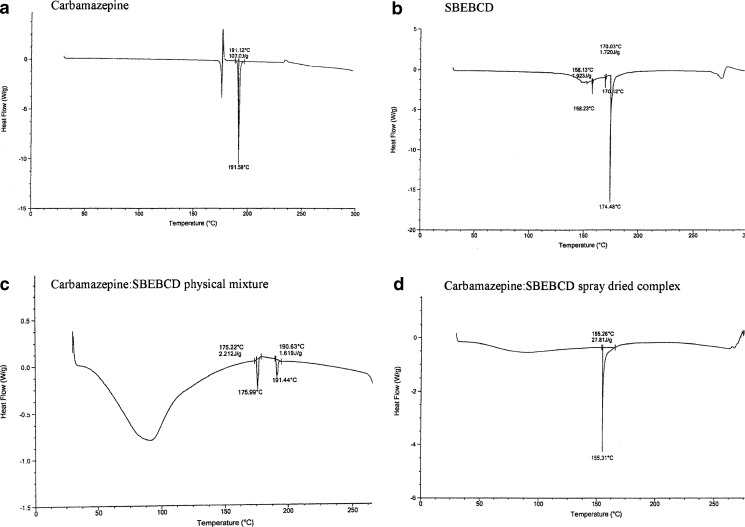
The differential scanning calorimetry (DSC) curve of CBZ (Fig. 3a) showed three distinct thermal events at 173°C, 178°C, and 191°C. This observation was in accordance with the reports by Zerrouk and coworkers (5). An endotherm at 173°C appeared due to the melting of form III of CBZ which was followed by an exothermic peak at 178°C due to its crystallization to form I. The third event observed at 191°C occurred due to the melting of form I of CBZ (23). The DSC curve of SBE7 β-CD showed a small endotherm at 158.23°C and a sharp endotherm at 174.48°C (Fig. 3b). A broad endotherm around 275°C was due to the beginning of decomposition events. The PM of SBE7 β-CD and CBZ (PM) showed an endotherm at 191.44°C which corresponded to the melting point of CBZ, but the intensity and height of the endotherm was reduced (Fig. 3c). This indicated partial complexation between CBZ and SBE7 β-CD in the PM. The DSC thermogram of the CBZ/SBE7 β-CD inclusion complex has been shown in Fig. 3d. It was evident that the endotherm corresponding to the melting point of CBZ disappeared suggesting that CBZ was completely entrapped in the SBE7 β-CD cavity. In the DSC curves of the complex, the SBE7 β-CD endotherm at 158°C was shifted to 155.31°C indicating formation of a stable complex.
Fig. 3.
DSC thermograms of a CBZ, b SBE7 β-CD, c CBZ/SBE7 β-CD PM, and d CBZ/SBE7 β-CD inclusion complex
IR Spectroscopy
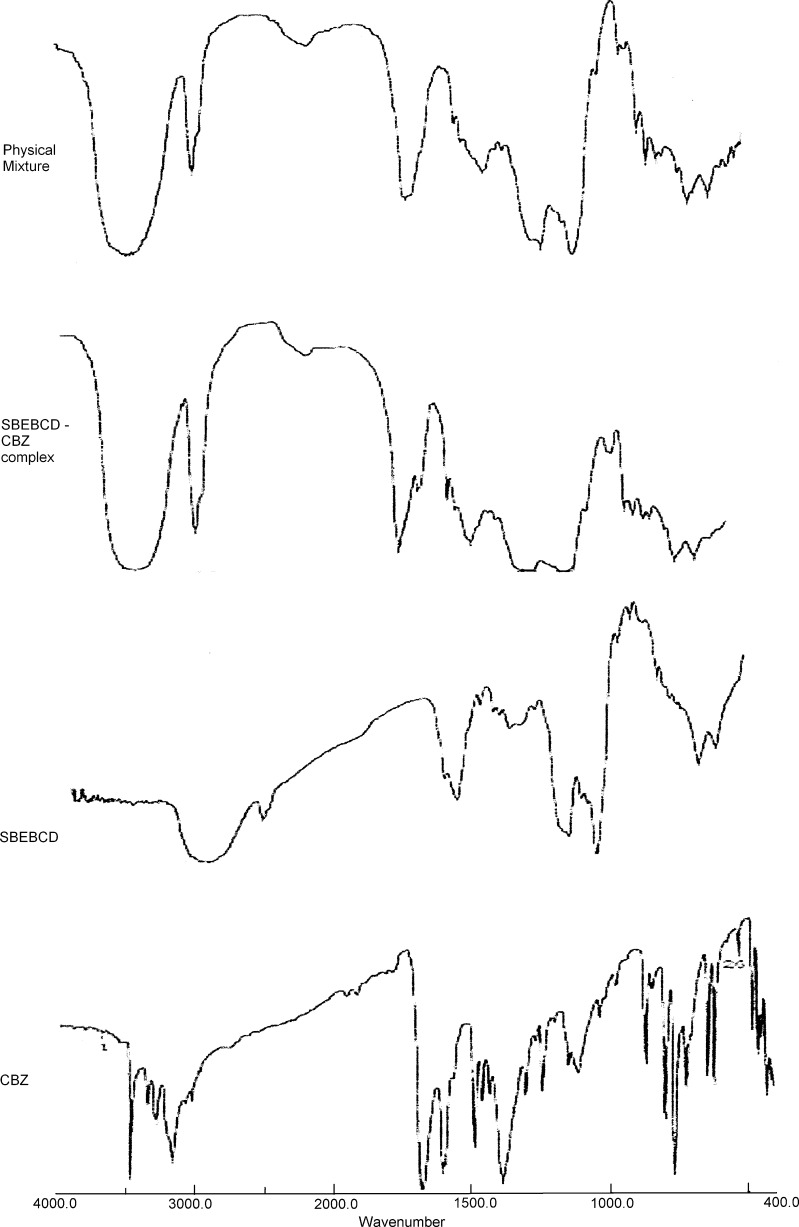
The results of IR spectroscopy have been shown in Fig. 4. The principal peak for hydrogens of the aromatic ring at 800.53 and 765.81 cm−1 for pure CBZ underwent a shift of 1.93 and 3.86 cm−1, respectively, in the CBZ/SBE7 β-CD inclusion complex. The PM of CBZ and SBE7 β-CD showed no shift for the absorption band at 800.53 cm−1, whereas for the band at 765.81 cm−1, a significant shift of 1.93 cm−1 was observed. The signal at 1,305 cm−1 was completely absent in the IR spectrum of the CBZ/SBE7 β-CD inclusion complex which was indicative of a strong interaction or bonding between CBZ and SBE7 β-CD; on the other hand, the IR spectrum of the PM showed no shift in this band. In the SBE7 β-CD spectrum, the peak at 3,445.18 cm−1 was characteristic of the alcoholic OH stretch which was shifted by 21.21 cm−1 in the CBZ/SBE7 β-CD complex and by 11.58 cm−1 in the PM. These results suggested that in both the complex and the PM, there was a strong interaction between SBE7 β-CD and CBZ, being more conspicuous in the complex. The shifts in the IR absorption band of the hydrogens of the aromatic ring and the HC=CH vinyl group in CBZ and the OH group of SBE7 β-CD were signposts of interaction between CBZ and SBE7 β-CD. Further evidence of this interaction has been shown in the NMR spectra. In the PM, the interaction between CBZ and SBE7 β-CD was of a random nature.
Fig. 4.
IR spectra of carbamazepine (CBZ), SBE7 β-CD, CBZ/SBE7 β-CD physical, and CBZ/SBE7 β-CD inclusion complex
NMR Studies
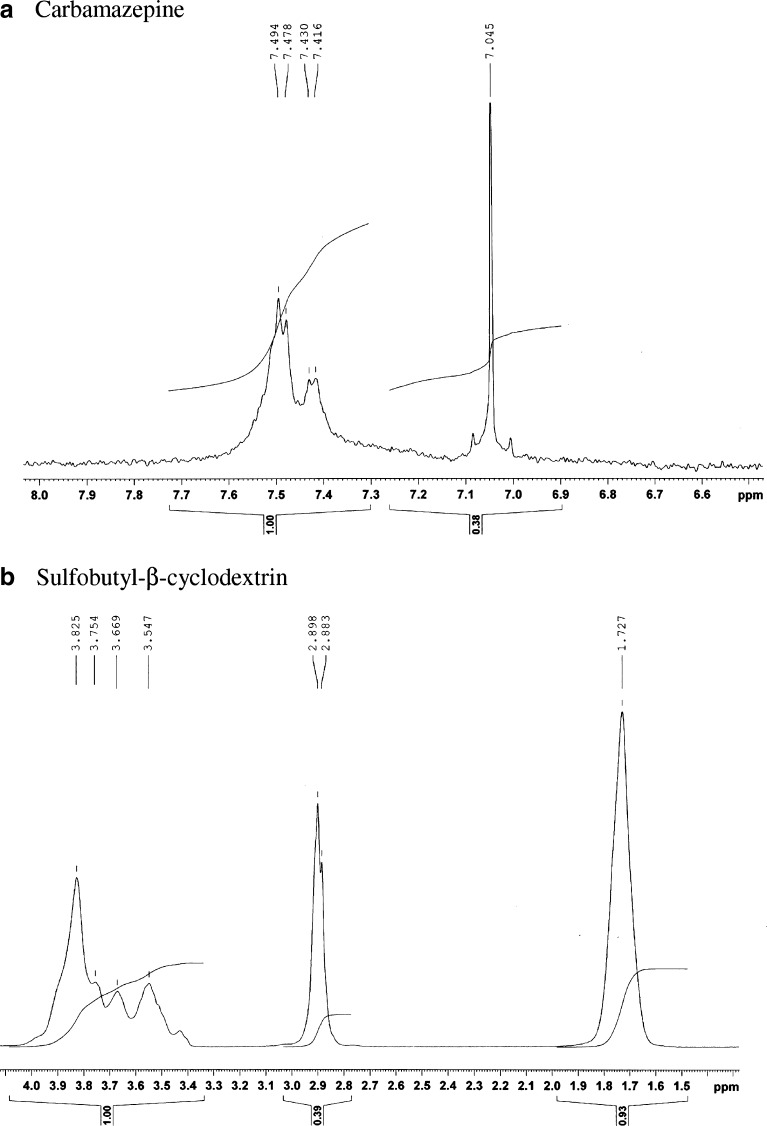
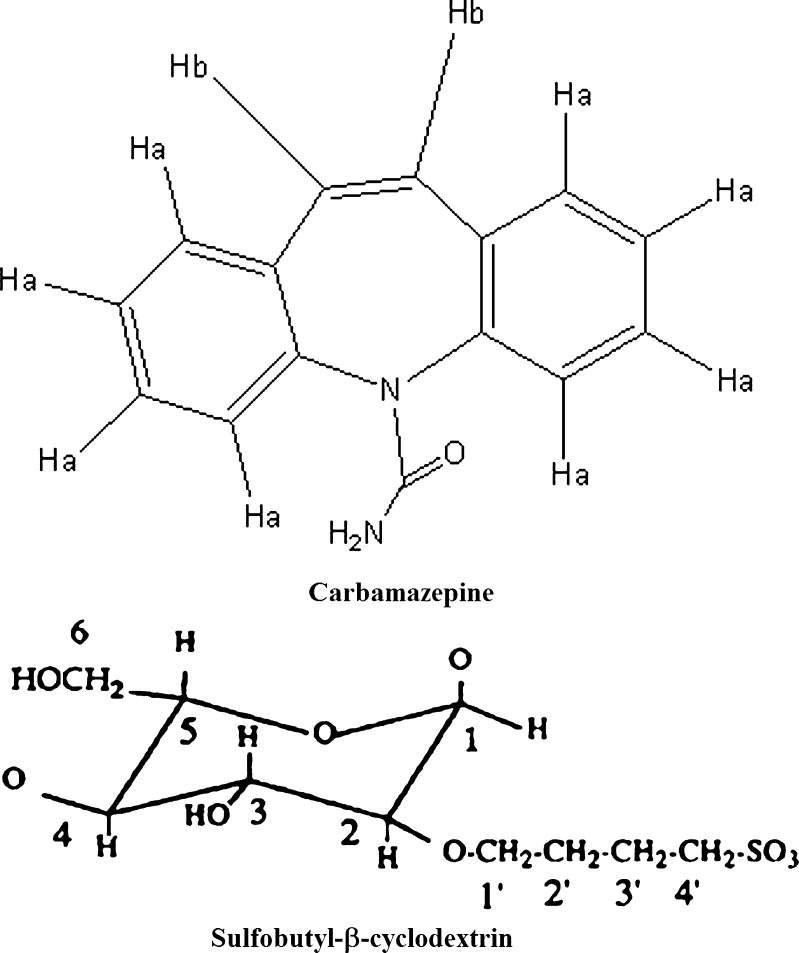
The 1H NMR spectra of CBZ and its dispersions were recorded to gain deeper insight into the interaction of the drug with cyclodextrin. The 1H NMR spectra of CBZ and SBE7 β-CD have been shown in Fig. 5. The assignment of various protons of CBZ and SBE7 β-CD has been shown in Fig. 6. The chemical shifts observed for CBZ and SBEBCD are given below
CBZ 1H-NMR (D2O): δ ppm 7.49, 7.47, 7.43, 7.41 (4 Ha, aromatic protons), and 7.04 (2 Hb, CH=CH protons)
SBEBCD 1H-NMR (D2O): δ ppm 3.82 (H5 proton), 3.75(H6 proton), 3.66 (H1′ proton), 3.54 (H2 proton), 2.89 (H4′ proton), and 1.72 (H3′ H2′ protons)
SBEBCD1H-NMR (CD3OD/D2O): δ ppm 5.04 (H1 proton), 4.03(H3 proton), and 3.57 (H4 proton)
Fig. 5.
1H NMR spectrum of a CBZ and b SBE7 β-CD
Fig. 6.
Proton assignment for a CBZ and b SBE7 β-CD
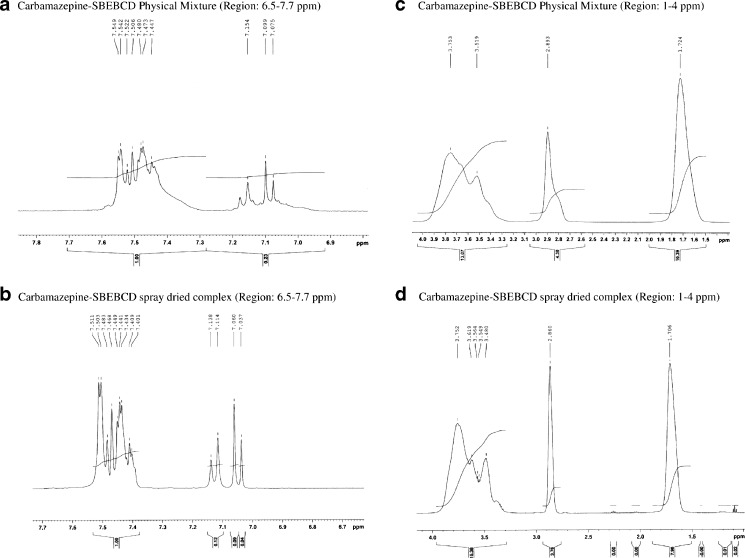
The 1H NMR spectra of the CBZ/SBE7 β-CD PM and CBZ/SBE7 β-CD inclusion complex have been shown in Fig. 7. It is evident from Fig. 5 that the characteristic protons of CBZ (Ha and Hb) and the protons in the cavity of SBE7 β-CD (H3, H4, H5, and H6) are distinct and can be used as probes to monitor the interaction between CBZ and SBE7 β-CD. It is evident in Fig. 7a, b that there were prominent changes in the pattern of the NMR signals of the protons Ha and Hb of CBZ for both the CBZ/SBE7 β-CD PM and the CBZ/SBE7 β-CD inclusion complex in comparison to Fig. 5. In addition, changes in the pattern of the NMR signals of the cavity protons of SBE7 β-CD were observed both for the CBZ/SBE7β-CD PM and the CBZ/SBE7 β-CD inclusion complex (Fig. 7c, d compared with Fig. 5b). These changes clearly indicated some form of interaction between SBE7 β-CD and CBZ in both the PM as well as the inclusion complex. However, intricate aspects of the interaction could not be deduced due to difficulties in extracting accurate changes in the chemical shifts.
Fig. 7.
1H NMR spectra of a CBZ/SBE7 β-CD PM and b CBZ/SBE7 β-CD inclusion complex
X-ray Diffraction Studies
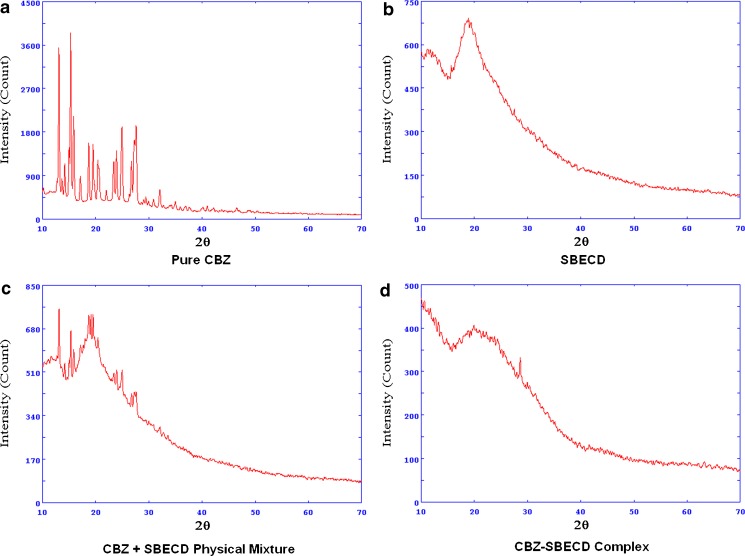
As shown in Fig. 8, the X-ray diffractogram of CBZ showed many sharp peaks which were indicative of its crystalline nature; some of these peaks are at ∼12°, 15°, 16°, 24°, and 27° on 2θ with intensity approximately 3,600, 4,050, 2,000, 1,850, and 1,900, respectively. The X-ray pattern of SBE7 β-CD showed no sharp peaks which indicated its amorphous nature. The CBZ/SBE7 β-CD complex had an XRD pattern very similar to that observed for pure SBE7 β-CD. All sharp peaks observed for pure CBZ had reduced in intensity to a great extent and were diffused. Only a single peak at ∼27° on 2θ was seen with a reduced intensity of ∼350. Most importantly, the highest peak of CBZ at 15° (∼4,050) on 2θ was absent in the XRD of the complex. The results of the XRD studies indicated that CBZ formed an inclusion complex with SBE7 β-CD and that CBZ existed in an amorphous state in the inclusion complex. The PM of SBE7 β-CD and CBZ also showed a reduction in peak intensities compared with the XRD pattern of pure CBZ, though peaks were seen at ∼12°, 15°, and 20° on 2θ with approximate intensities of 765, 680, and 760, respectively. This suggested that even physical mixing resulted in some interaction between CBZ and SBE7 β-CD resulting in reduction in crystalline nature of CBZ. However, a PM did not result in complete amorphization of CBZ unlike in the inclusion complex.
Fig. 8.
XRD spectra of a CBZ, b SBE7 β-CD, c CBZ/SBE7 β-CD PM, and d CBZ/SBE7 β-CD inclusion complex
SEM Studies
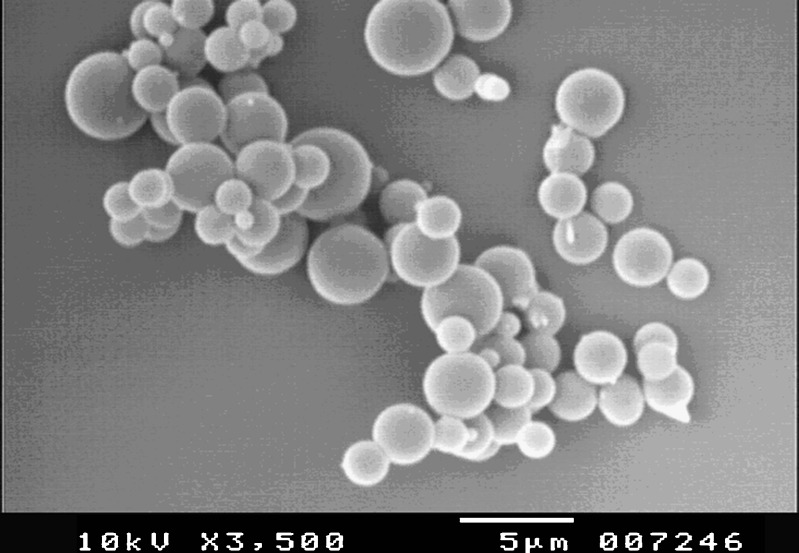
The SEM studies are useful in determining surface properties of powders. The SEM picture of spray-dried complex (Fig. 9) showed perfectly spherical particles. The microscopy studies of CBZ showed CBZ as irregular and prism shaped crystals (data not shown).
Fig. 9.
SEM image of CBZ/SBE7 β-CD inclusion complex
Molecular Modeling Studies
The process of synthesis randomly substitutes the OH groups in cyclodextrin with the sulfobutyl ether groups; the actual substitution pattern and the configurations have not been revealed by the manufacturer. This lack of information poses difficulties in molecular modeling. Although Blach et al., described molecular modeling of SBE7 β-CD with a catalyst, they considered only one isomer in their molecular modeling study (24). Recently, Jug et al., described a molecular modeling study of SBE7 β-CD with bupivacaine hydrochloride involving two isomers of SBE7 β-CD (25). In the present investigation, three possible isomers of SBE7 β-CD were employed for the molecular modeling studies so that sufficient representation of the hindrance induced by the sulfobutyl groups is considered. The three isomers of SBE7 β-CD considered in our molecular modeling studies have been shown in Fig. 1.
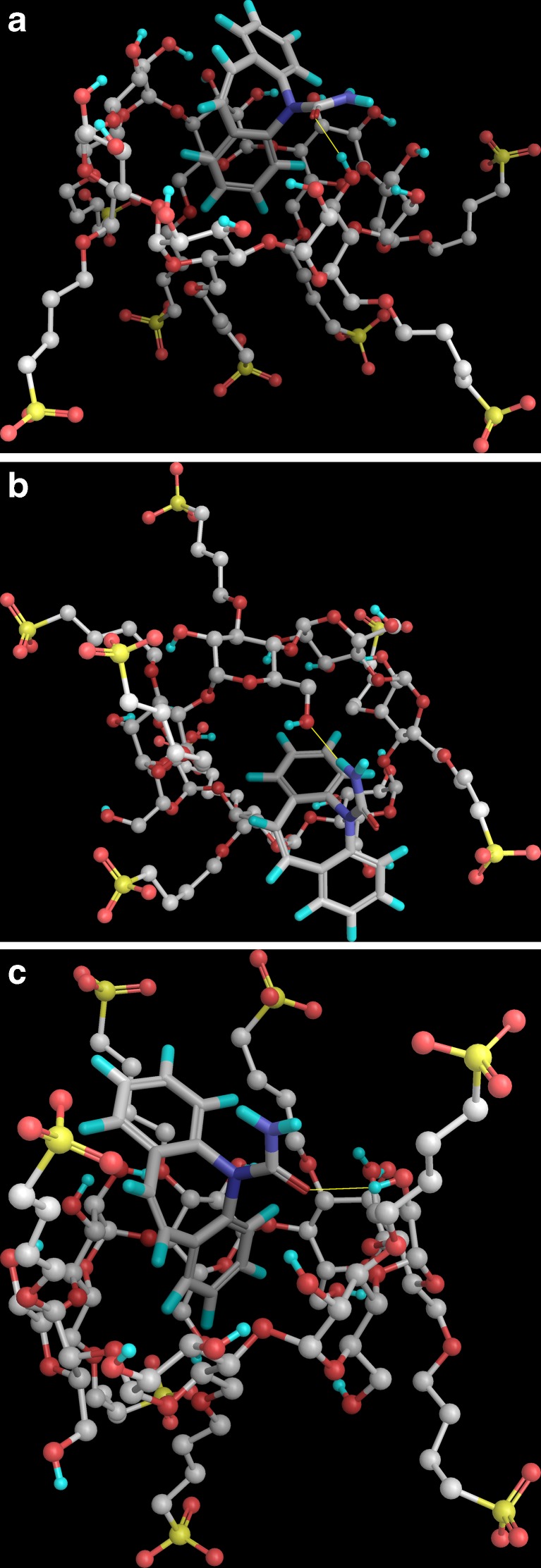
The results of the docking studies of CBZ with the three different SBE7 β-CD isomers have been shown in Fig. 10a–c. It was evident from Fig. 10 that CBZ interacted with SBE7 β-CD on the secondary face in isomers 1 and 3 (Fig. 10a, c) while in isomer 2 the interaction occurred at the primary face (Fig. 10b). It was also evident that in isomers 1 and 3, CBZ was partially embedded in the SBE7 β-CD cavity, i.e., one of the aromatic ring lies within the SBE7 β-CD cavity. This clearly explained the change in the pattern of the NMR signals of protons Ha and Hb of CBZ (Figs. 5 and 7) in the CBZ/SBE7 β-CD complex. It was evident from Fig. 10b that in isomer 2, CBZ interacted with the sulfobutyl ether arms of SBE7 β-CD. This surface interaction did constitute a part of the total interaction of CBZ with SBE7 β-CD and which would impact the outcome of the results of DSC, XRD, IR, and NMR more for the PM than for the inclusion complex. Interestingly, MD simulations revealed that the binding energies of the complex for all three isomers are nearly identical (Table I); however, their individual contributions to the overall binding energy would be weighted by the fractions of their population in the overall mixture.
Fig. 10.
Three-dimensional structures of the complexes between the carbamazepine and the three isomeric forms of sulfobutyl ether7 β-cyclodextrin obtained by molecular docking a isomer 1, b isomer 2, and c isomer 3. a 3D structure of the complex between the carbamazepine and the sulfobutyl ether7 β-cyclodextrin isomer 1 is referred to as case 1 in the manuscript. b 3D structure of the complex between the carbamazepine and the sulfobutyl ether7 β-cyclodextrin isomer 2 is referred to as case 2 in the manuscript. c 3D structure of the complex between the carbamazepine and the sulfobutyl ether7 β-cyclodextrin isomer 3 is referred to as case 3 in the manuscript
Table I.
Binding Energies Computed for the Three Carbamazepine-Sulfobutylether7-β-Cyclodextrin Complexes Post-5-ns MD Simulation
| Sr. no. | Complex | SBE substitution site on BCD | Binding energy (kcal/mol) |
|---|---|---|---|
| 1 | Case 1 | Interaction of carbamazepine with SBEBCD isomer 1 | −6.03 |
| 2 | Case 2 | Interaction of carbamazepine with SBEBCD isomer 2 | −6.04 |
| 3 | Case 3 | Interaction of carbamazepine with SBEBCD isomer 3 | −6.03 |
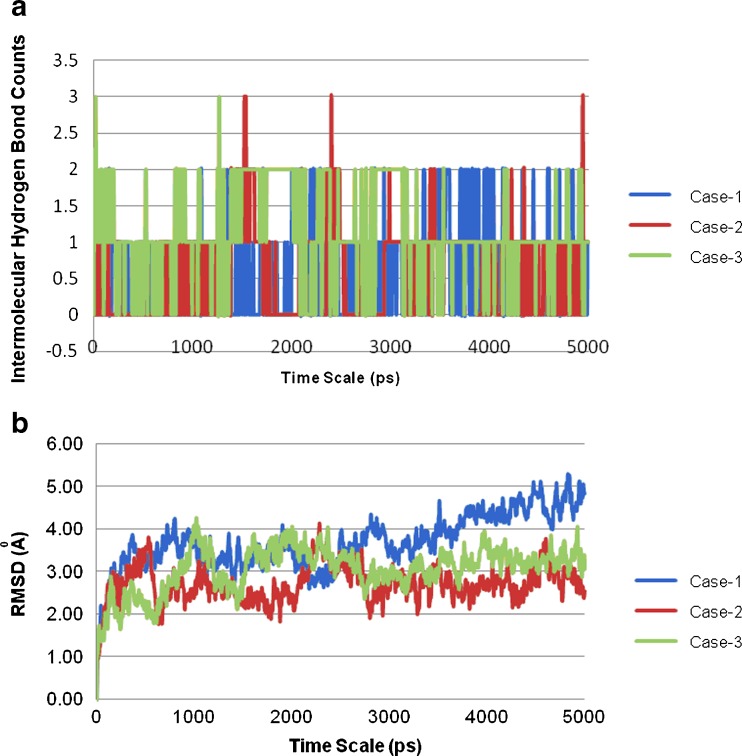
The MD trajectory depicted a persistent intermolecular hydrogen bond (Fig. 11a) between the carboxamide group of carbamazepine and the 3-OH of the glucose unit of SBE7 β-CD in complexes with isomers 1 and 3 (Fig. 10a, c). In contrast, in the complex with isomer 2, hydrogen bonding occurred between the NH of the carboxamide group of CBZ and the 6-OH group of the glucose unit of SBE7 β-CD as seen in Fig. 10b. The RMSD of the configuration of CBZ in the SBE7 β-CD cavity with time (Fig. 11b) was also constructed from the MD trajectory in water to evaluate the dynamics of the binding between CBZ and SBE7 β-CD (26). The RMSD analysis revealed that the CBZ/SBE7 β-CD complex was stable when soaked in the water during the entire simulation period. The RMSDs computed with respect to the initial structure fluctuated between 2.0 and 4.0 (Fig. 11b). The higher values in the RMSD were a result of the floppy motion of the sulfobutyl ether arms.
Fig. 11.
A 5-ns MD simulation trajectory for the complex formed between carbamazepine and the three isomeric states of sulfobutyl ether7 β-cyclodextrin a intermolecular hydrogen bonds (H-bonds) and b RMSD (heavy atoms)
Need for Bulking Agent
The yield obtained for the CBZ/SBE7 β-CD complex (without any bulking agent) obtained after spray drying was around 45 ± 5%. The incorporation of lactose as bulking material in the spray-drying process resulted in improvement of the CBZ/SBE7 β-CD complex yield (67 ± 2%) indicating utility of the bulking agent. However, incorporation of lactose did not affect the characteristics and strength of the CBZ/SBE7 β-CD complex (data not shown). Thus, use of lactose as a bulking agent would help to minimize wastage of both CBZ and SBE7 β-CD.
Dissolution Studies of Various CBZ Formulations
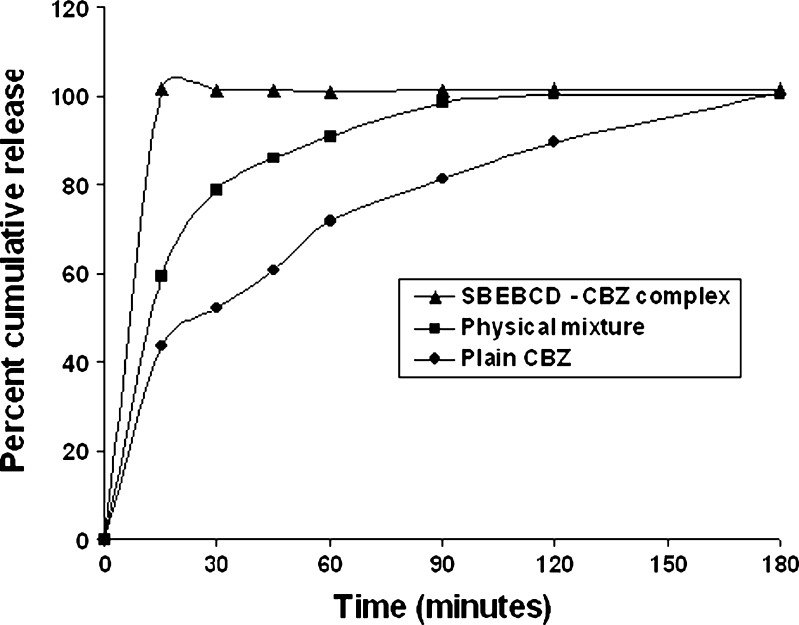
The in vitro dissolution profiles of pure CBZ, the PM of CBZ/SBE7 β-CD, and the CBZ/SBE7 β-CD inclusion complex have been shown in Fig. 12. As seen in Fig. 12, the spray-dried CBZ/SBE7 β-CD inclusion complex resulted in 100% drug release in just 15 min while pure CBZ took nearly 3 h for complete dissolution. Preparation of a PM of CBZ and SBE7 β-CD also enhanced the dissolution of CBZ. From the in vitro dissolution studies, it can be confirmed that CBZ does form an inclusion complex with SBE7 β-CD leading to a dramatic increase in its dissolution rate. The enhanced dissolution profile observed with the PM was also indicative of partial complexation between CBZ and SBE7 β-CD. From the in vitro dissolution studies, it can be assumed that the inclusion complex may display greater in vivo anti-epileptic activity.
Fig. 12.
In vitro dissolution profiles of a pure carbamazepine, b the PM, and c the CBZ/SBE7 β-CD inclusion complex
Evaluation of In Vivo Anti-epileptic Activity
Based on observations during the in vivo anti-epileptic study, behavioral changes were categorized into various phases as reported by Klioueva et al. (27)
Atypical behavior (e.g., intensive grooming, sniffing, and moving arrests), isolated myoclonic jerks, and ear and facial twitching;
Atypical minimal seizures, convulsive wave through the body;
Fully developed minimal seizures, clonus of the head muscles, and forelimbs, righting reflex was present
Major seizures (generalized without the tonic phase)
Generalized tonic-clonic seizures marked with running, followed by the loss of righting ability
Short tonic phase (flexion or extension of fore- and hindlimbs) progressing to the clonus of all four limbs finally leading to the death of the animal.
However, comparison among the groups was mainly done on basis of time required for onset of fully developed minimal seizures and occurrence of death of the animal (occurred just after extension of fore- and hindlimbs).
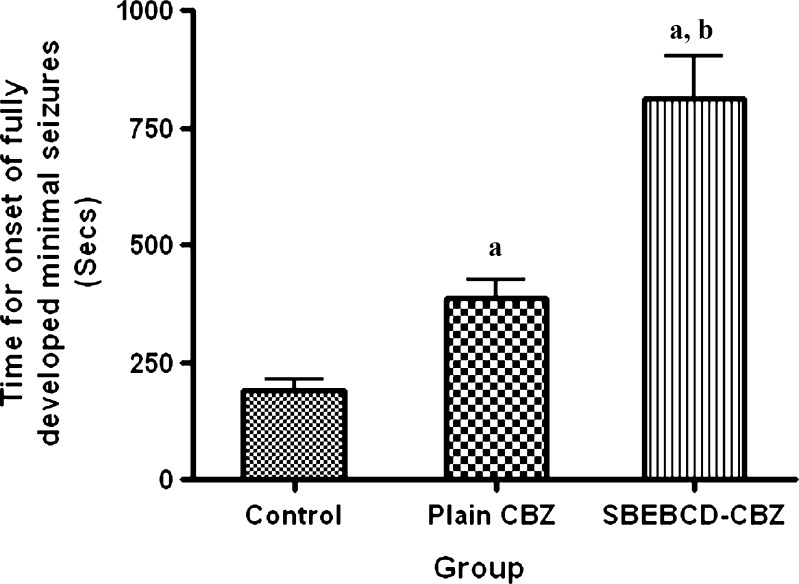
The results of in vivo anti-epileptic study have been shown in Fig. 13. It was evident that time required for onset of fully developed major seizures significantly increased for CBZ suspension as well as for the spray-dried CBZ/SBE7 β-CD inclusion complex compared with the control group (p < 0.05). This was indicative of the therapeutic efficacy of CBZ to treat PTZ-induced convulsions. It was noteworthy that the time required for onset of fully developed minimal seizures for the CBZ/SBE7 β-CD complex was significantly higher (p < 0.01) than that for the CBZ suspension. It was also observed that spray-dried CBZ/SBE7 β-CD inclusion complex offers better protection against seizures as compared with plain CBZ. This clearly indicated greater therapeutic efficacy of the CBZ/SBE7 β-CD inclusion complex as compared with pure CBZ suspension. Furthermore, administration of CBZ/SBE7 β-CD inclusion complex resulted in 50% mortality which was significantly lower than that for CBZ suspension (83%). It is noteworthy that the control (no treatment) group showed 100% mortality. The increased therapeutic efficacy of the inclusion complex was due to significant improvement in the solubility of CBZ in gastric fluids which in turn led to greater absorption and oral bioavailability.
Fig. 13.
In vivo anti-epileptic activity of control (no treatment), pure carbamazepine, and CBZ/SBE7 β-CD inclusion complex
CONCLUSIONS
It is possible to prepare CBZ/SBE7 β-CD inclusion complex by an industrially feasible spray-drying method. Various experimental techniques and molecular modeling studies confirmed the formation of the complex between CBZ and SBE7 β-CD. The spray-dried CBZ/SBE7 β-CD complex provided significantly higher protection against epileptic attacks compared with an orally administered CBZ suspension thus highlighting its usefulness as an oral drug delivery system.
Acknowledgments
Authors are thankful to Novartis India Ltd., for the gift sample of carbamazepine and to Signette Chemicals, Mumbai, India for the gift samples of lactose. The authors would also like to thank Mr. Nimish Vador and Dr. Aarati Jagtap, Department of Pharmacology, Bombay College of Pharmacy for useful suggestions and help while conducting animal studies. The NMR and XRD facility provided by the Tata Institute of Fundamental Research, Mumbai is gratefully acknowledged. A. Jain is thankful to AICTE for Junior Research Fellowship and A. Date is thankful to Indian Council of Medical Research for Senior Research Fellowship. The computational facilities supported by the Department of Biotechnology (BT/TF-8/BRB/2009), Council for Scientific and Industrial Research (file no. 01/2399/10/EMRII), and Department of Science and Technology (SR/FST/LSI-163/2003), New Delhi, are gratefully acknowledged.
References
- 1.Bauer J, Monika BM, Reuber M. Treatment strategies for focal epilepsy. Expert Opin Pharmacother. 2009;10:743–53. doi: 10.1517/14656560902772328. [DOI] [PubMed] [Google Scholar]
- 2.Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272:1–10. doi: 10.1016/j.ijpharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Barakat N, Omar S, Ahmed A. Carbamazepine uptake into rat brain following intra-olfactory transport. J Pharm Pharmacol. 2006;58:63–72. doi: 10.1211/jpp.58.1.0008. [DOI] [PubMed] [Google Scholar]
- 4.Kumineka G, Kratza J, Ribeiroa R, Kelmanna R, de Araújob B, Teixeirab H, et al. Pharmacokinetic study of a carbamazepine nanoemulsion in beagle dogs. Int J Pharm. 2009;378:146–8. doi: 10.1016/j.ijpharm.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Zerrouk N, Chemtob C, Arnaud P, Toscani S, Dugue J. In vitro and in vivo evaluation of carbamazepine-PEG 6000 solid dispersions. Int J Pharm. 2001;225:49–62. doi: 10.1016/S0378-5173(01)00741-4. [DOI] [PubMed] [Google Scholar]
- 6.El-Zein H, Riad L, Abd El-Bary A. Enhancement of carbamazepine dissolution: in vitro and in vivo evaluation. Int J Pharm. 1998;168:209–20. doi: 10.1016/S0378-5173(98)00093-3. [DOI] [Google Scholar]
- 7.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Miller D, Peppas N, McGinity J. Influence of sulfobutyl ether β-cyclodextrin (Captisol®) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int J Pharm. 2008;350:188–96. doi: 10.1016/j.ijpharm.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Nagase Y, Hirata M, Arima H, Tajiri S, Nishimoto Y, Hirayama F, et al. Protective effect of sulfobutyl ether β-cyclodextrin on DY-9760e-induced hemolysis in vitro. J Pharm Sci. 2002;91:2382–9. doi: 10.1002/jps.10236. [DOI] [PubMed] [Google Scholar]
- 10.Tötterman AM, Schipper NGM, Thompson DO, Mannermaa JP. Intestinal safety of water-soluble β-cyclodextrins in paediatric oral solutions of spironolactone: effects on human intestinal epithelial Caco-2 cells. J Pharm Pharmacol. 1997;49:43–8. doi: 10.1111/j.2042-7158.1997.tb06750.x. [DOI] [PubMed] [Google Scholar]
- 11.Okimoto K, Ohike A, Ibuki R, Aoki O, Ohnishi N, Irie T, et al. Design and evaluation of an osmotic pump tablet (OPT) for chlorpromazine using (SBE)7 m-beta-CD. Pharm Res. 1999;16:549–54. doi: 10.1023/A:1018827214223. [DOI] [PubMed] [Google Scholar]
- 12.Kale R, Saraf M, Tayade P. Cyclodextrin complexes of valdecoxib: properties and anti-inflammatory activity in rat. Eur J Pharm Biopharm. 2005;60:39–46. doi: 10.1016/j.ejpb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Kale R, Tayade P, Saraf M, Juvekar A. Molecular encapsulation of thalidomide with sulfobutyl ether-7 beta-cyclodextrin for immediate release property:enhanced in vivo antitumor and antiangiogenesis efficacy in mice. Drug Dev Ind Pharm. 2008;34:149–56. doi: 10.1080/03639040701484486. [DOI] [PubMed] [Google Scholar]
- 14.Smith JS, MacRae RJ, Snowden MJ. Effect of SBE-7-β-cyclodextrin complexation on carbamazepine release from sustained release beads. Eur J Pharm Biopharm. 2005;60:73–80. doi: 10.1016/j.ejpb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 16.Figueiras A, Carvalho A, Ribeiro L, Torres-Labandeira J, Veiga F. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur J Pharm Biopharm. 2007;67:531–9. doi: 10.1016/j.ejpb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Mark P, Nilsson L. Structure and dynamics of the TIP3P, SPC, and SPC/E Water models at 298 K. J Phys Chem A. 2001;105:9954–60. doi: 10.1021/jp003020w. [DOI] [Google Scholar]
- 18.Quigley D, Probert MIJ. Constant pressure Langevin dynamics: theory and application. Comp Phys Comm. 2005;169:322–5. doi: 10.1016/j.cpc.2005.03.072. [DOI] [Google Scholar]
- 19.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J Comput Phys. 1977;23:327–41. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 20.Vogel HG, Vogel WH, Schoelkens BA, Sandow J, Mueller G, Vogel WF, editors. Drug discovery and evaluation: pharmacological assays. New York: Springer; 2008. p. 613.
- 21.Moyano JR, Gines JM, Arias MJ, Robasco AM. Study of dissolution characteristic of oxazepam via complexation with β-cyclodextrin. Int J Pharm. 1995;114:95–102. doi: 10.1016/0378-5173(94)00220-Y. [DOI] [Google Scholar]
- 22.Yang B, Lin J, Chen Y, Liu Y. Artemether/hydroxypropyl-beta-cyclodextrin host-guest system: characterization, phase-solubility and inclusion mode. Bioorg Med Chem. 2009;17:6311–7. doi: 10.1016/j.bmc.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 23.Zerrouk N, Toscani S, Gines-Dorado J-M, Chemtob C, Ceólin R, Dugué J. Interactions between carbamazepine and polyethylene glycol (PEG) 6000: characterisations of the physical, solid dispersed and eutectic mixtures. Eur J Pharm Sci. 2001;12:395–404. doi: 10.1016/S0928-0987(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 24.Blach P, Landy D, Fourmentin S, Surpateanu G, Bricout H, Ponchel A, et al. Sulfobutyl ether-β-cyclodextrins: promising supramolecular carriers for aqueous organometallic catalysis. Adv Synth Catal. 2005;347:1301–7. doi: 10.1002/adsc.200505051. [DOI] [Google Scholar]
- 25.Jug M, Mennini N, Melani F, Maestrelli F, Mura P. Phase solubility, 1H NMR and molecular modelling studies of bupivacaine hydrochloride complexation with different cyclodextrin derivates. Chem Phys Lett. 2010;500:347–54. doi: 10.1016/j.cplett.2010.10.046. [DOI] [Google Scholar]
- 26.Zoppi A, Quevedo MA, Longhi MR. Specific binding capacity of β-cyclodextrin with cis and trans enalapril: physicochemical characterization and structural studies by molecular modeling. Bioorg Med Chem. 2008;16:8403–12. doi: 10.1016/j.bmc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Klioueva I, van Luijtelaar E, Chepurnova N, Chepurnov S. PTZ-induced seizures in rats: effects of age and strain. Physiol Behavior. 2001;72:421–6. doi: 10.1016/S0031-9384(00)00425-X. [DOI] [PubMed] [Google Scholar]