Abstract
The bioavailability of therapeutic agents from eye drops is usually limited due to corneal barrier functions and effective eye protective mechanisms. Therefore, the current study aims to enhance ocular bioavailability of brimonidine, a potent antiglaucoma drug, through the preparation of ocular inserts. Solvent casting technique was employed to prepare the inserts using polyvinylpyrrolidone K-90 (PVP K-90) as film-forming polymer blended with different viscosity grades of bioadhesive polymers namely hydroxypropyl methycellulose, carbopol, sodium alginate, and chitosan. The prepared ocular inserts were evaluated for various physicochemical parameters, swelling behavior, and in vitro release patterns. Sodium alginate-based ocular inserts revealed the most sustainment in drug release (99% at 6 h), so it was selected for further modifications via coating it, on one side or dual sides, using hydrophobic film composed of either ethylcellulose or Eudragit RSPO. The obtained in vitro release results for the modified ocular inserts revealed that ethylcellulose is superior to Eudragit RSPO in terms of brimonidine release sustainment effect. Ocular inserts composed of 7% PVP K-90, 1.5% low molecular weight sodium alginate with or without ethylcellulose coat were able to sustain the in vitro release of brimonidine. Their therapeutic efficacy regarding intraocular pressure (IOP) lowering effect when inserted in albino rabbits eyes showed superior sustainment effect compared with that of brimonidine solution. Furthermore, due to both the mucoadhesive property and the drug sustainment effect, the one-side-coated ocular insert showed more IOP lowering effect compared with that of its non-coated or dual-side-coated counterpart.
Key words: brimonidine, intraocular pressure, ocular insert, physicochemical characterization, sustained release
Introduction
The development of effective ophthalmic dosage forms faces many challenges due to physiological constrains imposed by the unique anatomic structure and efficient protective mechanism of the eyes (1). The majority of the ophthalmic drugs are administrated topically in the form of conventional eye drops (2). However, the rapid turnover of lacrimal fluid and extensive nasolacrimal drainage (3) along with eyes blinking reflex rapidly eliminate the administrated eye drops (4,5). This causes short pre-corneal residence time, which limits effective transcorneal drug absorption. Thus, frequent instillation of eye drops is required to achieve therapeutic effect (6). However, this usually results in pulsed administration and patient non-compliance. In addition, the topically applied drugs could enter the systemic circulation through the nasolacrimal duct system causing side effects that could sometimes extend to systemic toxicity (7,8).
In order to overcome the abovementioned drawbacks of the conventional eye drops, many researchers have attempted to increase the pre-corneal residence time by increasing the viscosity of ophthalmic delivery systems through inclusion of drugs in gels (3,4) and ointments (9). The latter dosage forms demonstrated substantially improvement in drug bioavailability when compared with their eye drops counterparts. However, they suffered from some disadvantages that include sticky sensation, blurred vision and induced reflex blinking which also caused patient non-compliance (10).
Generally, the main prerequisites for ideal ophthalmic drug delivery system that ensures effective ocular therapy is its ability to: (a) be administrated accurately without causing blurred vision or irritation, (b) have suitable mucoadhesive property to improve the drug retention in the pre-corneal area and thereby increase drug bioavailability, (d) have limited systemic absorption through nasolacrimal drainage, and (c) reduce the need for frequent dosing regimen leading to improved patient compliance (11).
Ocular inserts are solid or semi-solid devices, usually made of polymeric materials, meant to be placed in the conjunctival sac to deliver drugs to the ocular surface. The potential advantages offered by the inserts are the accurate dosing, increased ocular residence time, reduction in systemic side effects, better patient compliance due to reduced frequency of administration and possibility of releasing drugs at a slow and constant rate as well as increase shelf life stability. These advantages overall lead to effective ocular therapy (12).
In spite of the numerous advantages demonstrated by ocular inserts, its capital disadvantage is the foreign body sensation accompanied with its initial administration (13). After which, it exhibits a gelling behavior, resulting in an extended residence at the site of drug action. However, this disadvantage did not prevent the adoption of this technology in several successfully marketed ocular inserts (Ocusert®, Ocufit® SR, and Minidisc®) as their numerous advantages extremely supersede their sole disadvantage (14).
Glaucoma is the second leading cause of vision loss in the world after cataract (15). According to a published research in the British Journal of Ophthalmology, it is estimated that the number of people with glaucoma will be nearly 79.6 million worldwide by 2020 (16). This alarming high number of anticipated patients requires urgent improvement in the current therapeutic approaches adopted for treatment of this disease. Even though, the currently available eye drops for glaucoma treatment reduce its disability. Their long-term effectiveness and efficacy are being questioned due to poor patient compliance.
Brimonidine is among the most promising therapeutic agents for treatment of open-angle glaucoma and ocular hypertension (17). It is characterized by ultimate ocular hypotensive efficacy that is achieved through increasing uveoscleral outflow along with decreasing aqueous humor production. In several clinical trials, brimonidine showed intraocular pressure (IOP) lowering efficacy comparable to that of timolol and greater than that of betaxolol. Furthermore, the high selectivity of brimonidine for α2- versus α1-adrenergic receptors results in reduction in adverse pulmonary and cardiovascular effects when compared with other drugs namely clonidine and apraclonidine adopted for glaucoma treatment (18,19).
At present, brimonidine is commercially available in the form of eye drops and marketed under the name of Alphagan® (0.2%) or Alphagan® P (0.1% and 0.15%). For effective management of IOP, it needs to be administered 1 drop every 8 h (20). The drawbacks associated with the available eye drops are short pre-corneal retention time along with poor patient compliance.
In this regard, to improve patient compliance by lowering the frequency of administration and decreasing the incidence of side effects associated with brimonidine dosage regimen, this research aimed at formulating sustained release brimonidine-loaded ocular inserts. Initially, nine formulations were developed based on blends of polyvinylpyrrolidone K-90 (PVP K-90) with different concentrations of hydrophilic polymers that are of well-known biocompatibility, biodegradability and sustained drug release characteristics. These formulations were subjected to various physicochemical evaluations. The in vitro release profile of all the formulations was investigated. To effectively sustain brimonidine release, selected ocular inserts were coated (one side or two sides) using either ethylcellulose or Eudragit RSPO and then their in vitro drug release profiles were re-investigated. Before the in vivo study, the irritation potential of selected ocular inserts was evaluated. To ensure that the formulated ocular inserts attained the preset research goal, their IOP lowering effect were investigated in albino rabbits and compared with that of drug solution.
MATERIALS AND METHODS
Materials
Brimonidine tartarate was kindly provided by Allergan, Inc., Irvine, CA, USA. PVP K-90 was purchased from Sigma Chemical Co., St. Louis, MO, USA. Ethylcellulose (EC; Ethocel standard 100 Premium) and two different viscosity grades of hydroxypropyl methylcellulose (HPMC K4M and HPMC K100M with nominal viscosity values of 4,000 and 100,000 cP, respectively, when present in aqueous concentration of 2% at 20°C) were generously donated by Colorcon, Midland, MI, USA. Two grades of alginic acid sodium salts (low viscosity, ref. A2158 and medium viscosity, ref A2033 with approximate nominal viscosity of 250 and 2,000 cP, respectively, when present in aqueous concentration of 2% at 25°C) and two grades of chitosan (low molecular (LM) weight, ref. 448,869 and medium molecular (MM) weight, ref. 448,877 with viscosity values of 20–300 and 200–800 cP, respectively when present in 1% concentration in 1% acetic acid at 20°C) were obtained from Sigma-Aldrich, Tokyo, Japan. Two different viscosity grades of carbopol (carbopol 934 NF and carbopol 971 NF with viscosity values of 30,500–39,400 cP and 4,000–11,000 cP, respectively when present in concentration of 0.5% at pH 7.5) were purchased from BF Goodrich Chemical, Cleveland, OH, USA. Eudragit RSPO was generously donated from Rohm GmbH, Darmstadt, Germany. Triethanolamine, propylene glycol and glacial acetic acid were purchased from Adwic, El-Nasr Chemical Co., Cairo, Egypt. Double-distilled water was used for the preparation of the inserts and buffer solutions. All other chemicals and solvents were of analytical grade and were used as received.
Methods
Preparation of Brimonidine Ocular Inserts
Polymeric ocular inserts containing brimonidine were prepared using film-casting method (21). The percent composition (w/w) of the prepared polymeric ocular inserts is listed in Table I. PVP K-90 was used as an insert-forming polymer (22) and different viscosity grades of HPMC, chitosan, carbopol, and sodium alginate were employed as bioadhesive materials.
Table I.
Compositions, Drug Content, and Thickness of Different Formulations of Ocular Inserts Containing Brimonidine
| Formula code | Drug (%, w/w) | PVPK-90 (%, w/w) | Biodegradable polymer (1.5%, w/w) | Propylene glycol (%) | Drug content (% ± SD) | Thickness (mm ± SD) |
|---|---|---|---|---|---|---|
| F1 | 1 | 7 | 5 | n/a | n/a | |
| F2 | 1 | 7 | LM sodium alginate | 5 | 98.5 ± 0.2 | 0.49 ± 0.03 |
| F3 | 1 | 7 | MM sodium alginate | 5 | 96.2 ± 0.3 | 0.63 ± 0.05 |
| F4 | 1 | 7 | HPMC K4M | 5 | 97.3 ± 0.1 | 0.12 ± 0.03 |
| F5 | 1 | 7 | HPMC K100M | 5 | 98.1 ± 0.3 | 0.12 ± 0.03 |
| F6 | 1 | 7 | LM chitosan | 5 | 98.7 ± 0.5 | 0.33 ± 0.03 |
| F7 | 1 | 7 | MM chitosan | 5 | 96.9 ± 0.4 | 0.35 ± 0.06 |
| F8 | 1 | 7 | Carbopol 971 | 5 | n/a | n/a |
| F9 | 1 | 7 | Carbopol 934 | 5 | n/a | n/a |
Initially, PVP K-90 in concentration of (7%, w/w) was dissolved into the drug aqueous solution (1%, w/w) using a magnetic stirrer and then sonicated for 10 min in an ultrasonic water bath (Model 275T, Crest Ultrasonics Corp., Trenton, NJ, USA) to remove air bubbles. With the aid of magnetic stirrer, PVP K-90 polymeric solution was further mixed with the bioadhesive polymeric hydrogel. The latter was prepared by dispersing the bioadhesive polymers (1.5%, w/w) in distilled water using a magnetic stirrer adjusted to rotate at constant stirring speed of 400 rpm at room temperature. Following that, propylene glycol was employed as a plasticizer in concentration of 5% (w/w) to aid in the formation of flexible films as well as to protect the polymeric inserts from being brittle upon storage. In case of carbopol, the produced gel was neutralized to pH 6.9–7.2 using triethanolamine. On the other hand, chitosan hydrogel was prepared by dispersing the polymer in 1.5% acetic acid solution.
All of the resultant polymeric gels were then sonicated for 4 h in an ultrasonic water bath (Model 275T, Crest Ultrasonics Corp., Trenton, NJ, USA) to exclude entrapped air and then stored for 24 h at 4–8°C to ensure total hydration of the polymers. Before pouring into a plastic substrate (circular dish of 60 mm in diameter and 15 mm depth), the polymeric gels were brought back to ambient temperature. The cast polymeric gels were left to dry at room temperature for 48 h. Then, the dried membranes were carefully removed and circular inserts of 3 mm in diameter were punched out, using a stainless steel borer and stored in sealed containers until further use. Each ocular insert was prepared to contain 0.1 mg brimonidine tartarate.
Physicochemical Evaluation of Polymeric Ocular Inserts
Physical Characterization
The ocular inserts were evaluated for their physical characters such as color, texture, and appearance.
Drug Content Uniformity
Ocular insert belonging to each formulation was dissolved in suitable quantity of distilled water and the solution was filtered, suitably diluted and brimonidine content was analyzed spectrophotometrically (Shimadzu UV spectrophotometer, 1601-PC double-beam spectrometer, Kyoto, Japan) at 320 nm. This test was done on ten ocular inserts for each formulation.
Uniformity of Thickness
Inserts thickness was determined using a caliper (Vernier Caliper, Shanghai, China) and recorded as the mean of 20 measurements.
Swelling Test
Swelling test was investigated to measure the bulk hydrophilicity and hydration of polymers as it affects drug release from polymeric matrix. To test the swelling of brimonidine-loaded inserts, three inserts of each formulation were weighed and put inside an iron mesh basket which was inserted into phosphate-buffered saline (PBS) of pH 7.4 maintained at temperature of 32 ± 0.5°C. At specific time intervals of up to 30 min, the inserts were removed, wiped with lint-free tissue to remove excess surface PBS, weighed, and then returned back to the same container (23).
The degree of fluid uptake was calculated as swelling index using the following equation:
 |
where W0 is the initial weight of the sample and Wt its weight at t time.
In Vitro Drug Release Studies
Due to the absence of an official method reported for in vitro drug release studies of ocular inserts, a simple method was used to discriminate between the release patterns of the prepared inserts. Aiming to simulate the pH conditions of ocular cavity; inserts belonging to each formulation were placed in 15-mL glass vials containing 10 mL PBS of pH 7.4. The vials were placed in a thermostatically controlled shaking water bath (Model 1083, GLF Corp., Burgwedel, Germany) at 32 ± 0.5°C maintained at a speed of 25 strokes/min. At predetermined time intervals, aliquots from the release medium (2 mL) were withdrawn through Millipore® membrane filter of 0.45 μm pore size. Concentrations of brimonidine in the withdrawn samples were determined spectrophotometrically by measuring their absorbance using a UV spectroscopy (1601-PC Double-Beam Spectrometer, Shimadzu, Kyoto, Japan) at λmax value of 320 nm.
All of the withdrawn samples were replenished with equal volumes of same release medium to keep the release volume constant throughout the experiment. Release studies were carried out in triplicate and were expressed as percentage of drug loading versus time. Based on the resultant release data, further modification, by coating using ethylcellulose or Eudragit RSPO, was performed on selected ocular inserts for further sustainment of drug release.
The differential factor f1, defined by the following equation, was used to compare the difference between the drug release profiles of the tested formulations (24).
 |
where n is the number of dissolution sample times, and Rt and Tt are the individual percentages dissolved at each time point, t, for the reference and test dissolution profiles, respectively. f1 value indicates the percent difference between two profiles at each time point and is a measurement of the relative error between them. In general, to ensure similarity between the profiles, f1 should be in the range of 0–10.
Preparation of One-Side- and Dual-Side-Coated Polymeric Ocular Inserts
For further sustainment of brimonidine release, selected ocular inserts were coated using hydrophobic polymers namely ethylcellulose or Eudragit RSPO (Table II). Briefly, alcoholic solutions of the hydrophobic polymers in 10% (w/w) concentration were prepared. Propylene glycol in concentration of 10% (w/w) was added to the polymer alcoholic solution to aid in the formation of flexible and elastic coat. The selected inserts were coated by quickly immersing in the polymeric alcoholic solution for 5 s and were then left to dry at room temperature.
Table II.
Composition of the Coated Ocular Inserts Containing Brimonidine
| Formula code | Composition of the insert (%) | Composition of the coating polymeric solution (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | PVP K-90 | LM sodium alginate | MM sodium alginate | Propylene glycol | Ethyl cellulose | Eudragit RL PO | Propylene glycol | Coating layer | |
| F2a | 1 | 7 | 1.5 | 5 | 10 | 10 | One-side-coated insert | ||
| F2b | 1 | 7 | 1.5 | 5 | 10 | 10 | Dual-side-coated insert | ||
| F2c | 1 | 7 | 1.5 | 5 | 10 | 10 | One-side-coated insert | ||
| F2d | 1 | 7 | 1.5 | 5 | 10 | 10 | Dual-side-coated insert | ||
| F3a | 1 | 7 | 1.5 | 5 | 10 | 10 | One-side-coated insert | ||
| F3b | 1 | 7 | 1.5 | 5 | 10 | 10 | Dual-side-coated insert | ||
| F3c | 1 | 7 | 1.5 | 5 | 10 | 10 | One-side-coated insert | ||
| F3d | 1 | 7 | 1.5 | 5 | 10 | 10 | Dual-side-coated insert | ||
Drug–Polymer Compatibility Study
Apart from its physical characteristics, compatibility between the drug and polymer is an essential factor in determining the effectiveness of polymeric delivery systems. The possible drug–polymer interaction was studied via differential scanning calorimetry (DSC), Fourier transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD) (25) performed for brimonidine, pure polymers, physical mixture between drug and polymers, unmedicated ocular inserts, and medicated ocular inserts.
Differential Scanning Calorimetry
DSC analysis was performed using a Shimadzu differential scanning calorimeter (DSC-50, Shimadzu, Japan). The apparatus was calibrated with purified indium (99.9%). Samples (3–4 mg) were placed in flat-bottomed aluminum pan and heated at a constant rate of 10°C/min in an atmosphere of nitrogen in a temperature range of 20–400°C.
X-ray Diffractometry
The XRD patterns were recorded at room temperature using a Scintagdiffractometer (XGEN-4000, Scintag Corp., Waltham, MA, USA). The samples were irradiated with Ni-filtered Cu Kα radiation at 45 kV voltage and 40 mA current. The scanning rate employed was 2°/min over a diffraction angle of 2θ and range of 4–60°.
Fourier Transform Infrared Spectroscopy
The FTIR spectra were recorded using a Bruker FTIR spectrophotometer (Model 22, Bruker, UK) using the KBr disk technique. The FTIR measurements were performed in the scanning range of 4,000–400 cm−1 at ambient temperature.
In Vivo Tolerance Assay
An acute tolerance test was performed on New Zealand white albino rabbits of 1.55–1.65 kg weight to determine the ocular tolerance of inserts. All animals were healthy and free of clinically observable abnormalities. Animals were housed singly in standard cages, in a light-controlled room (12-h light and 12-h dark cycles) at 20–24°C and 30–75% relative humidity, with no restriction to food or water. Before conducting the experiment, the protocol was approved by the ethical scientific committee of the National Research Centre, Cairo, Egypt applied for the use of experimental animals.
Before commence of the study, an ophthalmological examination for rabbits’ eyes was conducted. Following that, the rabbits were randomly divided into four groups (six rabbits per each group). Based on the in vitro drug release studies, formulations that exhibited promising sustained release behavior, namely, uncoated ocular inserts (F2), one-side-coated ocular inserts (F2a), and the dual-side-coated ocular inserts (F2b) were selected for in vivo study. The first group (group I) received the eye drops solution (0.2% (w/v) brimonidine solution in pH 7.4), the second (group II) received the uncoated ocular inserts belonging to F2, and the third group (group III) received the one-side-coated ocular inserts F2a. However, the fourth group (group IV) received the dual-side-coated ocular inserts F2b. Each investigated ocular insert formulation or eye drops solution (50 μl) was placed in the lower conjunctival sac of the left eye of rabbits, while the right eyes (untreated eye) served as controls (Fig. 1). Subsequently, the ocular condition of both eyes of the rabbits were visually evaluated by examining the following parameters: redness, inflammation, surface of the cornea, and tear production using a slit lamp immediately after treatment and at 0.5, 1, and 2 h after insert application. The degradation and disappearance of the inserts was recorded by lifting the lower eyelid to determine their biodegradability (26). Moreover, the general behavior of the rabbits was also monitored. All observations were made by two independent operators.
Fig. 1.
Insertion of the ocular insert in the cul-de-sac of the rabbit’s left eye
Biological Studies
These studies were of a single-dose, cross over design and were performed on F2, F2a, F2b, and drug solution (0.2% brimonidine solution in pH 7.4). Male New Zealand albino rabbits weighing 2–2.5 kg were used; the animals were housed as previously described, and the experimental procedures conformed to the ethical principles of the National Research Centre, Cairo, Egypt for the use of experimental animals. IOP measurements were performed with a Schiötz Tonometer (Rudolf Riester GmbH & Co. KG, Germany). No more than three repeated readings for any eye were performed at each measurement. Only measurements in which two consecutive readings were identical were included. Animals showing a consistent difference of more than 2 mmHg between IOP of both eyes, any signs of irritation, or those which were agitated during handling were excluded. Observation for any fall out of the inserts was also recorded throughout the experiment.
Ocular inserts belonging to the investigated formulations or eye drops (50 μl) were placed in the lower conjunctival sac of the left eye of rabbits while the right eyes served as controls (Fig. 1). IOP was measured immediately prior drug administration and then at different time intervals following the treatment. All measurements were done three times at each interval, and the mean values were used to calculate the percentage decrease in IOP.
The pharmacodynamic parameters taken into consideration were maximum percentage decrease in IOP (Emax), time for maximum response (tmax), and area under percentage decrease in IOP versus time curve (AUC0−10 h). Mean residence times (MRT) were estimated from the quotient of AUMC and AUC, where AUMC is the area under the drug dynamic effect X time versus time curve. AUC and AUMC values were determined using the linear trapezoidal method (27). These parameters were calculated using WinNonlin® software (Pharsight Co., San Diego, CA, USA).
Statistical Analysis
Statistical analysis of the obtained results was performed using one-way analysis of variance followed by the least-significant difference test using SPSS® software (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Physical Characterization
It is reported in literature that the success of the film formation method is manifested from the fact that the prepared inserts are translucent, smooth in texture, and uniform in appearance without any visible cracks or imperfections (21). In this regard, all of the prepared ocuserts were visually inspected. With exception to the ocular inserts belonging to formulation F1 (contained only PVP K-90), F8 and F9 (contained PVP K-90 with different grades of carbopol) that were very soft and sticky. The rest of the investigated ocular inserts were homogeneous, translucent and elastic. In addition to that, their surface was homogeneous and continuous without any crack or phase separation between the matrix and drug, indicating uniform distribution of the components (drug and polymers).
Drug Content Uniformity
The drug content values for the prepared ocular inserts are depicted in Table I. As evident, the drug content was consistent in all batches and varied from 96.2 ± 0.3% to 98.7 ± 0.5%. This indicates that the adopted method of preparation gave reproducible results and that the drug was uniformly distributed in the polymeric matrix.
Uniformity of Thickness
The prepared ocular inserts had a diameter of 3 mm and thickness that ranges from 0.12 to 0.63 mm with low standard deviation values indicating the uniformity of the insert (Table I).
In Vitro Swelling Test
Hydrophilic polymers of different types and structures are likely to have different degrees of swelling depending on the relative resistance of the matrix network structure to the movement of water molecules. Polymer chains displaying weak hydrogen bonding may not be able to form a strong network structure that resists the rapid penetration of water. The larger the number and strength of hydrogen bonds between polymer chains, the slower is the diffusion of water molecules into the hydrated matrix (28). The swelling of the polymer is essential for initiating its bioadhesive character that starts shortly after the beginning of swelling by weak bonds (29). Following that, the adhesion increases with the increase in the polymer hydration until a certain point where excessive hydration leads to a sudden drop in adhesive strength as a result of disentanglement at the polymer tissue interface. Added to that, the rate and extent of insert hydration and swelling affect the drug release from the insert (30). Hence, this parameter is of paramount importance for predicting both drug release as well as bioadhesive potential of matrix.
Figure 2a illustrates the swelling behavior of the investigated polymeric ocular inserts. As far as the uncoated ocular inserts (F2–F7) are concerned, it is clearly manifest that the highest swelling capacity was observed for chitosan-based ocular inserts (formulations F6 and F7) where rapid increase in their swelling index accompanied by great expansion in size was initially observed during the first 25 min. After that, only a slight increase in the swelling index occurred. It is reported that the high swelling capacity of chitosan-based inserts is attributed to the extremely hydrophilic nature of chitosan as a consequence of the presence of hydroxy and amino groups in its structure that have the ability to interact with water molecules (31).
Fig. 2.
a Swelling profiles of ocular inserts containing brimonidine. b Swelling profiles of ethylcellulose-coated ocular inserts (one side or dual sides) containing brimonidine. c Swelling profiles of Eudragit RLPO-coated ocular inserts (one side or dual sides) containing brimonidine. (LM low molecular weight, MM medium molecular weight, EC ethylcellulose, ED Eudragit RLPO)
On the other hand, visual observation indicated that HPMC-based ocular inserts belonging to formulation F4 and F5 swelled rapidly and expanded in their size. The swollen ocular inserts failed to preserve their integrity and were easily fragmented upon removal from the swelling medium. Accordingly, they were discontinued from the swelling study.
On the contrary, it is evident that the sodium alginate-based ocular inserts belonging to F2 and F3 containing LM and MM weight sodium alginate, respectively, kept their integrity throughout the swelling study although their swelling indexed did not reach the values of the chitosan-based ocular inserts.
It was also evidenced by swelling study that both LM chitosan and LM sodium alginate ocular inserts showed higher swelling ability than that of their investigated higher molecular weight. This might be due to an increase in cross linking density between the polymer chains in the high molecular weight polymer which limits water penetration into the matrix system and thus decrease its swelling ability (28).
With reference to swelling behavior of the coated sodium alginate-based ocular inserts depicted in Fig. 2b, c, it is clearly evident that their swelling behavior manifest by the swelling index values varied from their uncoated counterparts (F2 and F3) and depended mainly on the type of hydrophilic polymer employed (ethylcellulose or Eudragit RSPO) as well as the number of sides coated by the hydrophobic polymer (one side or dual sides). It is worth mentioning that all of the coated ocular inserts maintained their integrity until the end of the experiment. Regarding the type of polymer employed in the coating membrane, the ocular inserts coated using Eudragit RSPO showed higher swelling index values in comparison to the ones coated using ethylcellulose. The latter maintained their original circular shape in swelling medium without any visible deformation throughout the swelling study. This observation was logically ascribed to the high hydrophobic nature of the coating film composed of ethylcellulose relative to that formed of Eudragit RSPO, which prevented water from rapidly penetrating into the ocular insert.
Concerning the number of coats, as expected, one-side-coated ocuserts showed higher swelling ability compared with the dual-side-coated ones.
In Vitro Drug Release Studies
Generally, drug release from the polymeric matrices is elicited by ease of water accessibility into the matrix, which breaks the polymer–polymer bonds and thus simultaneous leads to the creation of water–polymer bonds, separation of polymer chains, swelling to form a gel, and finally dispersion of polymer chains in the medium. The drug dissolves in the gel and diffuses to the exterior with a rate depending on its concentration gradients and its diffusion ability through the gel. Concurrently, the latter is eroded with a rate depending on polymer molecular weight and hydrodynamics of release medium. The drug release pattern depends on the relative rates of these processes (32).
In our study, all of the formulations that successfully formed ocular inserts, with proper consistency and thickness that allow easy insertion and patient acceptance, were subjected to in vitro drug release studies (F2–F7). Figure 3 graphically illustrates the obtained in vitro release profiles of brimonidine from the uncoated ocular inserts at prefixed time intervals. Drug release of brimonidine is expressed as a percentage of the drug loading.
Fig. 3.
Brimonidine release pattern from ocular inserts prepared from different viscosity grades of sodium alginate, HPMC, and chitosan. (LM low molecular weight, MM medium molecular weight)
It is quite evident that both HPMC and chitosan-based ocular inserts (irrespective of their viscosity grades) were not able to effectively modulate brimonidine release as more than 80% of the drug was delivered from their matrices within only 30 min and the remaining part of the drug was delivered in less than 1 h of the release experiment. This could be explained on the basis of the rapid polymer hydration and high swelling rate abilities of chitosan-based ocular inserts, as evident from the swelling study, which caused relaxation and disentanglement of polymer chains along with the formation of loose network through which brimonidine rapidly diffused to the release medium. On the other hand, the rapid release of brimonidine from HPMC-based matrices was a direct consequence of the rapid water uptake followed by the rapid erosion and dissolution of the hydrated matrices due to the high solubility of the used polymer. Analysis of the results revealed that there is similarity between the drug release profiles from the different viscosity grades polymers utilized (f1 values, <10).
On the other hand, when brimonidine was incorporated in sodium alginate-based ocular inserts, namely formulation F2 and F3, the drug release was delayed as the percent released at 30 min was approximately 40% and complete drug release was achieved after 6 h. It has to be mentioned that the viscosity grade of sodium alginate influenced the in vitro release behavior of brimonidine from the ocular inserts. Obviously, the amount of drug released from the medium viscosity grade sodium alginate-based films was lower than that released from the low-viscosity-grade counterpart (f1 = 18). A possible explanation for this observation is the difference in the swelling and erosion behavior between the different viscosity grades of sodium alginate. The low-viscosity-grade alginate contains polymers with shorter chain length with respect to medium viscosity grade alginate, which could result in a looser network and correspondingly less restriction to water acceptability and thus faster swelling and erosion of the polymeric matrix which facilitate rapid solute movement (13). It is reported that the increase in polymer molecular weight is usually coupled with an increase in the entanglement of the polymer macromolecules accompanied by a decrease in polymer dissolution rate. This collectively leads to decrease in water and drug diffusion coefficients and therefore decrease in drug release (33).
Additional control on drug release was executed for ocular inserts prepared using the two viscosity grades sodium alginate. The latter were selected for modification since they showed better sustainment for drug release compared with the ones prepared using chitosan or HPMC. In addition, they showed acceptable physicochemical and mechanical parameters. The selected ocular inserts were coated (either on one side or dual sides) using two different insoluble polymers namely ethylcellulose and Eudragit RSPO to form a rate controlling membrane to protect against rapid ocular inserts erosion and disintegration and consequently retard the drug release. The percent released of brimonidine from these modified ocular inserts as a function of time is illustrated in Figs. 4 and 5.
Fig. 4.
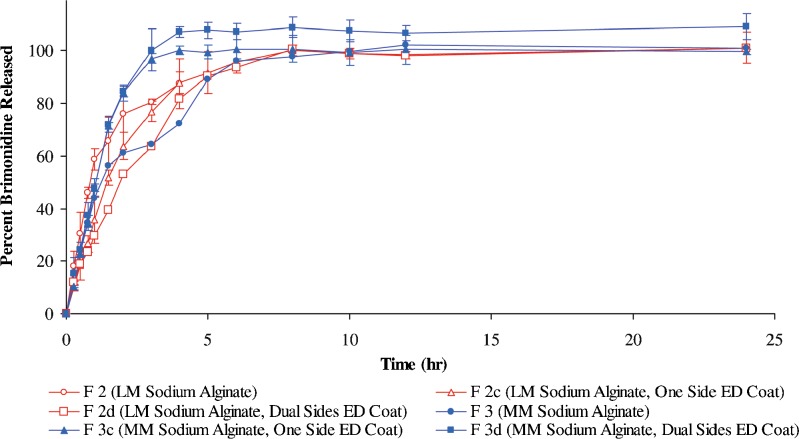
Brimonidine release pattern from ocular inserts prepared from different viscosity grades of sodium alginate coated with Eudragit RSPO. (LM low molecular weight, MM medium molecular weight, ED Eudragit RSPO)
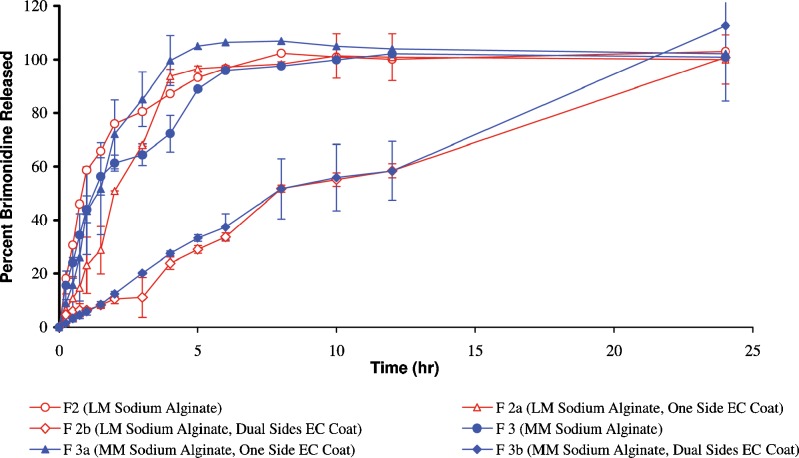
Fig. 5.
Brimonidine release pattern from ocular inserts prepared from different viscosity grades of sodium alginate coated with ethylcellulose. (LM low molecular weight, MM medium molecular weight, EC ethylcellulose)
It could be seen from the release figures that both type of polymer used for the coat as well as the number of sides applied generally affected the drug release from the ocular inserts.
Regarding Eudragit RSPO-coated medium-viscosity-grade sodium alginate-based ocular inserts; it is evident that the ocular inserts, after coating, exhibited a faster extent of drug release compared with that of the uncoated sodium alginate-based ocular inserts. This unexpected observation could be explained on the basis of the coat ability to adhere or tightly stick onto the ocular insert after its volume expands in the dissolution medium. Visual observation of these ocular inserts during the release study confirmed that their volume expansion lead to detachment of the coat film along with some parts of the polymeric matrix (bonded to the film). This causes the formation of wider meshes and allows the drug to diffuse more easily from the matrix. On the other hand, Eudragit RSPO-coated low-viscosity sodium alginate-based ocular inserts revealed slower drug release pattern compared with that of the uncoated sodium alginate-based ocular inserts. In addition, no differences in terms of brimonidine release were observed from Eudragit RSPO one-side- and dual-side-coated ocular inserts using the same viscosity grade polymer (f1 = 6.5). This could be explained on the observation that by the swelling of these ocular inserts during the dissolution study, a rupture of the dual coat film occurred.
Ethylcellulose-coated sodium alginate-based ocular inserts remained intact and were able to dramatically delay brimonidine release for more than 24 h which is probably due to the extreme hydrophobicity of EC when compared with Eudragit RSPO. Analysis of the release profiles revealed that there is a difference between the coated, one-side- and dual-side-coated sodium alginate ocular inserts using the same viscosity grade polymer (f1 values, >10). The drug release pattern was found to be: Uncoated ocular insert > one-side-coated ocular insert > dual-side-coated ocular insert.
Surprisingly enough, the coated (one side or dual sides) low-viscosity-grade sodium alginate-based ocular inserts showed lower drug release compared with that obtained from the high-viscosity counterparts. This can be ascribed according to our observation that the coating film was able to remain adherent to the swollen LM sodium alginate-based ocular inserts during drug release.
In view of the abovementioned results, the ocular insert composed of LM sodium alginate with dual side ethylcellulose coat (F2b) depicted more sustained drug release patterns compared to all of the other formulations. Accordingly, it was selected as optimized formulation for further investigations. Ocular inserts belonging to formulation F2 and F2a consist of LM sodium alginate, uncoated and single-side-coated using ethylcellulose, respectively, were also chosen for further investigations for comparative purposes.
Drug–Polymer Compatibility Study
Differential Scanning Calorimetry
DSC analysis was used to characterize the thermal behavior of brimonidine, individual polymers, physical mixture, plain, and medicated ocular inserts in order to evaluate the physical state of the drug in F2a ocular insert (Fig. 6). DSC thermogram of brimonidine was typical of a crystalline substance, exhibiting a sharp endothermic peak at 214°C (indicated by an arrow) corresponding to its melting (34). However, the DSC thermogram of PVP K-90 showed a broad endotherm over the temperature range from 89.7°C to 145.4°C and a peak at 115.8°C, which is typically present in amorphous hydrated substances and is attributed to the loss of residual moisture present in PVP K-90 (35). Also, the thermogram of sodium alginate showed a broad endothermic peak at 95°C attributed to the loss of water absorbed and an exothermic peak at 244°C due to the thermal degradation of intermolecular side chain (36). The thermograms of the physical mixtures of brimonidine with the investigated polymers (PVP K-90 and sodium alginate) showed the existence of brimonidine endothermic peak (214°C) but with marked reduction in its intensities which could be attributed to the low drug to polymers ratio. Significant changes were observed for the DSC thermograms of the prepared films when compared with the thermogram of the physical mixture. They showed a characteristic sharp peak at 140°C and at 122°C for the non-medicated and medicated films, respectively, which could be accredited to increase in molecular interaction via hydrogen bonding between PVP K-90 and sodium alginate molecules that resulted from polymers chains entanglement during film formation (37). It is worth mentioning that the drug sharp characteristic peak was completely broadened and hardly detected in the DSC thermograms of the medicated film which indicates the suppression of the drug crystallinity in the film. This is an indication of complete drug amorphization and/or well distribution of brimonidine in the film matrices (38). Therefore, X-ray powder diffractometry was considered in conjunction with DSC analysis to reach a definite conclusion.
Fig. 6.
DSC analysis of brimonidine, sodium alginate, PVP K-90, physical mixture of polymers, non-medicated F2a ocular insert and medicated ocular F2a insert
X-Ray Diffractometry
Figure 7 presents the XRD patterns for individual ocular insert components, their physical mixture, non-medicated F2a ocular insert and medicated F2a ocular insert. The diffraction pattern of brimonidine powder revealed several sharp high intensity peaks at diffraction angles 2θ of 11.7°, 24.0°, 24.4°, 26.6°, and 34.3°, suggesting that it existed as a crystalline material. Sodium alginate and PVP K-90 recorded halo patterns which confirm their amorphous nature. Generally, the diffraction pattern of the investigated physical mixture was corresponded to the superposition of those of its individual components and revealed that brimonidine was present in a crystalline state, as evidenced by the presence of its diffraction lines. The diffractogram of the medicated F2a ocular insert did not differ from the non-medicated F2a insert diffractogram as it showed a typical diffuse pattern indicating the complete conversion of brimonidine to an amorphous form and/or its uniform distribution in the medicated F2a ocular insert. This observation supports the results of DSC analysis presented earlier. It is reported that the absence of sharp peaks associated with crystalline drug molecules in XRD diffractogram of the medicated film verify that the drug is either molecularly dispersed or present in an amorphous state within the polymeric matrix (25). Moreover, the irregular molecular structure of the film that arises from the random entanglement of polymeric chains reduces drug crystalline character as well as prevents its re-crystallization during storage (39).
Fig. 7.
XRD diffractometry of brimonidine (1), sodium alginate (2), PVP K-90 (3), physical mixture of polymers (4), non-medicated F2a ocular insert, (5) and medicated ocular F2a insert (6)
Fourier Transform Infrared Spectroscopy
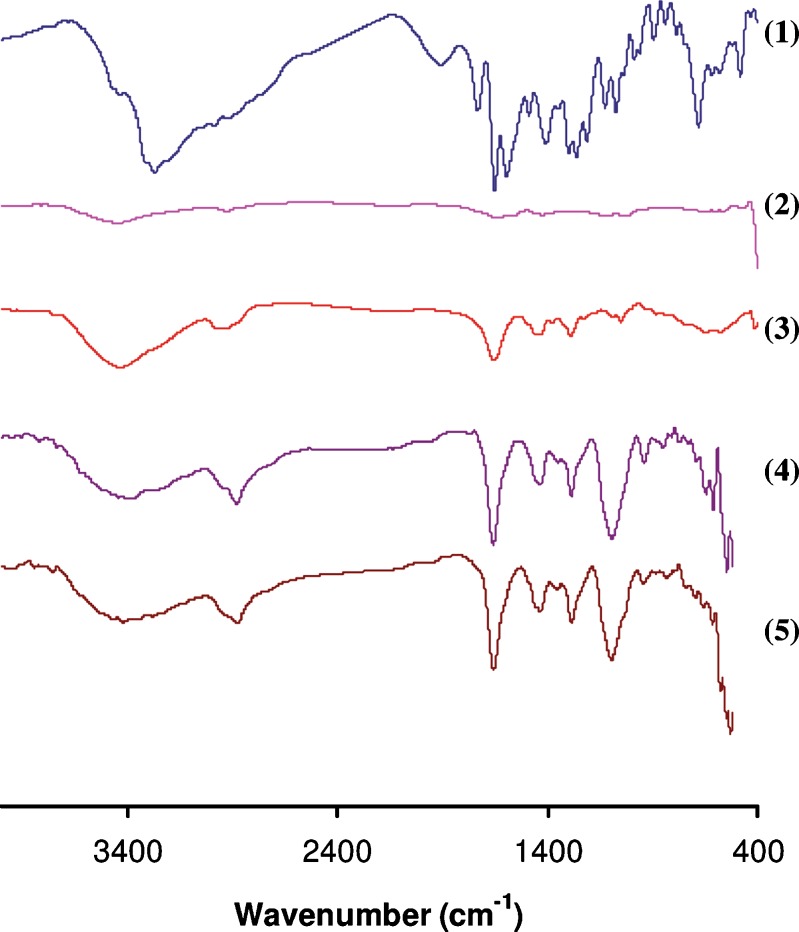
FTIR spectra were recorded to assess the interaction between the drug and the ocular insert components. The FTIR spectra for individual ocular insert components, their physical mixture, non-medicated F2a ocular insert and medicated F2a ocular insert was studied (Fig. 8). The FTIR spectrum of brimonidine showed a characteristic peak at 1,593 cm−1 corresponding to –NH bending vibration. Intense absorption peak due to the stretching vibration of the C=N and C=C groups was found at 1,651 cm−1. The stretching vibrations of the C=O group appeared at 1,732 cm−1. For the FTIR spectra of sodium alginate, it is obvious that it shows a broad peak at 3,448 cm−1 due to the stretching vibration of the –OH group, two peaks at 1,647 and 1,424 cm−1 for asymmetric and symmetric stretching vibration of the COO− group, and one sharp peak at 1,025 cm−1, which is for the C–O group. Regarding PVP’s FTIR spectrum, it showed a strong band for C=O stretching vibration at 1,655 cm−1, and a wide strong band due to –OH stretching vibration at 3,433 cm−1.
Fig. 8.
FTIR spectra of brimonidine (1), sodium alginate (2), PVP K-90 (3), physical mixture of polymers, and (4) medicated ocular F2a insert (5)
The FTIR spectra of the investigated physical mixture and medicated ocular insert revealed that brimonidine characteristic peaks are almost completely obscured by the very intense and broad ocular insert components bands, which makes the application of infrared spectroscopy insignificant in detecting any interactions. This masking of the brimonidine peaks was expected since brimonidine content was less than 10% in the mixture. Masking of drug peaks due to small amount of the drug in physical mixtures was also reported in previous studies (40–42). On the other hand, considering the interaction between sodium alginate and PVP K-90, a slight shift occurred in their characteristic peaks in their physical mixture where the COO− and C–O groups for sodium alginate appeared at 1,425 and 1,039 cm−1, respectively, while the C=O group for PVP K-90 appeared at 1,651 cm−1. The prepared film showed stronger shift for the COO− and C–O groups for sodium alginate and the C=O group for PVP K-90 to 1,434, 1,095, and 1,685 cm−1, respectively. This strongly supports the idea that a hydrogen bonding can form between –C=O groups of PVP K-90 and COO− and C–O groups of sodium alginate (37).
In Vivo Tolerance Study
The clinical acceptability of topically applied ocular inserts may be limited by their annoying ocular adverse effects, such as, irritation, burning, stinging, and tearing, that may provide a reason for patients to stop their medication. In general, the ocular inserts must be well tolerated in the eyes and should not cause any irritation or inconvenience to the patients. Accordingly, the potential ocular adverse and/or damaging effects of the ocular inserts under investigation were evaluated by observing the conjunctiva and cornea of rabbits’ eyes at specific time intervals after ocular insert administration.
It is worth mentioning that during and after ocular administration of different treatments, all animals were calm and did not show any signs of discomfort except for the animals belonging to group IV that were treated using the dual-side-coated ocular inserts (F2b). These animals were slightly agitated and showed an increase in reflex blinking which was related to ocular discomfort. However, their intake of food and water was normal during the study. The results of the in vivo tolerance behavior of the inserts in the conjunctival sac are summarized in Table III.
Table III.
In Vivo Behavior of the Ocular Inserts in the Conjunctival Sac on the Basis of Direct Visual Observation Using a Slit Lamp
| Animal group | Ocular treatment | Irritation signs | Insert behavior in conjunctival sac | ||
|---|---|---|---|---|---|
| After 0.5 h from insertion | After 1 h from insertion | After 2 h from insertion | |||
| Group I | Eye drops | Initial slightly reddening of the cornea associated with lacrimation | – | – | – |
| Group II | Uncoated ocular insert (F2) | Very mild lacrimation without corneal or conjunctivae reddening | Incomplete hydration and gelation | Complete hydration and gelation | Completely eroded |
| Group III | One-side EC-coated ocular insert (F2a) | Very mild lacrimation without corneal or conjunctivae reddening | Incomplete hydration and gelation | Incomplete hydration and gelation | Complete hydration and gelation without fragmentation |
| Group IV | Two-side EC-coated ocular insert (F2b) | Redness of the conjunctiva, cornea, and eyelid rim; irritation; and watering of the eyes | No gelation | No gelation | The insert still retained its shape without any apparent swelling or gelation |
Initial mild redness and lacrimation that lasted for less than 1 min was observed in rabbits eyes (group I) after direct instillation of eye drops. This was logically attributed to the high local drug concentration within the eye drops that induced tearing. This is expected to cause rapid flushing of the drug into the nasolacrimal gland with a probable decrease in the ocular efficacy.
Regarding the uncoated ocular inserts (F2) and one-side-coated ocular insert (F2a) applied in the lower conjunctival sac of rabbits belonging to group II and group III, respectively; they were in general well tolerated and didn’t show any visible redness or inflammation in the conjunctiva and cornea of rabbits eyes. This observation suggests the absence of any irritation potential associated with their ocular administration. However, minimal lacrimation without any redness occurred immediately after application of the previously mentioned ocular inserts. This was extremely advantageous as it initiated their rapid hydration and softening followed by their adherence onto the application site. The observed swelling in lacrimal fluids permitted the entanglement of the matrix polymeric chains with mucus on the surface of the corneal tissue by means of hydrogen bonds (43). Moreover, it is reported that the observed appropriate adhesion of alginate-based ocular insert is attributed to the lower surface tension (31.5 mN/m) of the alginate when compared with that of mucin coated cornea (38 mN/m) (44).
The time for complete gelation of the uncoated insert (~1 h) was shorter than that for the one-side-coated insert (~2 h). This result is in complete agreement with the ocular inserts swelling behavior, previously shown in Fig. 2b, which point to a faster hydration of the former. Also, the shorter residence time of the uncoated ocular inserts on the cornea compared with the one-side-coated ocular insert is in concordance with the in vitro data presented in Fig. 5, showing faster dissolution of the former.
It is of paramount importance to mentioning that the one-side-coated ocular insert was inserted in the lower conjunctiva with the coat side facing the palpebral conjunctiva and the uncoated side facing the bulbar conjunctiva. This specific insertion technique was adopted to increase the local absorption of the drug through bulbar conjunctiva and decrease the systemic absorption through the palpebral conjunctiva (45). Also, it ensures the minimum level of discomfort as the swelling soft part of the ocular insert will be facing the eyeball.
Even though, intact and very delicate ethylcellulose coating membrane of the one-side-coated ocular inserts was expelled from the rabbit eyes after complete erosion of the ocular insert drug matrix. The conjunctiva and cornea of rabbit’s eyes revealed excellent ocular tolerability without any observed redness or irritation. Furthermore, the animals were completely restful and did not show signs of anxiety manifested as tearing or increase in the reflex blinking.
On the other hand, the dual-side-coated inserts (F2b) produced local conjunctival hyperemia and redness at the site of the ocular insert placement. This might be due to the very poor swelling of the ocular inserts due to the presence of a hydrophobic ethylcellulose coat that prevented their hydration and softening. This poor swelling behavior did not favor good adhesion with the ocular mucosa and increased the risk of ocular insert ejection and slippage during blinking. Moreover, it may induce corneal injury due to the movement of the ocular insert.
Biological Studies
The mean percentage decrease in IOP profiles after the instillation of brimonidine solution or application of its optimized inserts (F2, F2a, and F2b inserts) into the rabbit’s eye until 8 h following administration, are shown in Fig. 9 while the relevant pharmacodynamic data are listed in Table IV. After administration of brimonidine aqueous eye drops, the tmax was reached after 1.6 h of instillation, followed by a rapid decline of the percentage decrease in IOP. Consequently, the efficacy of eye drops delivery system was low and of short duration. In contrast, topical administration of brimonidine using a long-acting ophthalmic insert improved the duration of drug action showing a significant higher values of MRT compared with that of drug solution (p < 0.05). The increase in residence time following insert application has also the potential to improve therapeutic effect of the administered drug, as larger AUC values were obtained by the optimized inserts compared with that of drug solution. Thus, the AUC after application of the brimonidine inserts until 8 h were 4.2-, 4.5-, and 2.1-fold higher than that of the brimonidine eye drops for F2, F2a, and F2b inserts, respectively. These findings suggested that the application of the brimonidine inserts enhanced its therapeutic efficacy compared with the eye drops. Thus, ocular inserts overcame the disadvantage of the rapid percorneal clearance of the eye drops by maintaining constant therapeutic effect for longer periods. Drug solution and F2b revealed high deviation in their pharmacodynamic parameters compared with that of F2 and F2a. This might be attributed to the rapid eye clearance for brimonidine eye drops and rapid insert expulsion for F2b due to the loss of insert bioadhesive properties of sodium alginate by the applied ethylcellulose coat.
Fig. 9.
Percentage decrease in intraocular pressure (IOP) after administration of brimonidine solution and inserts
Table IV.
Pharmacodynamic Parameters After Administration of Brimonidine Solution and Inserts (Mean ± SD)
| Formula code | Pharmacodynamic parameters | |||
|---|---|---|---|---|
| t max (h) | E max (%) | AUC | MRT | |
| Drug solution | 1.62 ± 0.75 | 17.90 ± 10.53 | 29.65 ± 22.90 | 1.81 ± 0.17 |
| F2 | 3.00 ± 1.41 | 40.13 ± 4.14 | 125.58 ± 25.29 | 2.74 ± 0.40 |
| F2a | 2.75 ± 0.95 | 44.73 ± 8.71 | 133.64 ± 11.75 | 2.85 ± 0.36 |
| F2b | 2.12 ± 1.18 | 35.99 ± 17.03 | 61.56 ± 32.88 | 2.19 ± 0.55 |
As shown in Fig. 9 and Table IV, it was found that F2a had the highest AUC and MRT values compared with the other investigated inserts. This result was not in agreement with the observed in vitro drug release data. Based on this evidence, bioadhesive properties combined by in vitro sustained drug release seem to be essential for maintaining prolonged therapeutic effect in the eye. Thus, although F2b achieved a sustained in vitro drug release, it failed to promote an in vivo sustained effect. This may be due to the limited bioadhesiveness of the insert, due to the presence of ethylcellulose coat, which encouraged rapid insert expulsion. For F2a, the sustained drug release of this insert seemed to be compensated by a higher mucoadhesive power. Thus, this insert has decreased the risk of expulsion and ensure prolonged residence in the eye, combined with controlled drug release.
This study indicated that the combination of two important features, long retention and sustained drug release can be essential and fruitful approach to provide the desired sustained drug release into the eye.
CONCLUSIONS
The results of the present study revealed that the types as well as the properties of the used polymer to formulate the ocular insert are important features which influence the swelling of the insert and thus affect the drug release from it. On the other hand, the coating of the ocular insert with different hydrophobic films is an important tool to modify the drug release from it. The ocular insert consisted of LM weight sodium alginate with single-side ethylcellulose-coated exhibiting better pharmacodynamic activity in terms of decreasing the intraocular pressure compared with that of the eye drops of brimonidine. Our studies suggested that the mucoadhesive feature of sodium alginate and the sustainment effect on drug release obtained by proper optimization using suitable one-side film coating may be exploited as a potential candidate to formulate sustained brimonidine release ocular inserts.
Acknowledgments
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Mundada AS, Shrikhande BK. Design and evaluation of soluble ocular drug insert for controlled release of ciprofloxacin hydrochloride. Drug Dev Ind Pharm. 2006;32(4):443–448. doi: 10.1080/03639040500534101. [DOI] [PubMed] [Google Scholar]
- 2.Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Adv Drug Deliv Rev. 2006;58(11):1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CG, Zhu YP, Frier M, Rao LS, Gilchrist P, Perkins AC. Ocular contact time of a carbomer gel (GelTears) in humans. Br J Ophthalmol. 1998;82(10):1131–1134. doi: 10.1136/bjo.82.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83(1):65–74. doi: 10.1016/S0168-3659(02)00175-X. [DOI] [PubMed] [Google Scholar]
- 5.Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2(1):67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 6.Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Exp Ophthalmol. 2000;28(2):113–117. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurtler F, Kaltsatos V, Boisrame B, Gumy R. Long-acting soluble bioadhesive ophthalmic drug insert (BODI) containing gentamicin for veterinary use: optimization and clinical investigation. J Control Release. 1995;33:231–236. doi: 10.1016/0168-3659(94)00096-D. [DOI] [Google Scholar]
- 8.Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnurch A. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): development and in vivo evaluation in humans. J Control Release. 2003;89(3):419–428. doi: 10.1016/S0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra M, Majumdar DK. Aqueous, oil, and ointment formulations of ketorolac: efficacy against prostaglandin E2-induced ocular inflammation and safety: a technical note. AAPS PharmSciTech. 2006;7(4):96. doi: 10.1208/pt070496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sintzel M, Bernatchez S, Tabatabay C, Gurny R. Biomaterials in ophthalmic drug delivery. J Pharm Biopharm. 1996;42:258–374. [Google Scholar]
- 11.Qi H, Chen W, Huang C, Li L, Chen C, Li W, et al. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm. 2007;337(1–2):178–187. doi: 10.1016/j.ijpharm.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Deliv Rev. 1995;16:95–106. doi: 10.1016/0169-409X(95)00014-X. [DOI] [Google Scholar]
- 13.Koelwel C, Rothschenk S, Fuchs-Koelwel B, Gabler B, Lohmann C, Gopferich A. Alginate inserts loaded with epidermal growth factor for the treatment of keratoconjunctivitis sicca. Pharm Dev Technol. 2008;13(3):221–231. doi: 10.1080/10837450801949566. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande PB, Dandagi P, Udupa N, Gopal SV, Jain SS, Vasanth SG. Controlled release polymeric ocular delivery of acyclovir. Pharm Dev Technol. 2010;15(4):369–378. doi: 10.3109/10837450903262017. [DOI] [PubMed] [Google Scholar]
- 15.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De TK, Rodman DJ, Holm BA, Prasad PN, Bergey EJ. Brimonidine formulation in polyacrylic acid nanoparticles for ophthalmic delivery. J Microencapsul. 2003;20(3):361–374. doi: 10.1080/0265204031000093591. [DOI] [PubMed] [Google Scholar]
- 18.Burke J, Manlapaz C, Kharlamb A, Runde E, Padillo E, Spada C, et al. Therapeutic use of α2-adrenoceptor agonists in glaucoma. In: Lanier S, Limbird L, et al., editors. α2-Adrenergic receptors: structure, function and therapeutic implications. Reading: Harwood Academic Publishers; 1996. pp. 179–187. [Google Scholar]
- 19.Munk SA, Harcourt D, Arasasingham P, Gluchowski C, Wong H, Burke J, et al. Analogs of UK 14,304: Structural features responsible for α2 adrenoceptor activity. Bioorganic Med Chem Lett. 1995;5(15):1745–1750. doi: 10.1016/0960-894X(95)00295-5. [DOI] [Google Scholar]
- 20.Alphagan product leaflet. Allergan New Zealand Ltd: Allergan, Inc 2009.
- 21.Gilhotra RM, Gilhotra N, Mishra DN. Piroxicam bioadhesive ocular inserts: physicochemical characterization and evaluation in prostaglandin-induced inflammation. Curr Eye Res. 2009;34(12):1065–1073. doi: 10.3109/02713680903340738. [DOI] [PubMed] [Google Scholar]
- 22.El-Gendy NA, Abdelbary GA, El-Komy MH, Saafan AE. Design and evaluation of a bioadhesive patch for topical delivery of gentamicin sulphate. Curr Drug Deliv. 2009;6(1):50–57. doi: 10.2174/156720109787048276. [DOI] [PubMed] [Google Scholar]
- 23.Juliano C, Cossu M, Pigozzi P, Rassu G, Giunchedi P. Preparation, in vitro characterization and preliminary in vivo evaluation of buccal polymeric films containing chlorhexidine. AAPS PharmSciTech. 2008;9(4):1153–1158. doi: 10.1208/s12249-008-9153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services. Guidance for industry: dissolution testing of immediate release solid oral dosage forms. Food and Drug Administration Center for Drug Evaluation and Research (CDER) 1997.
- 25.Chandak AR, Verma PRP. Development and evaluation of HPMC based matrices for transdermal patches of Tramadol. Clin Res Regul Aff. 2010;6:1370–1379. [Google Scholar]
- 26.Jain D, Carvalho E, Banerjee R. Biodegradable hybrid polymeric membranes for ocular drug delivery. Acta Biomater. 2010;6(4):1370–1379. doi: 10.1016/j.actbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Bialer M, Yacobi A, Moros D, Levitt B, Houle JM. Criteria to assess in vivo performance and bioequivalence of generic controlled-release formulations of carbamazepine. Epilepsia. 1998;39:513–519. doi: 10.1111/j.1528-1157.1998.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 28.Panomsuk SP, Hatanaka T, Aiba T, Katayama K, Koizumi T. A study of the hydrophilic cellulose matrix: effect of drugs on swelling properties. Chem Pharm Bull. 1996;44:1039–1042. [Google Scholar]
- 29.Eouani C, Piccerelle P, Prinderre P, Bourret E, Joachim J. In-vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur J Pharm Biopharm. 2001;52(1):45–55. doi: 10.1016/S0939-6411(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 30.Alanazi FK, Abdel Rahman AA, Mahrous GM, Alsarra IA. Formulation and physicochemical characterization of buccoadhesive films containing ketorolac. J Drug Del Sci. 2007;17(3):183–192. [Google Scholar]
- 31.Cafaggi S, Leardi R, Parodi B, Caviglioli G, Russo E, Bignardi G. Preparation and evaluation of a chitosan salt-poloxamer 407 based matrix for buccal drug delivery. J Control Release. 2005;102(1):159–169. doi: 10.1016/j.jconrel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Joshi HN, Wilson TD. Calorimetric studies of dissolution of hydroxypropyl methylcellulose E5 (HPMC E5) in water. J Pharm Sci. 1993;82(10):1033–1038. doi: 10.1002/jps.2600821011. [DOI] [PubMed] [Google Scholar]
- 33.Hamza Yel S, Aburahma MH. Design and in vitro evaluation of novel sustained-release double-layer tablets of lornoxicam: utility of cyclodextrin and xanthan gum combination. AAPS PharmSciTech. 2009;10(4):1357–1367. doi: 10.1208/s12249-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh KH, Shinde UA. Development and Evaluation of Novel Polymeric Nanoparticles of Brimonidine Tartrate. Curr Drug Deliv 2011; (in press). [DOI] [PubMed]
- 35.Patel RP, Patel MM. Physicochemical characterization and dissolution study of solid dispersions of Lovastatin with polyethylene glycol 4000 and polyvinylpyrrolidone K30. Pharm Dev Technol. 2007;12(1):21–33. doi: 10.1080/10837450601166510. [DOI] [PubMed] [Google Scholar]
- 36.Xiao C, Lu Y, Liu H, Zhang L. Preparation and physical properties of blend films from sodium alginate and polyacrylamide solutions. Macromol Sci Pure Appl Chem. 2000;A37(12):1663–1675. doi: 10.1081/MA-100102332. [DOI] [Google Scholar]
- 37.Çaykara T, Demirci S, Kantoğlu Ö. Thermal, spectroscopic, and mechanical properties of blend films of poly(N–vinyl-2-pyrrolidone) and sodium alginate. Polymer Plast Tech Eng. 2007;46(7):737–741. doi: 10.1080/03602550701273971. [DOI] [Google Scholar]
- 38.Phromsopha T, Baimark Y. Methoxy poly(ethylene glycol)-b-poly(d, l-lactide) films for controlled release of ibuprofen. Trends Appl Sci Res. 2009;4:107–115. doi: 10.3923/tasr.2009.107.115. [DOI] [Google Scholar]
- 39.Elmotasem H. Chitosan–alginate blend films for the transdermal delivery of meloxicam. Asian J Pharm Sci. 2008;3(1):12–29. [Google Scholar]
- 40.Badr-Eldin SM, Elkheshen SA, Ghorab MM. Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluation. Eur J Pharm Biopharm. 2008;70(3):819–827. doi: 10.1016/j.ejpb.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Ruan LP, Yu BY, Fu GM, Zhu DN. Improving the solubility of ampelopsin by solid dispersions and inclusion complexes. J Pharm Biomed Anal. 2005;38(3):457–464. doi: 10.1016/j.jpba.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Redenti E, Peveri T, Zanol M, Ventura P, Gnappi G, Montenero A. A study on the differentiation between amorphous piroxicam:β-cyclodextrin complex and a mixture of the two amorphous components. Int J Pharm. 1996;129:289–294. doi: 10.1016/0378-5173(95)04357-8. [DOI] [Google Scholar]
- 43.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28(4):353–369. doi: 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57(11):1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki H, Nagano T, Sakanaka K, Kawakami S, Nishida K, Nakamura J, et al. One-side-coated insert as a unique ophthalmic drug delivery system. J Control Release. 2003;92(3):241–247. doi: 10.1016/S0168-3659(03)00362-6. [DOI] [PubMed] [Google Scholar]