Abstract
The role of poloxamer 188, water and binder addition rate, on retarding dissolution in immediate-release tablets of a model drug from BCS class II was investigated by means of multivariate data analysis (MVDA) combined with design of experiments (DOE). While the DOE analysis yielded important clues into the cause-and-effect relationship between the responses and design factors, multivariate data analysis of the 40+ variables provided additional information on slowdown in tablet dissolution. A steep dependence of both tablet dissolution and disintegration on the poloxamer and less so on other design variables was observed. Poloxamer was found to increase dissolution rates in granules as expected of surfactants in general but retard dissolution in tablets. The unexpected effect of poloxamer in tablets was accompanied by an increase in tablet-disintegration-time-mediated slowdown of tablet dissolution and by a surrogate binding effect of poloxamer at higher concentrations. It was additionally realized through MVDA that poloxamer in tablets either acts as a binder by itself or promotes binder action of the binder povidone resulting in increased intragranular cohesion. Additionally, poloxamer was found to mediate tablet dissolution on stability as well. In contrast to tablet dissolution at release (time zero), poloxamer appeared to increase tablet dissolution in a concentration-dependent manner on accelerated open-dish stability. Substituting polysorbate 80 as an alternate surfactant in place of poloxamer in the formulation was found to stabilize tablet dissolution.
Key words: design of experiments (DOE), multivariate data analysis (MVDA), poloxamer 188 (pluronic/Lutrol F-68), quality by design (QbD), tablet dissolution
INTRODUCTION
A quality-by-design (QbD) approach comprises pre-defining a target product profile (TPP) for a product, identifying critical quality attributes of a drug product, and process parameters through experimentation and risk-assessment-based exercises. This eventually leads to establishing a design space, developing a control strategy around the design space and conducting continual improvement in accordance with ICH Q8, Q9, Q10, FDA PAT guidance and Quality by Design manufacturing paper (1–5). The QbD paradigm underlying pharmaceutical drug product development relies on multivariate data both from formulation and the process to help explain the multi-factorial relationship between the formulation variables, process variables, and the drug product attributes. Criticality analysis following a quality risk-assessment (QRA) exercise usually further results in assigning risk-based values to formulation and process parameters and drawing relationships between these two and the critical quality attributes (CQAs) of the drug product.
In line with drug product development utilizing QbD tenets, a QRA exercise of an existing drug product manufactured by high shear wet granulation (HSWG) process resulted in a formal design of experiments study to elucidate the effect of poloxamer, an excipient within the formulation along with water amounts for the binder solution and binder addition rates. These three design factors were identified at the time to be critical to tablet quality attributes in the HSWG process.
This case study discusses the use of a synergistic approach utilizing design of experiments effects analysis/response surface analysis (6) and multivariate data analysis to identify and understand the cause of slowdown in dissolution in tablets with increase in surfactant concentration. This combinatorial approach has only been explored by a few in the area of pharmaceutical and product development (7–14)
Surfactants used in tablet formulations usually tend to increase dissolution by promoting hydrophilicity within the tablet matrix and increased active pharmaceutical ingredient (API) solublization (15,16). Illustrated by example in this paper is the opposite effect, i.e., presence of a surfactant poloxamer 188 retarded tablet dissolution. This paradox is delved into deeper by the use of multivariate data analysis that helps to holistically explain the cause/s of the observed dissolution slowdown.
Apart from investigating into possible mechanisms for tablet dissolution slowdown, also obtained were the response surface and optimized solution/s for tablet dissolution by the combined use of DOE, optimization, and multivariate analysis. Multivariate techniques in this paper will refer to the principal components analysis (PCA) (17) and partial least squares (PLS) (18–21) in combination with the effects and response surface analysis. This is important as the effects and response surface analysis can only deal with a limited number of variables in contrast to the PCA and PLS techniques that can handle a larger number of variables in practice. This ability of the MVDA to handle virtually unlimited number of variables can provide important additional mechanisms to the ones obtained by the effects analysis. It has also been shown previously by our group (22) that when used in combination, multivariate data analysis can be considered a complementary tool to DOE effect and response surface analysis, providing additional information as well as confirmatory information about the product and processes and that the integrated approach may be used to an advantage to elucidate complex multivariate relationships in pharmaceutical product and process development.
The tablet formulation comprised 70% by weight of a BCS Class II API with pH-dependent solubility, microcrystalline cellulose (Avicel PH-101) as the diluent, poloxamer 188 as the wetting agent, povidone K25 as the binder, crospovidone as the disintegrant, and magnesium stearate as lubricant. Poloxamer 188 and povidone K25 were dissolved in a single solution for use as a binder/surfactant solution in the HSWG.
Poloxamer is a nonionic hydrophilic polyoxyethylene–hydrophobic polyoxypropylene copolymer. The content of polyoxyethylene in poloxamer 188 ranges from 79.9% to 83.7%. It is freely soluble in water and has a HLB value of 29. Its melting point is 52–57°C (23–25). The function of poloxamer in the current formulation was to act as a wetting agent for the high load BCS class II API as well as improve its solublization and bioavailability in vivo. In this case study, the wetting properties of the pre-blend were dictated by the API properties since the API comprised ∼70% by weight of the tablet formulation. The API was poorly water soluble and hydrophobic BCS class II compound. Poloxamer enabled the aqueous povidone-based binder solution to spread on primarily hydrophobic pre-blend with poor wetting characteristics by lowering the interfacial tension between the hydrophobic poorly soluble API particles.
MATERIALS and METHODS
Design of Experiments
Since a QbD approach was undertaken for this drug product, a quality TPP was finalized prior to undertaking any experimental work. In addition, small-scale experiments were carried out, and the results of which were analyzed and interpreted. Following this, a QRA exercise that identified and prioritized both the formulation and the manufacturing risks was carried out. It was then realized that HSWG process was the most critical unit operation in controlling the critical quality attributes of the drug product. The input-process-output diagrams that further broke down the HSWG unit operation into focus areas of interest mandated testing the effect of poloxamer, total water content in the binder/poloxamer solution, and the binder poloxamer addition rates. Thus, 13 DOE lots were prepared to evaluate the impact of preliminary critical process and formulation parameters on the preliminary critical quality attributes of the finished drug product.
The DOE employed was a hybrid RSM DOE (see Table I for DOE details) in which the poloxamer amounts, amount of water added to binder poloxamer, and the binder poloxamer addition rate were varied. The design space for evaluation of the three DOE factors was chosen based on:
Varying commonly used water amounts for wet granulation from 16% to 20% (w/w)
Poloxamer concentration was chosen based on ±3% of what was being used at the time as target (3%, w/w) poloxamer concentration
Binder addition rates were varied so as to achieve all of binder addition under 5 min, in this case between 3 and 4.5 min
Table I.
DOE Details, Tablet Disintegration Times, and Release on Stability
| DOE run number | Poloxamer 188 (% w/w) | Granulating water (g) | Binder addition rate (g/min) | Tablet DT range (N = 6) (min) | Timepoint, condition | Percent released at 15 min (+% change) (N = 6 tablets) |
|---|---|---|---|---|---|---|
| 1 | 1.5 | 162 | 58.5 | 10.6–12.9 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | 50 |
| 4–4.7 | 69 (+19) | |||||
| 5.5–7.0 | 72 (+22) | |||||
| 2 | 0 | 147 | 58.5 | 6.2–6.6 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | 66 |
| 3.7–4 | 69 (+3) | |||||
| 5.0–5.8 | 72 (+6) | |||||
| 3 (center point) | 3 | 147 | 58.5 | 11.5–13.1 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | 43 |
| 5.5–6.4 | 54 (+11) | |||||
| 8.3–10 | 74 (+31) | |||||
| 4 | 1.5 | 147 | 48 | 10–11.9 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | ND |
| 3.5–4.3 | ND | |||||
| 7.2–8.5 | ND | |||||
| 5 | 6 | 147 | 58.5 | 14.3–14.9 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | 38 |
| 11.3–12 | 51 (+13) | |||||
| 13–16 | 46 (+8) | |||||
| 6 (center point) | 3 | 147 | 58.5 | 10.2–11.1 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH: | ND |
| 3–3.3 | ND | |||||
| 8–8.5 | ND | |||||
| 7 | 4.5 | 158 | 66 | 14.1–15.6 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | 38 |
| 8.2–8.6 | 44 (+6) | |||||
| 12–13.8 | 52 (+14) | |||||
| 8 (center point) | 3 | 147 | 58.5 | ND | ND | ND |
| 9 | 4.5 | 158 | 51 | ND | ND | ND |
| 10 | 1.5 | 132 | 58.5 | ND | ND | ND |
| 11 | 1.5 | 147 | 69 | ND | ND | ND |
| 12 | 4.5 | 136 | 66 | 11.3–14.9 | Initial: 1 month, 40°C: 1 month, 40°C/75% RH | ND |
| 8–9.3 | ND | |||||
| 10–14 | ND | |||||
| 13 | 4.5 | 136 | 51 | ND | ND | ND |
ND not done
Hybrid designs are a combination of a central composite design (CCD) for the first k-1 factors and select values of the kth factor to create rotatable or nearly rotatable second-order designs. Rotatability or near rotatability is desirable in RSM designs since we want to optimize the response in the design space but do not know where the optimum is. Therefore, it is reasonable to want equal precision for the estimation of the response in all directions of a certain distance from the center of the design. In other words, a design is rotatable if the variance of the predicted response is the same for all values that are equally distant from the center. The hybrid designs are also better than a small central composite design in terms of prediction error at the design perimeter but are still highly sensitive to outliers and/or missing data (26,27). Estimation using a CCD versus a hybrid or a small CCD has less variability but at the cost of an increase in the number of runs. The DOE runs were performed in random order, the DOE created in Design Expert 7.13 (Stat-ease Inc., MN), and the DOE effect analyses, response surface analyses, and optimization were conducted in JMP 8 (SAS Institute Inc., NC)
API and Excipients
API (weak base, BCS Class II) with poor water solubility and good pH-dependent solubility below pH 5. The contact angle of the API as measured using an optical contact angle measuring system with glycerol was found to be 42°. The excipient Microcrystalline cellulose (Avicel PH-101) was obtained from FMC biopolymer; crospovidone, poloxamer 188 (Lutrol F68), and povidone K25 from BASF and magnesium stearate from Mallinckrodt.
Equipment and Process
The manufacturing process of this product involved HSWG, wet milling, drying, dry milling, blending, and compression. The DOE batches were conducted in small-scale equipment at a 1-kg scale. The API, a portion of crospovidone and microcrystalline cellulose were pre-blended in a turbula blender to obtain a uniform pre-mix. Post-pre-blending, the aqueous binder solution comprising of povidone/poloxamer in variable amounts of water was added to the pre-blend in a Lödige LFP mini one-bottom-driven granulator under high shear mixing with the chopper and the impeller on. The binder addition rate was varied per the DOE design. At the end of binder addition, any material adhering to the walls was scraped down and additional wet massing conducted with both the impeller and the chopper on. The wet massing time was held constant for all the DOE batches. After wet massing, the wet granules were manually screened through a 4.0-mm screen and dried in a fluidized bed drier. The dried granules were then manually dry screened through a 0.8-mm screen and blended with extragranular microcrystalline cellulose and crospovidone in a turbula blender. The resultant blend was then lubricated with magnesium stearate. The final tablet blend was compressed to tablets on a Riva-II Minipress.
RESULTS AND DISCUSSION
Observations
All DOE batches were manufactured without issues. It was however visually observed that the run without poloxamer (run 2) was a “noisy” run as seen by the fluctuating high impeller power readings on the granulator (data not shown). It was further seen that the granulation appeared to be non-uniformly wetted and that the binder solution had not spread well throughout the granulating mix. In addition, it was also observed that the compressed core tablets with no poloxamer appeared to have markedly non-glossy surface.
Granule and Tablet Dissolution
Following this observation, both granules and tablets were tested for dissolution. Since this was an oral tablet formulation with good API solubility at lower pH, a USP type II (paddle) apparatus at an agitation speed of 50 rpm with 900 ml in 0.1 N HCl medium was utilized as the dissolution medium. As the saturation solubility of the API at this pH in 0.1 N HCl was approximately 44 mg/ml, adequate sink conditions were thus maintained for a 500-mg tablet dose. Finished blend granules (N = 3 samples) containing 0%, 3%, and 6% (w/w) poloxamer (DOE runs 2, 6, and 5) passed through ASTM screen #20 and retained on ASTM screen #40 were tested for dissolution in 0.1 N HCl (Fig. 1). This particular size fraction of granules was chosen so that the granules were sufficiently dense/heavy and did not float to the top of the dissolution vessel during testing. The dissolution of the granules revealed that increasing poloxamer in the formulation increased dissolution.
Fig. 1.
Final blend granule dissolution in 0.1 N HCl using a USP type II apparatus (agitation speed of 50 rpm at 37°C; N = 3 samples). Error bars indicate standard deviation
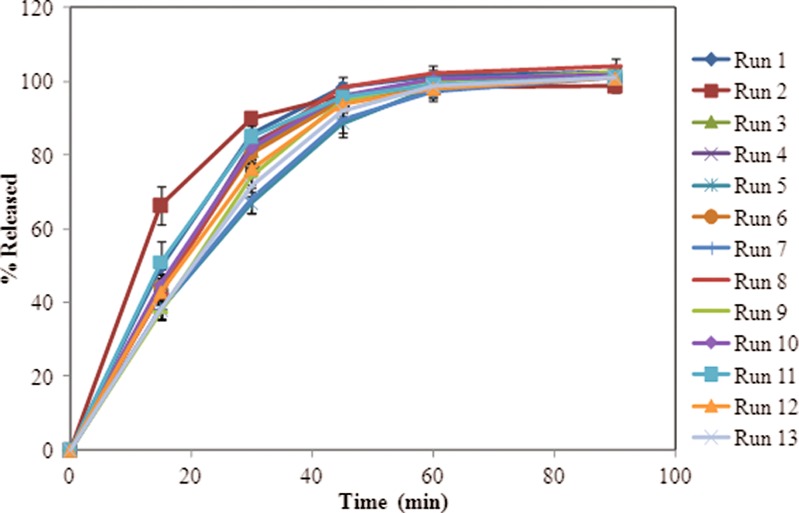
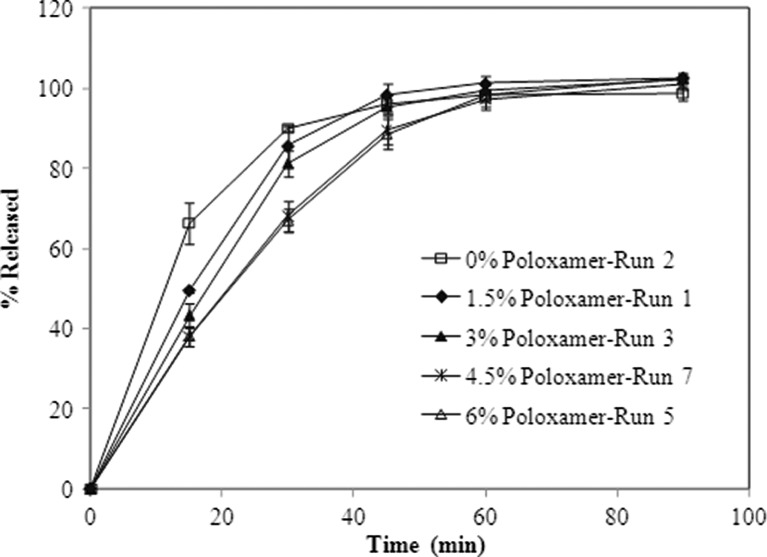
For the tablets (N = 6 tablets), it was observed from the dissolution profiles that the runs with high poloxamer appeared to dissolve slower especially at early timepoints (15 and 30 min, see Figs. 2 and 3).
Fig. 2.
Dissolution profiles of tablets in 0.1 N HCl using a USP type II apparatus (agitation speend of 50 rpm at 37°C; N = 6 tablets). Error bars indicate standard deviation
Fig. 3.
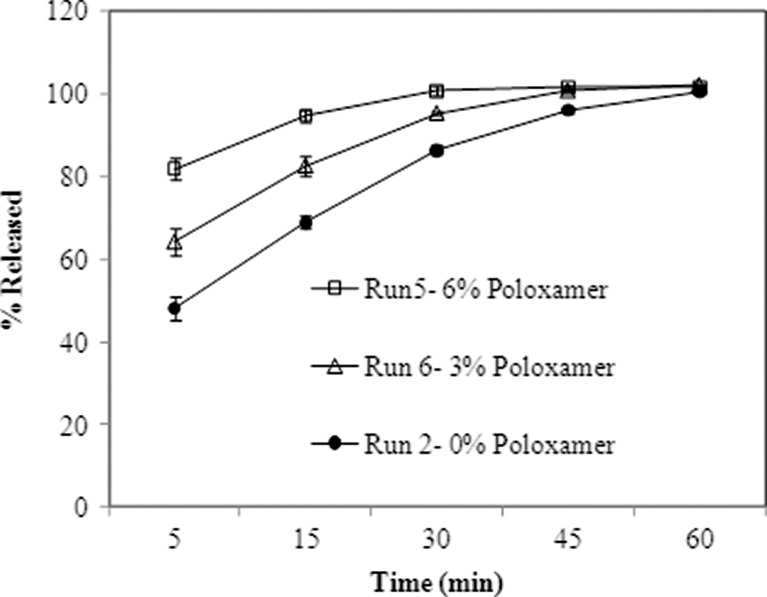
Effect of poloxamer on tablet dissolution in 0.1 N HCl using a USP type II apparatus (agitation speed of 50 rpm at 37°C; N = 6 tablets). Error bars indicate standard deviation
Granule Size, Tablet Disintegration, and Dissolution
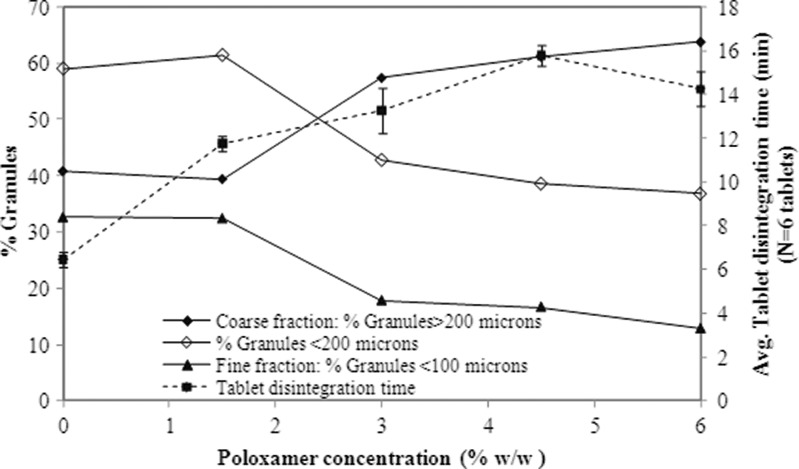
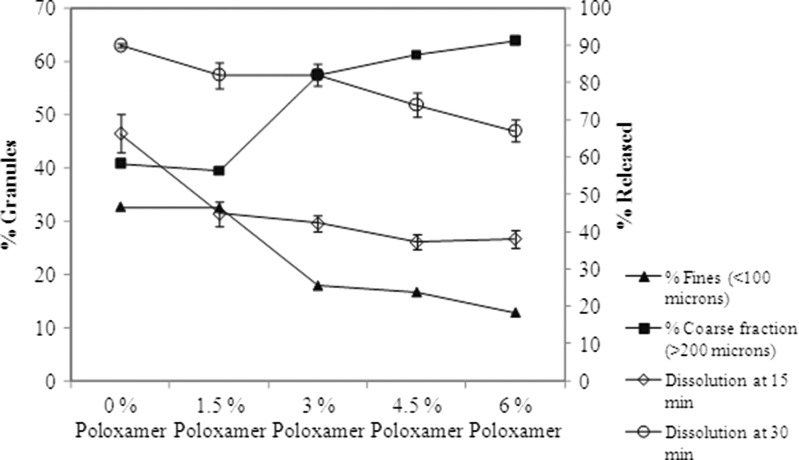
Figure 4 shows a plot of poloxamer concentration versus particle size fractions of the granules. It is observed that the coarse fraction (defined as % granules of >200 μm) increases as a function of poloxamer concentration. Commensurate to that is also the observation that fines (defined as % granules of <100 μm) tend to decrease with increase in poloxamer concentration in the blend. Figure 4 also indicates that tablet disintegration times (N = 6) tend to increase with poloxamer. Figure 5 shows a similar plot with tablet dissolution and indicates that dissolution at both 15 and 30 min decreases with increase in poloxamer and granule particle size. These observations would suggest that poloxamer may not only affect particle size of the granulation but also tablet disintegration times and through either one or both (i.e., granule particle size and/or tablet disintegration) mediate tablet dissolution.
Fig. 4.
Granule size fraction and tablet disintegration times as a function of poloxamer concentration
Fig. 5.
Granule size fraction and tablet dissolution as a function of poloxamer concentration
Tablet Dissolution on Accelerated Stability
In addition, the effect of poloxamer on tablets during an accelerated open-dish stability study was also studied for a select number of DOE runs. Contrary to the initial observation that poloxamer decreased tablet dissolution at release, it was observed from 1 month open-dish data at 40°C and 40°C/75% RH that poloxamer appeared to increase tablet dissolution on accelerated stability (Table I). This increase in tablet dissolution was again only observed at early timepoint of 15 min, suggesting possibly a disintegration associated mechanism for this observed change. Tablet disintegration testing on stability was conducted (Table I) to substantiate the observation that any dissolution increase on stability was indeed tablet disintegration time mediated.
Lastly, an alternate surfactant, polysorbate 80 was tried in the same formulation at 1% (w/w) concentration and studied under open-dish accelerated stability studies. The initial dissolution of the 1% polysorbate-80-containing formulation was found to be comparable to the 3% poloxamer-containing formulation (data not shown). It was further observed that no dissolution shift on the accelerated stability was seen when polysorbate 80 was used as a surfactant (data not shown).
DOE Effect and Response Surface Analysis
The effect of poloxamer (0–6%, w/w), a wetting and solubilizing agent was studied along with water amounts (132–162 g) and binder poloxamer addition rates (48–69 g/min) in a hybrid response surface design DOE. This was designed so as to evaluate the effect of the two critical process parameters and one critical formulation parameter on tablet dissolution (CQA). All tablets were manufactured at a tablet hardness ranging between 140 and 160 N, with a target of 150 N. While tablet ejection force was not measured, the compression force was monitored throughout the entire runs. The compression force was allowed to vary for the 13 DOE runs to achieve target hardness tablets of 150 N. It was found that compression force for all the DOE runs varied between 11.5 and 19 kN. While most of the poloxamer-containing DOE runs had compression forces in the range of 15–19 kN, the no poloxamer formulation could be compressed at relatively lower compression forces of 11.5 kN to achieve target hardness. No trends were observed between compression forces and poloxamer content of the DOE runs. Tablet dissolution for target hardness tablets (150 N) at release and on accelerated open-dish stability studies was conducted in 0.1 N HCl.
The target specification for tablet dissolution in 0.1 N HCl was set at Q NLT 70% in 30 min. Some of the DOE runs at release (Time Zero) were found to either to barely or not comply at all with the set specifications.
Tablet disintegration testing (with disks) was also conducted for target hardness tablets (150 N) in 0.1 N HCl. The requirement for tablet disintegration time was set as NMT 15 min in 0.1 N HCl.
Other tablet in-process testing like tablet weight variation, friability, etc., was also conducted. In addition, compression force and hardness profiles were also generated for the tablets.
DOE effects analysis was conducted on these variables, but only tablet dissolution and disintegration on release (at time zero) and accelerated stability will be discussed in this paper.
Effects on Granule and Tablet Dissolution at Release
Poloxamer 188 was found to have the expected effect on uncompressed tablet final blends. A specific size fraction of final blend granules sieved over screen #20 and retained on screen #40 were used for dissolution. Granule dissolution increased with increase in poloxamer content from 0% to 6% for the 5, 15, and 30 min timepoints (refer to Fig. 1). The tablet dissolution however was found to be very different from that of the granule blends. The dissolution profiles are captured in Figs. 2 and 3 (Fig. 3 shows the effect of varying poloxamer with some runs omitted). It is obvious that the poloxamer at concentrations of 1.5% and above tends to slow down dissolution below 45 min. The extent of slowdown could be correlated to the poloxamer concentration in the formulation. The tablet disintegration time data in Table I and Fig. 4 and dissolution data in Fig. 5 additionally suggest that the dissolution slowdown in tablets at 15 and 30 min is most likely related to increases in the disintegration time of tablets.
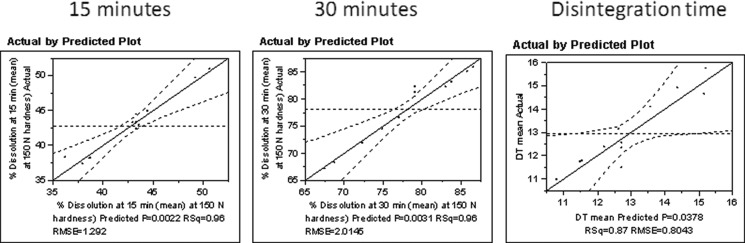
A model was fit and the analysis of variance and the model statistics are summarized in Fig. 6 and Tables II and III. Figure 6 shows the actual-statistics by predicted plots for dissolution at 15 and 30 min and disintegration times of tablet. The evaluative statistics in Table II show good model fits with high adjusted R2 and low root mean square error for the three response variables, dissolution at 15 and 30 min, respectively, and disintegration. Additionally, the models are significant with p values of 0.0022, 0.0031, and 0.0378 for the dissolution and the disintegration times (Table III).
Fig. 6.
Actual by predicted plots for tablet dissolution and disintegration time models
Table II.
Summary of Fit Table for Tablet Dissolution and Disintegration
| Summary of fit | Dissolution at 15 min | Dissolution at 30 min | Disintegration time |
|---|---|---|---|
| R 2 | 0.9613 | 0.9554 | 0.8724 |
| Adjusted R 2 | 0.9149 | 0.9020 | 0.7195 |
| Root mean square error | 1.2919 | 2.0145 | 0.8043 |
| Mean of response | 42.7058 | 78.0592 | 12.9417 |
| Observations (or sum of weights) | 12 | 12 | 12 |
Table III.
Analysis of Variance for Tablet Dissolution and Disintegration
| Response variable | Source | Degrees of freedom | Sum of squares | Mean square |
|---|---|---|---|---|
| Dissolution at 15 min | Model | 6 | 207.3713 | 34.5619 |
| Error | 5 | 8.3458 | 1.6692 | |
| C. total | 11 | 215.7171 | ||
| F ratio | 20.7061 | |||
| Prob > F | 0.0022* | |||
| Dissolution at 30 min | Model | 6 | 435.3454 | 72.5576 |
| Error | 5 | 20.2907 | 4.0581 | |
| C. total | 11 | 455.6361 | ||
| F ratio | 17.8795 | |||
| Prob > F | 0.0031* | |||
| Disintegration time | Model | 6 | 22.1298 | 3.6883 |
| Error | 5 | 3.2343 | 0.6468 | |
| C. total | 11 | 25.3642 | ||
| F ratio | 5.7018 | |||
| Prob > F | 0.0378* |
*P value
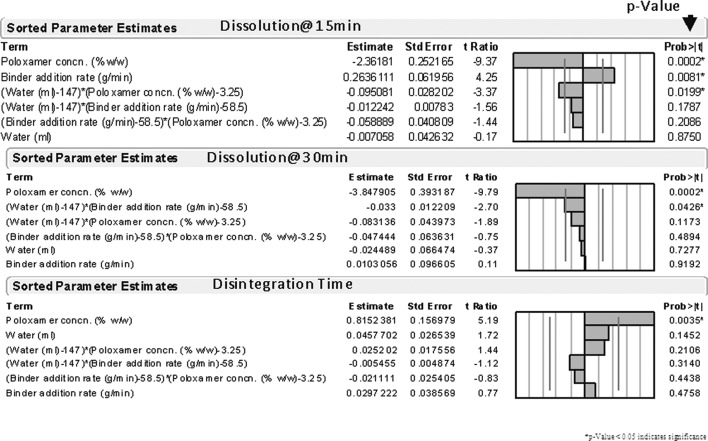
DOE effects analysis (Fig. 7) for time zero (release) dissolution in 0.1 N HCl was carried out to find out if the process variables (water amounts and binder addition rates) also played a role in dissolution slowdown. Figure 7 displays the sorted estimates of the DOE model terms on tablet disintegration and dissolution. It was revealed that the poloxamer content was a major variable controlling the disintegration and dissolution of tablet. Poloxamer was also found to be the most influential variable controlling the disintegration time of tablet with a p value of 0.0035. Increasing poloxamer concentration increased the disintegration times for the tablets. As a result of disintegration-mediated dissolution slowdown, poloxamer was also found to adversely affect initial dissolution at both 15 and 30 min. All terms having a p value of <0.05 are the terms that significantly influence disintegration or dissolution of tablet. Poloxamer shows the highest effect at 15 min dissolution followed by the binder addition rate. Poloxamer appeared to have a negative impact on dissolution of tablet at 15 min, mediated most likely by its effect on disintegration times of tablet (Fig. 7). Binder addition rate may also play a modest role in dissolution of tablet at early timepoints.
Fig. 7.
Sorted DOE effects analysis
Increasing binder rate addition was found to moderately increase tablet dissolution at 15 min and a second-order interaction between binder rate addition and water amounts was found to have a minor effect of slowing down dissolution at 30 min. This increase in tablet dissolution may have to do with granule nucleation and granule size build up. The magnitude of effect of the poloxamer indicated by t-ratio (Fig. 7) appeared to be greater than the other two design factors under test (binder addition rate and water) under test indicating that the poloxamer appeared to play a major role in tablet dissolution and disintegration under the DOE test conditions.
It was hypothesized that the slowdown in tablet dissolution was most likely related to poloxamer-mediated increase in tablet matrix hydrophilicity which then adversely affected the disintegrant action. Disintegrant crospovidone is mainly thought to promote disintegrant action by virtue of its wicking and a moderate swelling action, which may be exhausted early in case of extreme hydrophilicity within the tablet matrix due to the poloxamer (28). Apart from this mechanism, it was also proposed that poloxamer may either itself act as a binding agent or promote the binding action of the binder (povidone K25) by improving wetting of the API and excipient blend within the tablet, which results in increased particle size of the resulting granulation (Figs. 4 and 5).
Any or both of these actions would then retard tablet disintegration and dissolution. A verification of poloxamer affecting the crospovidone efficacy was indirectly obtained from the observation that poloxamer did not affect either disintegration or dissolution of an equivalent croscarmellose sodium-based tablet formulation (data not shown). The croscarmellose sodium disintegrant action is mediated by a quicker wicking and a more powerful swelling based mechanism than that of crospovidone.
The binder action of poloxamer was also later verified after conducting a thorough multivariate data analysis.
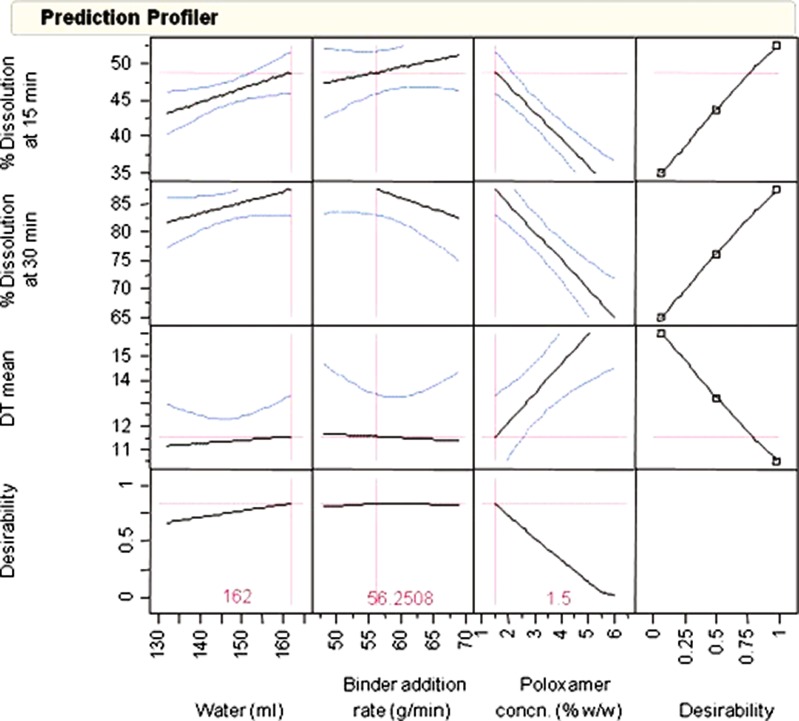
Figure 8, a prediction profiler shows the dependence of both tablet dissolution (15 and 30 min) and disintegration on the poloxamer and less so on water amounts and the binder addition rates, as indicated by steeper slope for poloxamer and less so for the other two factors. Prediction profiler based on response surfaces suggested reducing the poloxamer to a lower concentration in order to achieve good dissolution and disintegration time (DT) since poloxamer appeared to control most of the effects observed on tablet disintegration and dissolution. Once response models had been established, optimization was performed to find optimal combination of design factors in order to achieve desired responses—dissolution and disintegration time in this case. It is critical to define appropriate desirability functions for both responses and design factors during optimization. Desirability value ranges from 0 to 1, with 1 representing most importance. For instance, higher desirability was given to higher dissolution in the desirability function for dissolution at 15 and 30, whereas higher desirability was preferred for lower disintegration time. Constraints in this case were simply the boundaries/ranges for each factor. After the objective function, constraints and desirability were specified, an optimization algorithm was then applied to search along response surfaces or multi-dimensional space for optimal solutions that would satisfy optimization criteria. Design space constituted these optimal solutions derived from optimization. A number of optimal solutions were found to maximize dissolution at 15 and 30 min while minimizing disintegration time.
Fig. 8.
Optimization of DOE factors to maximize dissolution and minimize disintegration time
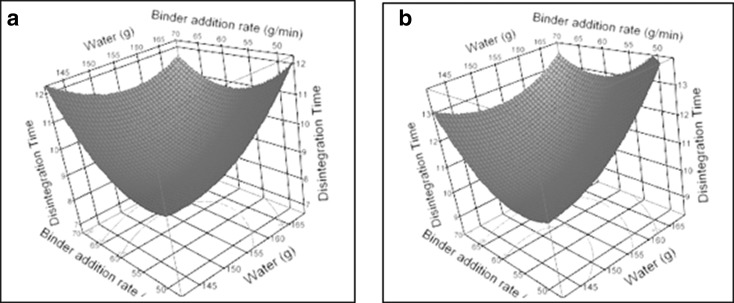
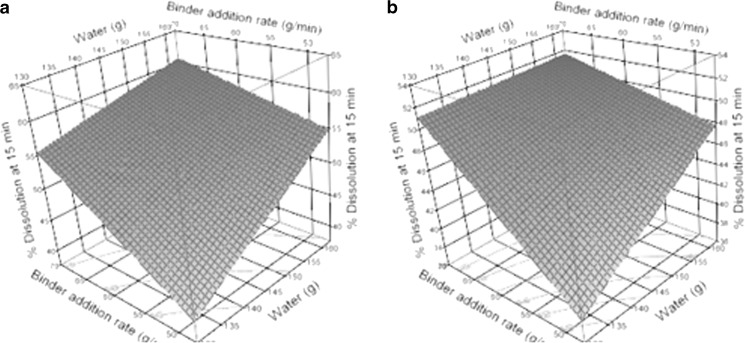
The response surface of tablet disintegration and dissolution at 15 min as a function of water and binder addition rate (holding poloxamer constant at 0% and 1.5%) is shown in Figs. 9 and 10, respectively. An optimal solution with poloxamer = 1.5%, water = 162 g, and binder addition rate = 56.25 g/min, was selected to run an independent test batch for confirmation. The predicted dissolutions at 15 and 30 min from their corresponding response surface models are 48.8% and 87.5%, respectively, when using this optimal solution. A confirmatory batch using this optimal setting showed actual dissolutions at 15 and 30 min are 52% and 89%, respectively.
Fig. 9.
Response surface plots for tablet disintegration time with poloxamer concentration at 0% (a) and 1.5% (w/w) (b)
Fig. 10.
Response surface plots for tablet dissolution at 15 min with poloxamer concentration at 0% (a) and 1.5% (w/w) (b)
Effects on Tablet Dissolution on Accelerated Stability
The effect of poloxamer on accelerated stability was also evaluated by conducting tablet disintegration and dissolution testing. Table I illustrates the percent increase in tablet dissolution on accelerated stability at 15 min with a commensurate decrease in tablet disintegration times. It is seen that the greatest increase in dissolution change at 15 min on open-dish stability occurs at concentrations of 1.5% and 3% (w/w). No dissolution shifts were observed when poloxamer in the tablet formulation was substituted with polysorbate 80, a liquid surfactant at room temperature and above in place of poloxamer 188 to see its effect on dissolution shift on stability. It could be concluded from the comparative studies above that the dissolution shift on stability probably occurred as a result of poloxamer in the formulation.
Visual Evaluation
Poloxamer was also found to affect appearance of the tablets. Tablets without poloxamer were found to have rough surfaces with picking observed on some tablets. In addition, the tablets with no poloxamer were markedly non-glossy with poor appearance. The tablets containing poloxamer were glossy and shiny and did not have any of the surface defects observed in tablets without poloxamer. This is a confirmation on a work by Desai et al. (29) that poloxamer may also act as lubricant in tablets.
Multivariate Data Analysis (PCA and PLS) of Variables
In order to explore the underlying mechanisms that caused tablet disintegration and dissolution changes at release, it was decided to study all variables captured in the DOE study that included process parameters and quality attributes of the resulting intermediates within unit operations and also the quality attributes of the drug product. An attempt was then made to correlate not only variables from same unit operations but also downstream process variables, with the understanding that upstream processing exerted much influence on downstream manufacturing.
An attempt to better understand the complex and multivariate tablet manufacturing process was made by employing multivariate PCA and PLS methods jointly to provide complementary and holistic results to the existing understanding obtained from the DOE effects and response surface analysis methods. These methods offer the advantage of being used either on individual unit operations or on combined processes, depending on the study objective. All multivariate analyses were performed on SIMCA-P+12 (Umetrics Inc., NJ).
Principal Component Analysis
Approximately 40 variables were analyzed including process parameters from unit operations like HSWG, blending, milling, drying, and tablet compression. Quality attributes of the intermediates and the final tablet that were studied included power consumption of the granulator, particle size distribution of granules and blends as measured by sieve analysis, loss on drying, water by KF, bulk and tapped densities of blends and granules, product temperature during drying, and compression force to achieve target hardness to name a few. PCA was performed to study combined multiple unit operations as well as to individually study unit operations.
Inter-batch Relationships
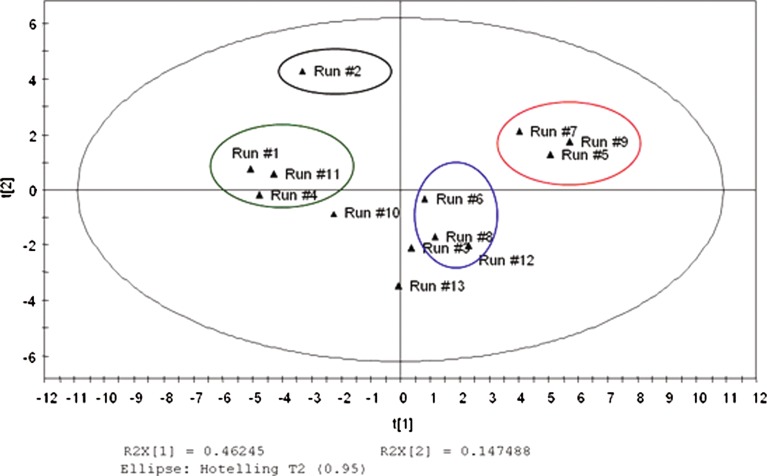
A PCA plot in Fig. 11 shows the relationships between the 13 DOE runs by examining both sample and variable relationships in the DOE. The scores t1 and t2 are the orthogonal latent variables or the principal components that sum up the x variables. The score t1 or the first component explains majority of the variation in x space at 46.24%, followed by t2 that explains 14.74% of the variation. Taken together, the two latent variables explain 61% variation. The observations close to each other in the PCA plot are similar while those farther away more dissimilar. The PCA plot shows good variation amongst the DOE batches and there are at least three obvious groupings that can be observed for the 13 DOE runs. Group 1 comprised runs 1, 4, and 11; the second group, group 2 of runs 5, 7, and 9; and the third group comprised the center point runs—runs 3, 6, and 8. None of the groups or individual batches was outside the 95% confidence interval ellipse. In addition, the three center points, runs 3, 6, and 8, were near the center indicating good reproducibility. The most obviously different run appears to be run 2 that appears to be located in the upper left quadrant.
Fig. 11.
PCA Scores plot (t1-t2) describing batch relationships
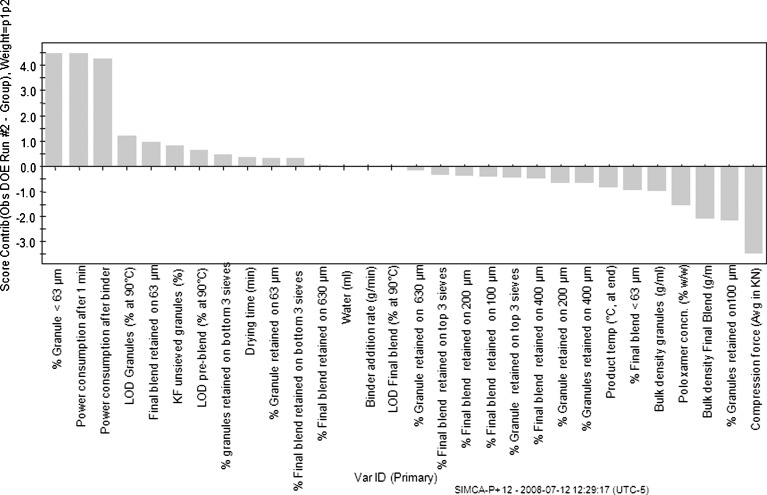
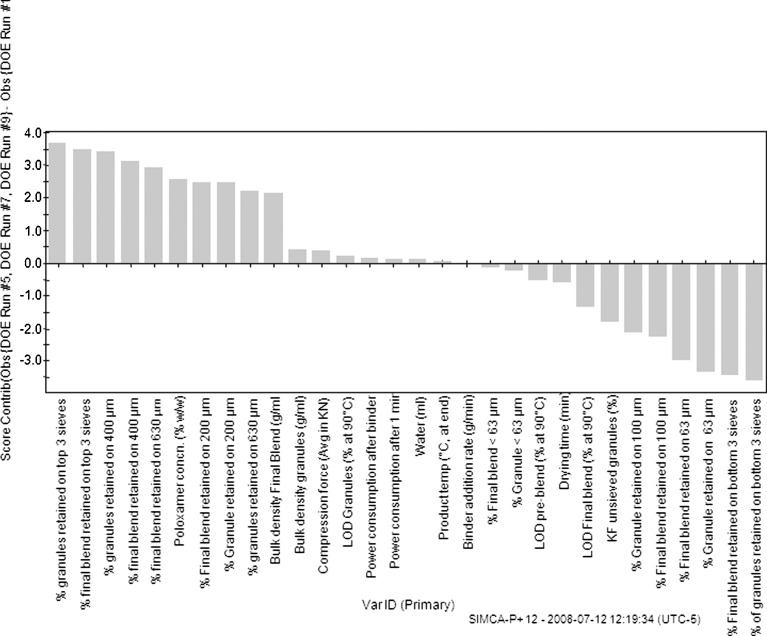
To elucidate why run 2 (no poloxamer run) was different from the other groups of runs, a score contribution plot (Fig. 12) was used to identify variables contributing to the differences. The score contribution plot displayed the contributing variables in a sorted order, with the variables with larger positive and lesser negative values being more important in differentiating run 2 from the rest of the runs. It is observed that run 2 exhibits larger percentage of granule and blend particle sizes below 63 μm, i.e., it exhibits a larger percentage of “fines” in the blend. In addition, due to underwetting of the primarily hydrophobic pre-blend in the granulator and non-uniform binder solution spread, confirmed by visual observations, surges were observed in power consumption as the granulation continues, resulting in higher than other power consumption. The higher percentage of fines in the batch with no poloxamer suggests that the poloxamer does indeed act as a binder/surrogate binder in addition to povidone and thus DOE runs with no poloxamer appear to have more fines. The presence of finer undergranulated material could then also help explain the relatively faster dissolution of the no poloxamer, run 2 compared with others. In addition, it is also observed that run 2 tablets could be compressed at far lower compression forces (11.5 kN) to a target hardness of 150 N than the runs with poloxamer (15–19 kN) and this is reflected in Fig. 12 as well. Higher compression forces of runs with poloxamer may suggest that poloxamer when present may alter compactibility of a granulation, although no specific trends were observed in compression forces as a function of poloxamer concentration.
Fig. 12.
Score contribution plot to explain differences in run 2
An additional attempt to explain differences between high and low poloxamer runs was made via Fig. 13—also a score contribution plot. This plot revealed that group 2 runs (5, 7, and 9—high poloxamer runs) appeared to have larger percentage of coarser particles in the granules and tablet blend relative to the group 1 runs (1, 4, and 11). Thus, it is observed that the runs with high poloxamer result in coarser granulation due to binder or binder-promoting action of the poloxamer that increases pre-blend wettability and promotes intra-particle cohesion with the blender by improving wettability.
Fig. 13.
Score contribution plot to explain differences between group 1 and 2 runs
To summarize the results, it can thus be concluded that the poloxamer action on tablet disintegration and dissolution is mediated by either its action as a binder and/or its ability to promote binder properties when in solution with povidone. These results are largely consistent with the DOE effects results. Compression force was not found to be a reason for differentiating high and low poloxamer runs probably because either the variation in compression force was not enough to explain differences between them or else it was interacting with the poloxamer concentration in the formulation.
Inter-variable Relationship
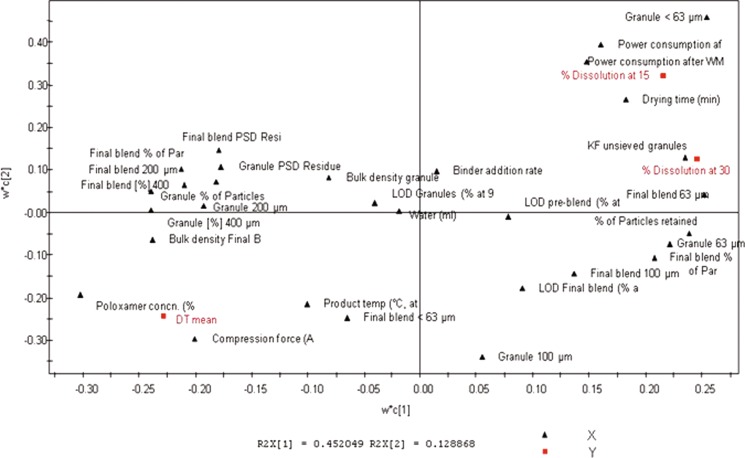
A loading plot in Fig. 14 shows the relationships between the X-matrix variables. It is further known that variables near each other are positively correlated and those across from each other with respect to the origin are negatively correlated. This plot further substantiates the observation that poloxamer does increase disintegration time of tablets (DT mean) and decreases tablet dissolution at release (% dissolution at 15 and 30 min). In addition, it also shows that water and binder addition rate do not have much influence on either drug product quality attributes.
Fig. 14.
Loading plot showing variable relationships
PLS Regression Analysis
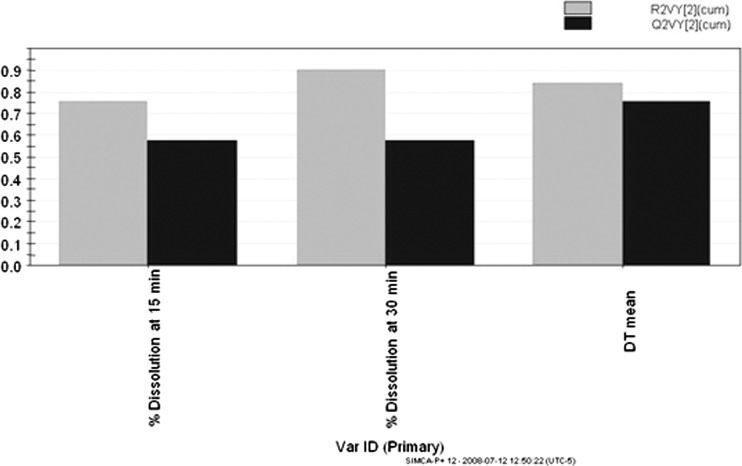
The objective of the PLS in this example was to figure out the effect of input variables (material attributes and process parameters) on both intermediate and final product quality attributes. The primary purpose here was to study relationships between X and Y variables. The PLS model (Fig. 14) was used to establish relationship between the 40 X variables and three Y variables. The Y variables under analysis were tablet disintegration and dissolution at 15 and 30 min. From Fig. 14, it was observed that the poloxamer concentration was strongly positively correlated to tablet disintegration times and strongly negatively correlated to tablet dissolution at both 15 and 30 min. Figure 15 explains the cumulative R2 and Q2 for the Y matrix using two components. R2Y is the percent of variation in Y explained by the model indicating model fitness, and Q2Y is the percent variation in Y predicted by the model. A model is deemed good if both R2Y and Q2Y are above 0.5, which is indeed the case here.
Fig. 15.
Cumulative R2 (R2VY[2](cum)) and Q2 R2 (Q2VY[2](cum)) for tablet dissolution and disintegration
It is seen that good correlation exists for tablet disintegration and dissolution, but further model validation would be required for future prediction. In addition, predicted versus measured plots were constructed for tablet disintegration time and dissolution from the calibration model. High R2 (0.9–0.95) values indicate good model fit for all (data not shown).
CONCLUSIONS
Tablets were manufactured using HSWG process, followed by drying, milling, blending, and compression to tablet cores. Poloxamer was added to the formulation to improve process manufacturability by decreasing surface tension of the binder solution and promoting binder spread over the hydrophobic high load API-based (∼70% by weight) pre-blend in the formulation.
Increasing poloxamer was found to enhance the drug dissolution from granules as expected but appeared to retard dissolution from tablets in a concentration-dependent manner, when the same granules were compressed into tablets. While compression force was not found to be a major variable in distinguishing the various poloxamer-containing DOE runs, it was found to be one of the differentiating variables between the no poloxamer and the poloxamer runs.
There was no in vivo data at the time to support bio-relevance of either of the two dissolution methods used (0.1 N HCl and pH 4 acetate buffer—data not shown), significantly low F2 similarity factors (F2 < 50) between no poloxamer run and others, as well as dissolution differences between the poloxamer runs themselves warranted an investigation into the reason/s/probable mechanisms of dissolution differences.
The 15- and 30-min timepoints were considered important for comparison as approximately 75% drug is expected to be released at 30 min (dissolution specification, Q NLT 70% in 30 min).
An additional impetus for the study was based on the observation that the tablet formulation with any appreciable poloxamer showed dissolution shifts on accelerated stability. The calculated F2 similarity factors between dissolution at initial timepoints and on accelerated stability in 0.1 N HCl were also found to be sufficiently low (F2 = 32–40, F1 = 12–33 for 1.5%, 3%, and 6% (w/w) poloxamer-containing formulations) to moderate (F2 = 53 for the 4.5% formulation) to warrant an investigation into reasons for dissolution shift.
The first step was therefore to establish the reason for the dissolution shift as a function of both formulation composition and process parameters. This study provided valuable input in establishing the role of poloxamer in not only causing dissolution changes as a function of the surfactant’s concentration but also pointed towards the possibility of poloxamer playing a role in mediating dissolution shifts on accelerated stability.
DOE effects analysis for initial/time zero dissolution of tablets in 0.1 N HCl was carried out that revealed that the percent poloxamer content was the major variable controlling both tablet disintegration and thus dissolution. Increasing poloxamer was found to not only increase granule particle size but also increase tablet disintegration and retard early time point dissolution.
-
The increase in granule particle size as a function of poloxamer concentration may be explained by the action of poloxamer- a wetting agent in improving both povidone solution spread on the surface of the dry and hydrophobic pre-blend as well as by improving wettability of the blend by reducing intra-particular cohesion. Increased particle size of the granulation as a result of particle aggregation and coalescence would then result in larger and denser granules that would show lower dissolution especially at 15 and 30 min. Multivariate analysis also attested to the binder/binder-promoting properties of poloxamer by showing evidence of granule particle size increase with increase in poloxamer concentration.
Electrostatic (if the polymer and surfactant are oppositely charged) and hydrophobic interactions (between hydrophobic domains of the polymer and surfactant) are the two main kinds of interactions between polymers and surfactants in solution (30–32). Generally, the presence of polymers reduces the CMC concentration of a surfactant, more so if they are oppositely charged. The presence of polymeric chains induces formation of micelles and the similarities between the surfactant and the polymer attract the surfactant molecules to certain positions in the polymer. Combinations of polymers and surfactants are commonly used to improve desired properties of a product for instance, in this study povidone was added because of its adhesive/binding and granule promoting properties, and the surfactant poloxamer improved binder spread over the blend by reducing the surface tension of the binder solution thus promoting wetting of the powder blend.
Tablet disintegration times may be affected by poloxamer-mediated reduction in disintegrant efficacy of crospovidone. Increased hydrophilicity of the tablets with poloxamer may retard wicking capacity of the disintegrant which then retards tablet disintegration times. Replacing crospovidone with croscarmellose sodium, a disintegrant with higher swelling capacity than crospovidone was found to remedy this disintegration slowdown. This was also confirmed by the fact that the tablets with no poloxamer exhibited the lowest DT time and that the tablets with increasing amounts of poloxamer showed high disintegration times. Higher DT may also be explained by action of poloxamer as a binder.
The decrease in early time point dissolution as a function of poloxamer increase is believed to be disintegration time mediated.
DOE effects analysis suggested lowering the poloxamer to achieve acceptable dissolution. The solution was independently verified by a confirmatory experiment, and there was good agreement between the predicted and the actual dissolution.
Thus, an overall effect of increase in granule particle size at the granulation level and increased tablet disintegration times at the tablet stage as a result of poloxamer were proposed to be the reasons for tablet dissolution slowdown. However, once the tablets disintegrate, the effect on the poloxamer on disintegrated granules is as expected, i.e., higher the poloxamer, faster the dissolution.
While additional studies may be required to explain the effect of poloxamer on stability in a more complete manner, the observations that substituting crospovidone in the formulation by a quick swelling disintegrant like croscarmellose sodium or by substituting poloxamer with an alternate surfactant like polysorbate 80 may help prevent dissolution shifts on stability will be important in exploring poloxamer-mediated mechanism of action on stability and subsequent control strategies.
This case study illustrated in this paper exemplifies the use of tools in the QbD armamentarium to study drug products and processes that affect them. It was demonstrated by the combination approach of using both DOE effects/response surface analysis and multivariate data analysis techniques like the PCA and PLS that valuable insight may be provided into hither to unknown and complex cause-and-effect mechanisms during drug product development.
The effects analysis gave us the variables affecting the final product quality attributes and also revealed the mechanism for dissolution slowdown. The multivariate approach further improved our understanding of the underlying mechanism causing the dissolution slowdown. Thus, using an integrated approach of experimental design, response surface modeling, and optimization and multivariate approaches of PLS/PCA, we were able to facilitate our mechanistic understanding of the process and formulation.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Na Zhao, Andrew McKeen, and Brent Harrington for help during tablet manufacture and constructive input in statistical analysis.
REFERENCES
- 1.ICH Q8 (R2), Pharmaceutical development. Part I: Pharmaceutical development, and. Part II: annex to pharmaceutical development, August 2009. (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf). Accessed 15 February 2011.
- 2.ICH Q9, quality risk management, November 2005. (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf). Accessed 15 February 2011.
- 3.ICH Q10. Pharmaceutical quality systems, June 2008. (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf). Accessed 15 February 2011.
- 4.Food and Drug Administration. Guidance for industry PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance, September 2004 (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070305.pdf). Accessed 15 February 2011.
- 5.Cook GD, Venkateshwaran, Simmons SP. Quality by design manufacturing paper. World pharmaceutical frontiers, March 2009. (http://www.worldpharmaceuticals.net/editorials/015_march09/WPF015_quality.pdf). Accessed 15 February 2011.
- 6.Lundstedt T, Abramo L, Seifert E, Thelin B, Nystrom A, Pettersen J, Bergman R. Experimental design and optimization. Chemometrics Intell Lab Syst. 1998;42:3–40. doi: 10.1016/S0169-7439(98)00065-3. [DOI] [Google Scholar]
- 7.Naelapaa K, Alleso M, Kristensen HG, Bro R, Rantanen J, Bertelsen P. Increasing process understanding by analyzing complex interactions in experimental data. J Pharm Sci. 2008;98:1852–1861. doi: 10.1002/jps.21565. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg NO, Lundstedt T. Application of multivariate analysis in pharmaceutical development work. Drug Dev Ind Pharm. 1995;21:987–1007. doi: 10.3109/03639049509069801. [DOI] [Google Scholar]
- 9.Bolhuis G, Duineveld CAA, de Boer JH, Coenegracht PMJ. Simultaneous optimization of multiple criteria in tablet formulation: part I. Pharm Technol Eur. 1995;8:42–50. [Google Scholar]
- 10.Hwang RC, Geemoules MK, Ramlose DS, Thomason CE. A systematic formulation optimization process for a generic pharmaceutical tablet. Pharm Technol. 1998;22(5):48–64. [Google Scholar]
- 11.Voinovich D, Campisi B, Moneghini M, Vincenzi C, Phan-Than-Luu R. Screening of high shear mixer melt granulation process variables using an asymmetrical factorial design. Int J Pharm. 1999;190:73–81. doi: 10.1016/S0378-5173(99)00278-1. [DOI] [PubMed] [Google Scholar]
- 12.Westerhuis JA, Coenegracht PMJ. Multivariate modeling of the pharmaceutical two-step process of wet granulation and tabletting with multiblock partial least squares. J. Chemometrics. 1999;11(5):379–392. doi: 10.1002/(SICI)1099-128X(199709/10)11:5<379::AID-CEM482>3.0.CO;2-8. [DOI] [Google Scholar]
- 13.Gabrielsson J, Lindberg N, Lundstedt T. Multivariate methods in pharmaceutical applications. J Chemometrics. 2002;16:141–160. doi: 10.1002/cem.697. [DOI] [Google Scholar]
- 14.Xie L, Wu H, Shen M, Augsburger LL, Lyon RC, Khan MA, Hussain AS, Hoag SW. Quality-by-design (QbD): effects of testing parameters and formulation variables on the segregation tendency of pharmaceutical powder measured by the ASTM D 6940-04 segregation tester. J Pharm Sci. 2007;97(10):4485–4497. doi: 10.1002/jps.21320. [DOI] [PubMed] [Google Scholar]
- 15.Lee KR, Kim EJ, Seo SW, Choi HK. Effect of poloxamer on the dissolution of felodipine and preparation of controlled release matrix tablets containing felodipine. Arch Pharm Res. 2008;31(8):1023–1028. doi: 10.1007/s12272-001-1263-9. [DOI] [PubMed] [Google Scholar]
- 16.Quadir A. Characterization of newly developed micronized poloxamers for poorly soluble drugs. In: BASF at releasing technology workshops, controlled release society meeting, Miami, June 2005
- 17.Wold S, Esbensen K, Geladi P. Principal component analysis. Chemometrics Intell Lab Syst. 1987;2:37–52. doi: 10.1016/0169-7439(87)80084-9. [DOI] [Google Scholar]
- 18.Esbensen KH. An introduction to multivariate data analysis and experimental design: multivariate data analysis—in practice, 5th ed. CAMO Process AS; 2002. ISBN 82-993330-3-2:2002
- 19.Martens H, Naes T. Multivariate calibration. Oxford: Wiley; 1989. [Google Scholar]
- 20.Miller CE. The use of chemometric techniques in process analytical method development and operations. Chemometrics Intell Lab Syst. 1995;30:11–22. doi: 10.1016/0169-7439(95)00026-7. [DOI] [Google Scholar]
- 21.Kourti T. Process analytical technology and multivariate statistical process control. Wellness index of process and product—part I. Process Analytical Technology. 2004;1(1):13–19. [Google Scholar]
- 22.Huang J, Kaul G, Cai C, Chatlapalli R, Hernandez-Abad P, Ghosh K, Nagi A. Quality by design case study: an integrated multivariate approach to drug product and process development. Int J Pharm. 2009;382:23–32. doi: 10.1016/j.ijpharm.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Martindale W. Martindale: the extra pharmacopoeia. 31. King of Prussia: Rittenhouse Book Distributors; 1996. [Google Scholar]
- 24.Kabanov A, Batraoka E, Alakhov V. Pluronic block copolymers as novel polymer therapeutics for oral and gene delivery. J Control Rel. 2002;82:189–212. doi: 10.1016/S0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 25.Lange KR. Surfactants: a practical handbook. Cincinnati: Hanser Gardner; 1999. [Google Scholar]
- 26.Anderson MJ, Whitcomb PJ. RSM Simplified: optimizing processes using response surface methods for design of experiments. New York: Productivity Press; 2005. [Google Scholar]
- 27.Myers RH, Montgomery DC, Anderson-Cook CM. Response surface methodology: process and product optimization using designed experiments. 3. New York: Wiley; 2009. [Google Scholar]
- 28.Bühler V. Kollidon: polyvinylpyrollidone for the pharmaceutical industry. 4. Ludwigschafen: BASF Aktiengesellschaft, Feinchemie; 1999. [Google Scholar]
- 29.Desai D, Zia H, Quadir A. Evaluation of selected micronized poloxamers as tablet lubricants. Drug Deliv. 2007;14(7):413–426. doi: 10.1080/10717540701203042. [DOI] [PubMed] [Google Scholar]
- 30.Stilbs P. NMR studies of polymer-surfactant systems. Surfactant Sci Ser. 1998;77(1):239–266. [Google Scholar]
- 31.Veggeland K, Nilsson S. Polymer–surfactant interactions studied by phase behavior, GPC and NMR. Langmuir. 1995;11(6):1885–1892. doi: 10.1021/la00006a012. [DOI] [Google Scholar]
- 32.Philip P, Gnanaprakash G, Jayakumar T, Kalyanasundaram P, Raj B. Three distinct scenarios under polymer, surfactant and colloidal interaction. Macromolecules. 2003;36:9230–9236. doi: 10.1021/ma0342628. [DOI] [Google Scholar]