Abstract
Orodispersible film (ODF) technology offers new possibilities for drug delivery by providing the advantages of oral delivery coupled with the enhanced onset of action and convenience to special patient categories such as pediatrics and geriatrics. In this study, mosapride (MOS) was formulated in an ODF preparation that can be used for treatment of patients who suffer from gastrointestinal disorders, especially difficulty in swallowing due to gastroesophageal reflux disease. Poloxamer 188 was used to solubilize MOS to allow its incorporation into the film matrix. The films were prepared by solvent-casting method using different polymer ratios of maltodextrin and hydroxypropyl methylcellulose and plasticizer levels of glycerol and propylene glycol. A D-optimal design was utilized to study the effect of polymer ratio, plasticizer type, and level on film mechanical properties, disintegration time, and dissolution rate. Statistical analysis of the experimental design showed that the increase of maltodextrin fraction and plasticizer level conferred optimum attributes to the prepared films in terms of film elasticity, film disintegration time, and MOS release rate. The ODF formulations were further tested for moisture sorption capacity, with formulations containing a higher ratio of maltodextrin and percent plasticizer showing more moisture uptake. The optimum film composition was also tested in vivo for film palatability and disintegration time. An optimized mosapride orodispersible film formulation was achieved that could be of benefit to patients suffering from gastrointestinal disorders.
Key words: design of experiments (DOE), dysphagia, fast-dissolving films, GERD, mosapride, patient compliance
INTRODUCTION
The oral route of drug administration is the most common and convenient for patient use. Tablets and capsules represent the most commonly used solid oral dosage forms. However, many patients suffer from dysphagia or difficulty in swallowing, which can pose a compliance problem for such patients when medications are prescribed. It is estimated that 35% of the general population, 30–40% of elderly nursing home patients, and 25–50% of patients hospitalized for acute neuromuscular disorders and head injuries have dysphagia (1). The disease can be the result of multiple causes including gastroesophageal reflux disease (GERD), cardiovascular conditions, autoimmune diseases, autoimmune deficiency syndrome, thyroid surgery, radiation therapy to head and neck or oral cavity, as well as other neurological diseases such as cerebral palsy (2). Additionally, oral solid dosage forms are not ideal for pediatrics, geriatrics, supine patients, or when water is inaccessible.
Fast-dissolving oral delivery systems are novel solid dosage forms, which disintegrate or dissolve in a few seconds after placement in the mouth. They offer substantial advantages over ordinary oral dosage forms such as ease of administration, lack of requirement for drinking water, and improved compliance in individuals who fit in one of the aforementioned categories (3). In addition, sublingual and buccal routes enhance the onset of action and improve the efficacy and safety profile of medicaments (4). They can be used for local and systemic delivery. Fast-dissolving oral delivery systems include tablets, caplets, wafers, films, granules, and powders.
There is a rising interest in the development of orodispersible films (ODFs) as an alternative to fast-dissolving tablets (5), which is attributed to their faster dissolution rate, higher durability, and better patient compliance. Recently, research work on the use of ODFs as promising carriers for multiple active pharmaceutical ingredients has emerged (5–11). Marketed ODF products have also become available including Listerine®, Chloraseptic®, Triaminic®, and multivitamins (12).
The backbone of an ODF is generally formed of a plasticized polymer or a mixture of polymers that provide the necessary elasticity and shape of the film. Examples of polymers that have been used in making films include hydrocolloids or povidone K-90 (13), maltodextrin (MDX) (5), hydroxypropyl methylcellulose (HPMC) (14), or blends of polymers (15). Films can be prepared using a solvent-casting, rolling, extrusion, or solid dispersion methods (16).
Mosapride citrate (MOS) (4-amino-5-chloro-2-ethoxy-N-((4-((4-fluorophenyl) methyl)morpholin-2-yl)methyl) benzamide citrate) is a new gastroprokinetic agent that is used for the enhancement of upper GI motility through selective stimulation of 5-hydroxytryptamine (5-HT4) receptors, without cardiac and central nervous system side effects (17–19). MOS is reported to improve overall symptoms in patients suffering from gastrointestinal disorders, including chronic gastritis, functional dyspepsia, and GERD. The dose of MOS is either 5 or 10 mg given three times daily. MOS is a weakly basic drug that is currently available on the market as sustained release tablet, melt in mouth tablet and chewable tablet.
MOS is an ideal drug candidate for an ODF application because of its special indication in patients with swallowing problems and its low-dose requirement. The formulation of MOS as a strip film to be placed on the patient’s tongue for dose administration, without the need to swallow, would significantly facilitate dose administration, with subsequent improvement in patient compliance.
Thus, the aim of this work was to design and characterize fast-dissolving films of MOS formulated using blends of two polymers: MDX and HPMC. A D-optimal experimental design was used to evaluate the influence of formulation parameters including polymer type and ratio, as well as the concentration and type of plasticizer used on the film’s physicochemical and mechanical properties, disintegration time, and dissolution rate. This study also assessed the palatability and in vivo disintegration time of the optimum formulation by administration to healthy volunteers.
MATERIALS AND METHODS
Materials
MOS citrate dihydrate (lot # 20070701) was purchased from Shanghai Ruyoung Industrial Co., China. MDX, dextrose equivalent value (DE; 13–17), and HPMC E15 and K4M were obtained from Aldrich (Germany) and Colorcon (Italy), respectively. Poloxamer 188 (P188) and 407 (P407) were a kind gift from BASF (Germany). Glycerol (GLY) and propylene glycol (PG) were obtained from Reidel-Rohm (Germany) and Adwic (Egypt), respectively. Potassium dihydrogen phosphate, sodium dihydrogen phosphate, and sodium chloride, of analytical grade, were obtained from El-Nasr Company for Chemicals (Egypt).
UV Analytical Method
The UV calibration curve of MOS was constructed in distilled water and in phosphate-buffered saline (PBS) at pH 6.8 (2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl/1 L of distilled water). Stock solutions were prepared by dissolving 10 mg MOS in 25 ml of one of the abovementioned media with the aid of minimum amount of dimethyl formamide. Serial dilutions of the stock solutions were prepared and their absorbance values were measured using an ultraviolet–visible (UV–VIS) spectrophotometer (Shimadzu, UV-1601 PC, Japan) at λmax 271 nm (Gasmotin(R) monograph, Dainippon Sumitomo Pharma Co., Ltd., Tokyo, Japan). No interference from excipients used was noticed at that wavelength. Linearity was observed over a concentration range of 4–48 μg/ml, with an R2 = 0.998.
Saturated Solubility
The saturated solubility of MOS alone and in the presence of P188 or P407 in three different concentrations (0.5, 2, and 4%, w/v) was determined in distilled water. A known excess of MOS (100 mg) was mixed with 10 ml distilled water in glass vials, followed by shaking in a thermostatically controlled mechanical shaker (Shaking water bath, Barloworld Scientific Ltd., UK) at 100 rpm for 72 h at 37 ± 0.5°C. Subsequently, the suspension was filtered through 0.45-μm membrane filter (Millipore Corp., Bedford, MA, USA), diluted, and the concentration of the drug in solution was measured spectrophotometrically at 271 nm. The experiment was performed in triplicate and the mean concentration was calculated.
Film Preparation
The films were prepared by a solvent casting method in which a mixture of HPMC E15 and MDX in different weight ratios (9:1, 7:3, and 5:5) was used as the film forming ingredient, with PG or GLY as plasticizers (15%, 20%, or 25% (w/w) of the total film weight). Briefly, HPMC E15 was soaked in 5 ml of distilled water containing the desired plasticizer concentration for 1 h, followed by the addition of MDX. Finally, a 5-ml alcoholic solution containing 0.5% (w/v) P188 and 10 mg MOS was added to the polymeric solution and stirred. The resulting solution was cast onto a glass container and dried under ambient conditions. The films were then carefully removed from the container and cut into strips of dimension 2 × 3 cm, wrapped in aluminum foil, and stored in an air tight glass container at ambient conditions for 1 week before subsequent characterization.
D-optimal Experimental Design
The effect of MDX/HPMC polymer ratio (1:9, 3:7, and 5:5), plasticizer type (PG and GLY), and plasticizer concentration (15%, 20%, and 25%, w/w) was evaluated through a D-optimal experimental design summarized in Table I. The levels chosen for each variable in the experimental design are based on preliminary studies. Statistical analysis of the data generated was performed using JMP® (SAS Institute, Inc., Cary, NC).
Table I.
D-optimal Design of ODF Formulations
| ODF | Plasticizer type | Plasticizer (%) | Polymer ratio (MDX/HPMC) |
|---|---|---|---|
| F1 | Glycerol | 15 | 3:7 |
| F2 | Glycerol | 20 | 5:5 |
| F3 | Glycerol | 20 | 3:7 |
| F4 | Glycerol | 20 | 5:5 |
| F5 | Glycerol | 20 | 1:9 |
| F6 | Glycerol | 25 | 3:7 |
| F7 | Glycerol | 25 | 1:9 |
| F8 | Glycerol | 15 | 5:5 |
| F9 | Glycerol | 25 | 3:7 |
| F10 | Glycerol | 15 | 1:9 |
| F11 | Glycerol | 20 | 1:9 |
| F12 | Glycerol | 25 | 5:5 |
| F13 | Glycerol | 15 | 3:7 |
| F14 | PG | 25 | 1:9 |
| F15 | PG | 25 | 3:7 |
| F16 | PG | 15 | 3:7 |
| F17 | PG | 20 | 5:5 |
| F18 | PG | 20 | 3:7 |
| F19 | PG | 25 | 5:5 |
| F20 | PG | 15 | 1:9 |
| F21 | PG | 25 | 5:5 |
| F22 | PG | 20 | 1:9 |
| F23 | PG | 15 | 1:9 |
| F24 | PG | 15 | 5:5 |
Characterization of MOS Films
Film Weight and Thickness
The weight of prepared films was recorded. Film thickness was measured by means of a micrometer (precision ± 0.0001 mm, Mitutoyo Corporation, Japan) at three different positions on the film.
Drug Content
The drug content uniformity of each film was tested by dissolving the film in 10 ml of PBS, followed by filtering through 0.45 μm membrane filter. The filtrate was appropriately diluted, and the mean content of MOS was determined at 271 nm using UV spectroscopy.
Differential Scanning Calorimetry
Samples of pure film components, the physical mixture of the drug and the excipients, in addition to F11, which was selected as a representative formulation for comparison purposes, were analyzed by differential scanning calorimetry (DSC) using a Shimadzu DSC-60 (Kyoto, Japan). Samples of 5 mg were crimped in a standard aluminum pan and heated under nitrogen atmosphere in the range between 30°C and 400°C, at a heating rate of 10°C. The characteristic peaks were recorded.
Mechanical Film Properties
The mechanical properties of the prepared films including tensile strength (TS) and percent elongation at (E%) break of the film formulations were determined using Zwick 1425 material testing machine (Germany) according to American Standards for Testing Materials D624 (2007). Briefly, the film formulations were cut into dumbbell-shaped specimens using appropriate punching dies with a width of 4 mm and a neck length of 15 mm. The film specimens were tested at a crosshead speed of 50 mm/min, with a load cell of 10–20 N.
TS, also known as stress at rupture, is calculated by dividing the maximum load by the original cross-sectional area of the specimen and is expressed in force per unit area (MPa). The E%, also known as strain at rupture, is calculated according to the following equation:
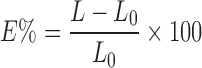 |
where L0 is the initial gauge length of the specimen and L is the length at the moment of rupture.
Determination of Moisture Uptake
Prior to the test, the film strips (2 × 3 cm) were placed in a dessicator for 24 h to ensure complete drying of the films. The film strips were then weighed and exposed to 75% RH, at room temperature for 1 week. The film strips were reweighed and the percentage increase in weight as a result of moisture uptake was recorded.
In vitro Disintegration of Films
The in vitro disintegration time of film strips was determined by the visual method described earlier (20,21). The film strip was placed in a glass Petri dish (6.5 cm in diameter) containing 25 ml of distilled water at 37°C, with swirling every 10 s. The disintegration time was recorded as the time at which the film starts to break or disintegrate.
Release Studies of MOS from Films
The in vitro dissolution test was carried out in a paddle dissolution apparatus (Vankel Industries VK 700, USA). In order to mimic the in vivo adhesion and to prevent the film strips from floating, each film strip was fixed to a rectangular glass slab and placed at the bottom of the dissolution vessel prior to starting the dissolution test.
The dissolution medium used was 250 ml of PBS at pH 6.8, maintained at 37 ± 0.5°C and stirred at 50 rpm. Samples of 5 ml were withdrawn at 1, 3, 5, 10, 15, 20, 25, 30, and 45 min, and the same volume was replenished with fresh buffer maintained at 37 ± 0.5°C. The samples were filtered through a 0.45-μm membrane filter and analyzed by UV–VIS spectroscopy for MOS concentration at 271 nm. The release mechanism of MOS was determined by fitting the release data to different kinetic models (zero order (22), first order (23), and Higuchi (24)).
Determination of Water Content in Selected ODF Formulations
Selected film formulations: F2, F12, F17, and F19 were stored either in a dessicator for 1 week or in a humidity chamber at ambient temperature and 60% RH. Subsequently, the water content of the films was determined using Karl-Fischer titration (787 KF Titrino, Metrohm, Switzerland). The device was first calibrated with anhydrous methanol, then film samples of 0.15 g were placed in the titrator, and the water content was determined by measuring the amount of iodine consumed as a result of reaction with water in the film samples.
Evaluation of Selected ODF Formulations in Human Volunteers
The film formulations F2, F12, F17, and F19, listed in Table I, were evaluated in twelve healthy human volunteers (eight females and four males, age 25–40) for in vivo disintegration time, palatability, ease of administration without water, and sensation after film disintegration. The study protocol was approved by the Research Ethics Committee of Faculty of Pharmacy, Cairo University, Egypt and complied with the principles of the Declaration of Helsinki. All subjects were completely informed about the study and signed a written consent form before the administration of the film strips.
The films used in the study had dimensions of 2 × 3 cm and average weight of 204.88 ± 6.07 mg. The volunteers were divided into two equal groups for testing each pair of ODF formulations simultaneously. The volunteers were asked to place the film strip on the tongue and were not restricted with respect to tongue movement later on. The time required for the complete disintegration of each film in the oral cavity was recorded. The other tested parameters were evaluated based on a scoring system that was scaled from 1 to 3, where 1 and 3 correspond to highest satisfaction and dissatisfaction, respectively. Sensation of the oral films was evaluated considering residues of the film left in the mouth after administration. Each film was tested in three different subjects.
RESULTS AND DISCUSSION
The choice of the components of an ODF formulation is critical because they influence its properties. A suitable drug candidate depends in part on its dosage, which is generally limited to a maximum of 30% (w/w) with respect to the weight of the film (25). The dose of MOS is 5 to 10 mg three times daily and so is a good candidate for this type of dosage form. In addition, its particular indication for GERD patients renders an ODF MOS product a convenient dosage form that should alleviate the patient’s suffering from swallowing problems, thereby improving patient compliance.
The choice of the polymer(s) to be included in the formulation and its amount is equally important because it is typically the major component in the formulation (at least 45% of the dry weight). Polymers play an important role, not only in imparting the necessary mechanical properties to the oral strip, but also influencing the release of the active ingredient into the oral cavity through disintegration. MDXs are mixtures of poly- and oligo-saccharides that are produced by hydrolysis of starch and differ in their “DE” value, which represents the reducing power of all sugars present relative to glucose (26). The DE value for MDXs is generally <20 and is a function of the degree of hydrolysis, with higher DE values corresponding to MDX of lower molecular weight and vice versa. The physicochemical properties of MDX are in turn affected by the DE value. In this study, a relatively high DE value (DE = 13–17) was chosen to impart high solubility and low viscosity to the film formulation. Meanwhile, HPMC is known to have film forming abilities (27), and thus a mixture of HPMC and MDX was selected for film formation to take advantage of the excellent film forming capability of the former and the improved dissolution characteristics of the latter. Preliminary studies were conducted to assess the feasibility of preparing MOS as an ODF and to assist in selecting the levels of variables to include in the D-optimal design.
Two different grades of HPMC were compared: E15 and K4M, and it was found that the rate of MOS released from the HPMC K4M films was significantly slower than that of HPMC E15 (data not shown). This is attributed to the swelling of the high viscosity HPMC K4M upon contact with the dissolution medium, resulting in the formation of a thick matrix gel, thereby hindering the release of the drug from the film. Hence, only the low viscosity HPMC E15 grade was used as film forming polymer.
A D-optimal design was implemented to select the optimum MDX/HPMC polymer ratio. The plasticizer type and level affect the mechanical properties of the film by reducing the glass transition temperature (Tg) as well as influencing the disintegration and dissolution characteristics. Earlier work has shown that hydroxyl-containing plasticizers perform better with cellulose containing polymers and are compatible with MDXs as well (25). Therefore, GLY and PG were selected as the two plasticizer types to include in the optimization work at three levels each.
Saturated Solubility
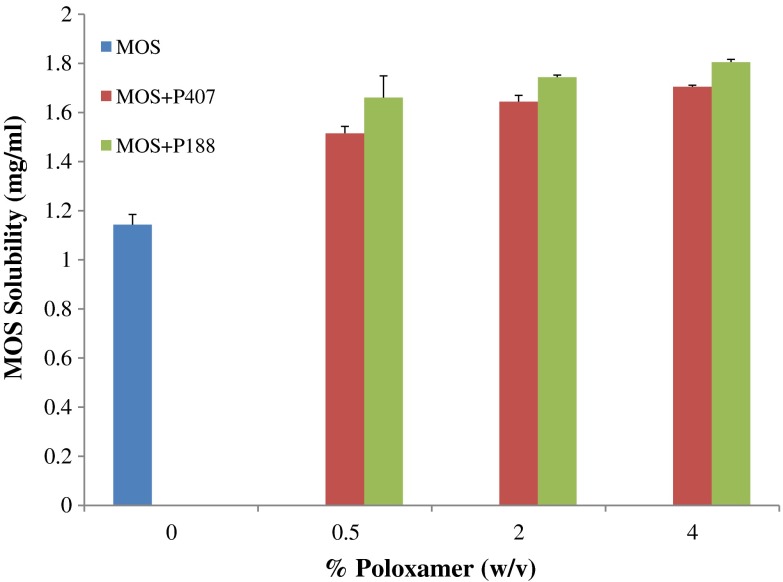
The saturated solubility of MOS in distilled water was found to be 1.143 ± 0.042 mg/ml as can be seen in Fig. 1. In order to facilitate ODF incorporation of MOS in a solubilized form, two different grades of poloxamer: P188 and P407 were investigated. Poloxamer was chosen as a solubilizer because of its bland taste.
Fig. 1.
Saturated solubility of MOS in distilled water at 37°C (n = 3)
The increase in saturated solubility of MOS with the use of poloxamer is illustrated in Fig. 1. It can be seen that the addition of 0.5% (w/v) of either P407 or P188 significantly increased the solubility of MOS in distilled water to 1.515 ± 0.051 and 1.660 ± 0.032 mg/ml, respectively (p < 0.05). However, the use of 2% and 4% (w/v) of either P407 or P188 did not show a significant increase in MOS solubility in distilled water beyond the level achieved for 0.5% (w/v) as seen in Fig. 1 (p > 0.05). A t test showed that at the 0.5% (w/v) level, P188 significantly increased the solubility of MOS compared to P407 (p < 0.05). Hence, P188 was selected for use as a solubilizer for MOS at 0.5% (w/v) in all film formulations.
Physicochemical Characterization of MOS Films
The formulated oral films were transparent, flexible, and showed no blooming. The physicochemical ODF attributes are described in this section. The weight and thickness values of prepared films are summarized in Table II. The weight of ODF preparations ranged from 185.14 to 211.18 mg. As can be seen in Table II, the mean thickness of the oral films ranged from 0.17 ± 0.09 to 0.35 ± 0.08 mm. Table II also summarizes the results of MOS loading content in ODF formulations and was found to be uniform and ranged from 98.65 to 100.14.
Table II.
Weight, Thickness and Content Uniformity of ODF Formulations
| ODF | Weight of films (mg) | Thickness of films (mm) | Drug content (%) |
|---|---|---|---|
| F1 | 187.14 | 0.21 ± 0.06 | 98.85 |
| F2 | 199.12 | 0.29 ± 0.09 | 100.08 |
| F3 | 198.66 | 0.31 ± 0.09 | 100.10 |
| F4 | 199.05 | 0.28 ± 0.09 | 99.14 |
| F5 | 200.00 | 0.17 ± 0.06 | 98.68 |
| F6 | 210.44 | 0.35 ± 0.03 | 99.13 |
| F7 | 210.00 | 0.25 ± 0.04 | 98.81 |
| F8 | 187.22 | 0.30 ± 0.02 | 98.65 |
| F9 | 210.07 | 0.31 ± 0.06 | 100.10 |
| F10 | 187.12 | 0.22 ± 0.01 | 99.17 |
| F11 | 199.45 | 0.19 ± 0.05 | 99.15 |
| F12 | 210.15 | 0.24 ± 0.07 | 100.11 |
| F13 | 198.38 | 0.23 ± 0.06 | 100.14 |
| F14 | 211.18 | 0.32 ± 0.04 | 100.11 |
| F15 | 211.18 | 0.24 ± 0.05 | 99.61 |
| F16 | 188.12 | 0.25 ± 0.08 | 98.78 |
| F17 | 200.23 | 0.31 ± 0.06 | 98.75 |
| F18 | 200.23 | 0.30 ± 0.04 | 100.07 |
| F19 | 210.08 | 0.35 ± 0.08 | 100.05 |
| F20 | 187.00 | 0.17 ± 0.09 | 100.04 |
| F21 | 200.08 | 0.33 ± 0.06 | 98.95 |
| F22 | 199.00 | 0.24 ± 0.07 | 99.54 |
| F23 | 185.14 | 0.19 ± 0.09 | 100.05 |
| F24 | 200.24 | 0.35 ± 0.06 | 99.23 |
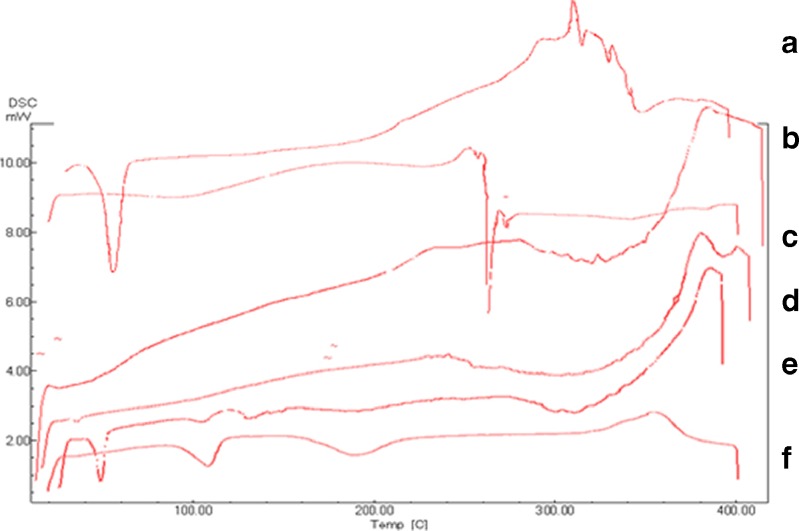
Figure 2 illustrates the DSC thermograms of MOS, film excipients, physical mixture of film components, and F11, which was selected as a representative formulation for comparison purposes. The thermogram of MOS included an endothermic peak at 115°C that corresponds to the melting point of the drug (28). Similarly, the thermograms of P188 and MDX showed endothermic peaks at 56°C and 272°C that correspond to their melting points, respectively (27).
Fig. 2.
DSC thermogram of a P188, b MDX, c HPMC E15, d F11, e physical mixture, and f MOS
It can be observed that the peaks of MOS, P188 and MDX completely disappeared in the thermogram of F11. In contrast, the respective peaks of the aforementioned compounds could still be detected in the physical mixture. This indicates the formation of a uniform dispersion with complete molecular miscibility of the different film components in the ODF preparation.
Mechanical Properties of the Films
The measurements of film mechanical properties for the different formulations are summarized in Table III. The increase of the MDX ratio relative to HMPC in the films, led to a decrease in TS and an increase in the E% of the film strips. This is consistent with earlier work in which the mechanical properties of HPMC films were studied, and it was shown that films made of HPMC alone were hard and glassy in nature (29). In contrast, MDX polymers with higher DE values were shown to impart greater ductility to strip films (6), which supports the observed mechanical properties of the different film formulations. Table III also shows that the increase in plasticizer level decreases the TS and increases the E% values, respectively. Plasticizers are known to act by inserting themselves between the polymer strands, thereby breaking the polymer–polymer interactions and increasing the molecular mobility of the polymer strands (30). Thus, it is to be expected that as the concentration of the plasticizer increases, the degree of film stiffness decreases, whereas the film ductility increases.
Table III.
Mechanical Properties of ODF Formulations
| ODF | Tensile strength (MPa) | % Elongation |
|---|---|---|
| F1 | 12.2 | 23.3 |
| F2 | 6.6 | 43.1 |
| F3 | 11.4 | 23.4 |
| F4 | 9.1 | 40.0 |
| F5 | 10.6 | 61.9 |
| F6 | 3.1 | 64.6 |
| F7 | 10.1 | 75.9 |
| F8 | 29.9 | 38.5 |
| F9 | 3.54 | 49.3 |
| F10 | 12.0 | 36.3 |
| F11 | 10.5 | 63.7 |
| F12 | 2.6 | 82.8 |
| F13 | 13.4 | 17.3 |
| F14 | 11.4 | 60.9 |
| F15 | 10.3 | 49.3 |
| F16 | 24.8 | 32.3 |
| F17 | 8.2 | 69.3 |
| F18 | 12.2 | 37.9 |
| F19 | 3.3 | 75.6 |
| F20 | 20.7 | 45.4 |
| F21 | 3.7 | 73.7 |
| F22 | 13.8 | 58.5 |
| F23 | 23.0 | 41.3 |
| F24 | 10.9 | 71.4 |
Statistical analysis of TS measurements showed that% plasticizer had a significant effect on the TS of the prepared films (p < 0.01). Tukey HSD was used for comparison of the means and it showed that using 15% plasticizer resulted in significantly higher film stiffness as can be seen from the higher TS values compared to films containing 20% and 25% plasticizer. This can be seen in Table III for formulations containing GLY or PG at different polymer ratios. At 5:5 MDX/HPMC for example, F8 (15% GLY) has TS of 29.9 MPa compared to 9.1 and 2.6 MPa for F4 (20% GLY) and F12 (25% GLY), respectively. A similar trend is observed with PG, where F24 (15% PG) has a TS of 10.9 MPa compared to 8.2 and 3.7 MPa for F17 and F21, respectively.
Determination of Moisture Uptake
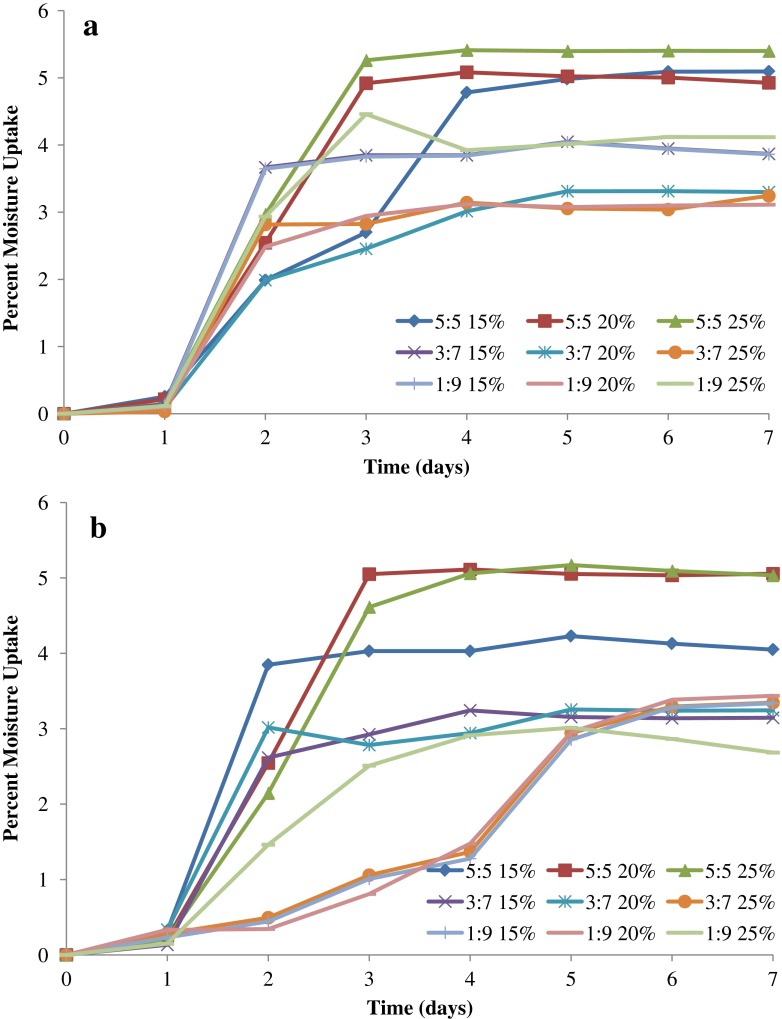
The polymers used in the ODF formulations are expected to affect their moisture sorption properties. This is because of the presence of HPMC, which is known to be moderately hygroscopic based on the classification by Callahan et al. (31). In addition, the presence of MDX in the formulation will influence the moisture uptake of the films as well. This is attributed to the higher hygroscopicity of MDXs with higher DE values (26). The percent moisture uptake is illustrated in Fig. 3a, b for ODF formulations containing GLY and PG, respectively. The percentage moisture uptake varied between approximately 3% and 5%, with an overall trend of increase in moisture uptake with the increase in both plasticizer level and MDX ratio. It can be seen that the percent plasticizer in the ODF formulation affects the percent moisture uptake, particularly as the ratio of MDX increases in the formulation. This can be attributed to the increase in MDX mobility, with the plasticizer getting between the polymer strands, thereby exposing more of its chains for moisture sorption. It can be seen that the equilibrium moisture content for all film formulations is attained within 5 days. The maximum % moisture uptake was 5.4% (w/w) and was achieved by F12, which contains 5:5 MDX/HPMC and 25% GLY.
Fig. 3.
Percent moisture uptake by ODF formulations containing different levels of a glycerol and b propylene glycol as plasticizer
In vitro Disintegration Time of the Films
The volume of saliva in the human buccal cavity is less than 6 ml and so the conventional disintegration tester that uses 900 ml of solution will not be representative of actual disintegration rate in vivo (32). Therefore, a Petri dish with a 6.5-cm diameter was used in this method to evaluate the in vitro disintegration rate, which is comparable to that of the sublingual area with a diameter of approximately 3–4 cm. Furthermore, the volume of the liquid medium as well as the relatively low agitation employed during the test closely resemble the volume of saliva and the relatively static environment in the buccal cavity, respectively.
Table IV summarizes the in vitro disintegration time of ODF preparations, which ranged from 1 to 11 s. Statistical analysis of the results was conducted and showed that plasticizer type and polymer ratio have a significant effect on disintegration time (p < 0.01). In regards to plasticizer type, it was seen that GLY resulted in significantly shorter disintegration time than PG. Tukey’s HSD test showed that 1:9 MDX/HPMC films possessed significantly increased disintegration time compared to the 3:7 and 5:5 MDX/HPMC formulations. This could be attributed to the higher fraction of MDX in the latter that facilitates water penetration into the film structure due to its high water solubility.
Table IV.
In vitro Disintegration and Release Results of ODF Formulations
| ODF | Disintegration time (s) | Total % dissolved (after 5 min) | Dissolution rate (in 5 min) |
|---|---|---|---|
| F1 | 1 | 23.3 | 2.6 |
| F2 | 2 | 43.1 | 3.1 |
| F3 | 3 | 23.4 | 2.7 |
| F4 | 5 | 40.0 | 2.7 |
| F5 | 5 | 61.9 | 2.6 |
| F6 | 6 | 64.6 | 2.9 |
| F7 | 7 | 75.9 | 3.6 |
| F8 | 5 | 38.5 | 3.9 |
| F9 | 6 | 49.3 | 2.8 |
| F10 | 11 | 36.3 | 2.9 |
| F11 | 6 | 63.7 | 2.6 |
| F12 | 3 | 82.8 | 3.6 |
| F13 | 2 | 17.3 | 2.3 |
| F14 | 10 | 60.9 | 3.6 |
| F15 | 6 | 49.3 | 3.3 |
| F16 | 9 | 32.3 | 2.5 |
| F17 | 6 | 69.3 | 6.1 |
| F18 | 8 | 37.9 | 2.3 |
| F19 | 5 | 75.6 | 4.9 |
| F20 | 11 | 45.4 | 2.5 |
| F21 | 6 | 73.7 | 4.6 |
| F22 | 9 | 58.5 | 2.7 |
| F23 | 10 | 41.3 | 2.6 |
| F24 | 9 | 71.4 | 3.2 |
Dissolution Studies
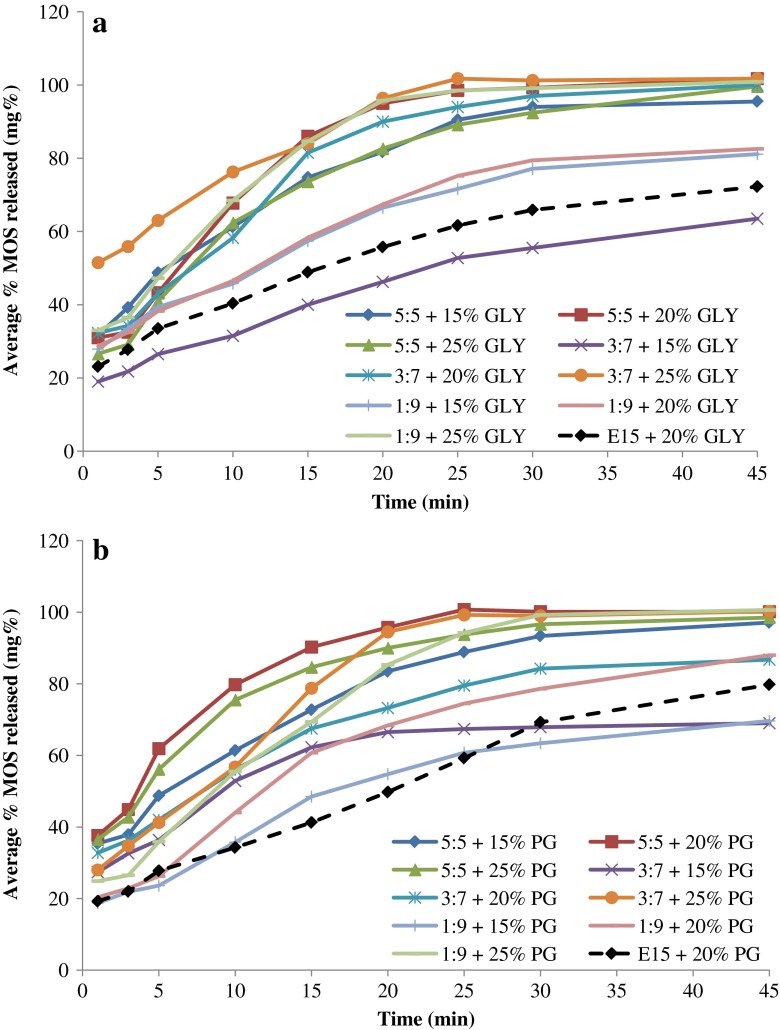
The release of MOS from ODF preparations using GLY or PG as plasticizers is depicted in Fig. 4a, b, respectively. In addition to D-optimal ODF preparations composed of HPMC and MDX, films were also prepared using just HPMC E15 as the polymer with 20% of each plasticizer type and their release profiles are also shown in Fig. 4 for comparison. It can be observed that the ODF prepared with HPMC E15 and 20% plasticizer showed slower release profile than most of ODF preparations utilizing MDX as part of the polymer matrix. This further supports the important role of MDX in enhancing the dissolution of MOS from prepared films.
Fig. 4.
MOS release profiles from ODF formulations containing different levels of a glycerol and b propylene glycol as plasticizer
The dissolution rate of MOS in the first 5 min was employed as the criterion for comparing the release results of different films, owing to the importance of rapid drug release in case of an ODF preparation. Statistical analysis of MOS release data demonstrated that polymer ratio has a significant effect on dissolution rate in 5 min (p < 0.01). Upon comparison of the means using Tukey HSD test, it was shown that the use of MDX/HPMC in the ratio of 5:5 as the film forming polymer resulted in significantly higher dissolution rate compared to formulations containing 3:7 and 1:9 MDX/HPMC.
There was a good linear correlation (R2 = 0.9896–0.9999) obtained by plotting the percent of MOS released from all the ODF formulations against the square root of time (data not shown). Thus, it was concluded that the release of MOS from ODF formulations followed a diffusion-controlled drug release profile and is in agreement with the Higuchi model (24).
Further Evaluation of Selected ODF Formulations
Based on the statistical analysis of ODF preparations, it can be concluded that the optimum formulations were those prepared using 5:5 MDX/HPMC as the polymer composition due to the improved mechanical properties and enhanced dissolution characteristics. Furthermore, using a plasticizer level at 20% or 25% improved the film elasticity. Consequently, F2, F12, F17, and F19 were selected as the optimum formulations based on in vitro experimentation and were further characterized with respect to their water content. Also, their in vivo disintegration time and palatability was tested in human volunteers.
In vivo Disintegration Time and Palatability
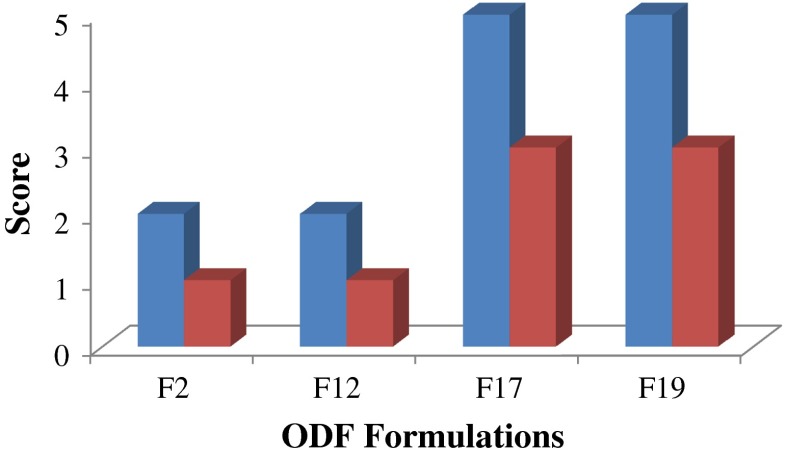
All tested films dissolved in the oral cavity within 5 s as shown in Fig. 5. The results showed that the in vivo disintegration time was shorter than that of the in vitro test, though not statistically significant (p > 0.01). This might be attributed to the extra agitation effect in the mouth by the tongue.
Fig. 5.
Mean in vivo disintegration time in seconds (blue bars) and (red bars) palatability of selected ODF formulations
Regarding film palatability, F2 and F12 were given a score of 1 (very satisfied) compared to F17 and F19 that were given a score of 3 (very dissatisfied) as shown in Fig. 5. This is due to the sweeter taste of GLY that is used as the plasticizer in the former case, which provides film sweetness compared to PG-containing films. Regarding the ease of administration and sensation thereafter, all the film specimens were given a score of 1 in both cases due to the ease of administration without the need for water, and the absence of any residue in the mouth following film dissolution, respectively.
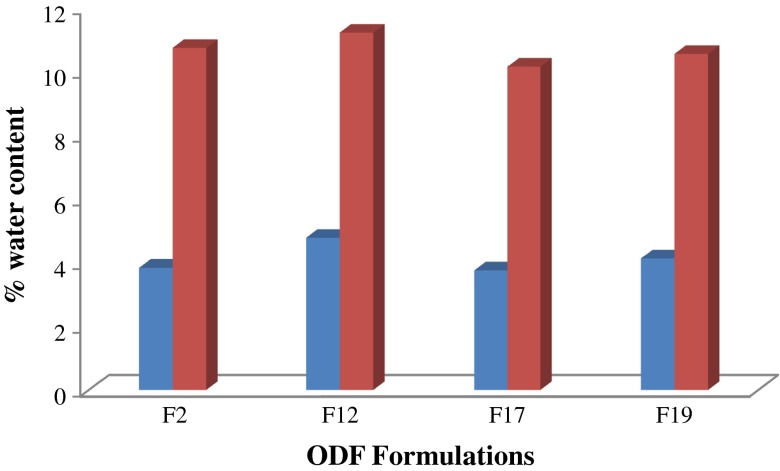
Determination of Water Content
The water content of F2, F12, F17, and F19, after storing in a dessicator or a humidity chamber at 60% RH, is plotted in Fig. 6. The water content in the films stored in a dessicator for 1 week was approximately the same. However, leaving the films at elevated humidity conditions led to a significant increase in their water content as shown by a paired t test (p < 0.01). It is interesting to note that the maximum water content measured at both ambient and high humidity conditions was that for F12, which also displayed the highest percent moisture uptake as can be seen in Fig. 3a.
Fig. 6.
Percent water content in ODF formulations after (blue bars) placing in dessicator and (red bars) 60% RH for 1 week
Owing to the hygroscopic excipients used in film formulations and the observed changes in percent moisture uptake and water content, it is advised that the prepared ODF be stored in air-tight containers to avoid possible subsequent effects on film properties and drug stability. Future studies will be aimed at studying the effect of storage on film characteristics and active ingredient stability.
CONCLUSIONS
In this work, the formulation of MOS in an orodispersible film preparation was achieved. An optimization approach through design of experiments was adopted to select the optimum formulation parameters that produce ODF of mosapride with desirable properties. The physicochemical properties of the prepared formulations were characterized using different techniques. The desired mechanical and drug release attributes were achieved through the use of equimolar ratio of MDX and hydroxypropyl methylcellulose, with a minimum of 20% GLY as plasticizer. Special attention is required during the storage and packaging of these ODF to avoid moisture uptake. This dosage form could be of particular benefit to patients treated with mosapride for gastrointestinal disorders owing to the anticipated relief of associated disease symptoms, which inherently pose a hurdle in administering any other oral form of this medication.
ACKNOWLEDGMENT
The authors would like to thank Prof. Dr. Doaa El-Nashar (National Research Center) for the help provided in tensile strength measurements.
REFERENCES
- 1.Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50–79-year-old men and women in urban population. Dysphagia. 1991;6:187–192. doi: 10.1007/BF02493524. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE. Approach to the patient with dysphagia or odynophagia. In: Yamada T, editor. Textbook of Gastroenterology. 4. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 682–687. [Google Scholar]
- 3.Ayra A, Chandra A, Sharma V, Pathak K. Fast dissolving oral films: an innovative drug delivery system and dosage form. Int J ChemTech Res. 2010;2:576–583. [Google Scholar]
- 4.Sharma K, Pfister WR, Ghosh TK. Quick-dispersing oral drug delivery systems. In: Ghosh TK, Pfister WR, editors. Drug delivery to the oral cavity. Florida: Taylor & Francis; 2005. pp. 262–287. [Google Scholar]
- 5.Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm. 2008;70:895–900. doi: 10.1016/j.ejpb.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Cilurzo F, Cupone IE, Minghetti P, Buratti S, Selmin F, Gennari CG, et al. Nicotine fast dissolving films made of maltodextrins: a feasibility study. AAPS PharmSciTech. 2010;11:1511–1517. doi: 10.1208/s12249-010-9525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Setouhy D, El-Malak NSA. Formulation of a novel tinapetine sodium orodispersible film. AAPS PharmSciTech. 2010;11:1018–1025. doi: 10.1208/s12249-010-9464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malke S, Shidhaye S, Desai J, Kadam V. Oral films-patient compliant dosage form for pediatrics. Internet J Ped Neonatol. 2010;11.
- 9.Martineau B. A look at fast-dissolving drug delivery systems. Drug Deliv Technol. 2009;9:36–38. [Google Scholar]
- 10.Mashru RC, Sutariya VVB, Snkalia MMG, Parikh PP. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev Ind Pharm. 2005;31:25–34. doi: 10.1081/ddc-43947. [DOI] [PubMed] [Google Scholar]
- 11.Shimoda H, Taniguchi K, Nishimura M, Matsuura K, Tsukioka T, Yamashita H, et al. Preparation of a fast dissolving oral thin film containing dexamethasone: a possible application to antiemesis during cancer chemotherapy. Eur J Pharm Biopharm. 2009;73:361–365. doi: 10.1016/j.ejpb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Corniello CM. Quick dissolve strips: from concept to commercialization. Drug Del Technol. 2006;6:68–71. [Google Scholar]
- 13.Ali S, Quadir A. High molecular weight povidone polymer-based films for fast-dissolving drug delivery application. Drug Deliv Technol. 2007;7:36–43. [Google Scholar]
- 14.Kulkarni KS, Deokule HA, Mane MS, Ghadge DM. Exploration of different polymers for use in the formulation of oral fast dissolving strips. J Curr Pharm Res. 2010;2:33–35. [Google Scholar]
- 15.Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech. 2008;9:349–356. doi: 10.1208/s12249-008-9047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel R, Shardul N, Patel J, Baria A. Formulation development and evaluation of mouth melting film of ondansetron. Arch Pharm Sci Res. 2009;1:212–217. [Google Scholar]
- 17.Curran MP, Robinsion DM. Mosapride in gastrointestinal disorders. Drugs. 2008;68:981–991. doi: 10.2165/00003495-200868070-00007. [DOI] [PubMed] [Google Scholar]
- 18.Devault KR, Castello DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:1434–1442. doi: 10.1111/j.1572-0241.1999.1123_a.x. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700–1707. doi: 10.1056/NEJMcp0804684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra R, Amin A. Formulation development of taste-masked rapidly dissolving films of citirizine hydrochloride. Pharm Technol. 2009;33:48–56. [Google Scholar]
- 21.Park JH, Holman KM, Bish GA, Kreiger DG, Ramlose DS, Herman CJ, et al. An alternative to the USP disintegration test for orally disintegrating tablets. Pharm Technol. 2008;32:54–58. [Google Scholar]
- 22.Shan-Yang L. Effect of excipients on tablet properties and dissolution behavior of theophylline-tableted microcapsules under different compression forces. J Pharm Sci. 1988;77:229–232. doi: 10.1002/jps.2600770309. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JG. Interpretation of percent dissolved-time plots derived from in-vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;52:1145–1149. doi: 10.1002/jps.2600581021. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi T. Mechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 25.Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139:94–107. doi: 10.1016/j.jconrel.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Chronakis IS. On the molecular characteristics, compositional properties, and structural–functional mechanisms of maltodextrins: a review. Crit Rev Food Sci Nutr. 1998;38:599–637. doi: 10.1080/10408699891274327. [DOI] [PubMed] [Google Scholar]
- 27.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 5. London: Pharm Press; 2005. [Google Scholar]
- 28.Mosapride (2009). http://www.druglead.com/cds/mosapride.html. Accessed 12 Sep 2010.
- 29.Aulton ME, Abdul-Razzak MH. The mechanical properties of hydroxypropylmethylcellulose films derived from aqueous systems. Part 1: the influence of plasticizers. Drug Dev Ind Pharm. 1981;7:649–668. doi: 10.3109/03639048109055689. [DOI] [Google Scholar]
- 30.Entwistle CA, Rowe RC. Plasticization of cellulose ethers used in the film coating of tablets. J Pharm Pharmacol. 1979;31:269–272. doi: 10.1111/j.2042-7158.1979.tb13499.x. [DOI] [PubMed] [Google Scholar]
- 31.Callahan JC, Cleary GW, Elefant M, Kaplan G, Kensler T, Nash RA. Equilibrium moisture content of pharmaceutical excipients. Drug Dev Ind Pharm. 1982;8:355–369. doi: 10.3109/03639048209022105. [DOI] [Google Scholar]
- 32.Shukla D, Chakraborty S, Singh S, Mishra B. Mouth dissolving tablets II: an overview of evaluation techniques. Sci Pharm. 2009;77:327–341. doi: 10.3797/scipharm.0811-09-02. [DOI] [Google Scholar]