Abstract
The purpose of this study was to develop a novel method to inhibit the formation of acylated peptide impurities in poly(d,l-lactide-co-glycolide) (PLGA) formulations by reversely blocking the amino groups of octreotide with maleic anhydride (MA). Two mono-MA conjugates with different modification sites (N terminus and Lys residue) and di-MA conjugate of octreotide were prepared and isolated by reversed-phase high-performance liquid chromatography (RP-HPLC). The polymer interaction of peptides and the formation of acylated peptides were monitored by RP-HPLC. The stability of MA-octreotide conjugates in PLGA films was studied in 0.1 M phosphate buffer (pH 7.4) at 37°C. The conjugation of MA to octreotide substantially inhibited the interaction of peptide with PLGA polymer and the subsequent formation of acylated peptide impurities. The MA-octreotides were successfully converted to intact octreotide as pH drops by PLGA hydrolysis. In PLGA films, MA-octreotide also showed complete inhibition of peptide acylation. In conclusion, MA conjugation provides a viable approach for stabilizing peptides in PLGA delivery systems.
Key words: maleic anhydride, octreotide, peptide acylation, poly(lactide-co-glycolide), reversible blocking
INTRODUCTION
For the controlled and prolonged release delivery of peptide drugs, poly(d,l-lactide-co-glycolide) (PLGA), one of the few polymers approved by the FDA, has been widely used as an attractive vehicle owing to its good biodegradability and biocompatibility (1,2). Various peptides have been incorporated into PLGA microparticles and several drugs, such as, the Lupron Depot® (leuprolide acetate), Zoladex® implant (goserelin acetate), and Sandostatin LAR® (octreotide acetate), have been commercialized as sustained-release formulations (3). However, instability of therapeutic peptides in the PLGA matrix during manufacture, storage, and in vivo release remains as one of the major obstacles in the development of PLGA depot formulations (4–6). Among several instability issues of peptides in PLGA matrix, the formation of acylated peptide impurities has emerged as significant instability mechanism in PLGA formulations (5,6). The acylated peptides are formed as a result of acylation of peptide’s amines with ester backbone of PLGA followed by release of peptides conjugated with lactic or glycolic acid units by polymer hydrolysis (7–9). Nucleophilic groups of peptides, such as the primary amino groups present in the N terminus and Lys residue, have been reported to be the major targets for peptide acylation (8). The peptide acylation in PLGA formulations may result in the incomplete release of the peptide from the matrix and can affect the biological activity, pharmacological effect, and toxicity of peptide drugs (10–12).
Octreotide is a synthetic octapeptide analogue of the naturally occurring hormone somatostatin and exerts pharmacologic actions similar to the somatostatin (13). Compared with somatostatin, octreotide has a longer half-life, greater selectivity for inhibiting glucagon, growth hormone and insulin release, and a lower incidence of rebound hypersecretion following discontinuation. Currently, octreotide has been commercially formulated in PLGA microspheres (Sandostatin LAR depot, Novartis Pharma, Basel, Switzerland) as a monthly dosage form for the treatment of acromegaly (14). In a previous study, the octreotide in Sandostatin LAR has been also shown to form acylated peptide impurities after in vitro incubation in phosphate-buffered saline (15).
Several strategies for minimizing and preventing the peptide acylation in PLGA formulations have been proposed and studied. Lucke et al. proposed that the use of block copolymer of poly(lactic acid) and polyethylene glycol (PEG) would prevent peptide acylation in PLGA microspheres, however, the block copolymer did not show favorable effect on inhibition of the peptide acylation (16). Houchin et al. studied the effects of pH-modifying excipients, such as, proton sponge, magnesium hydroxide, ammonium acetate, and magnesium acetate, on the chemical stability and acylation of peptides in PLGA films (17). Recently, the efficacy of water-soluble divalent cationic salts was proposed for inhibition of acylation of octreotide in PLGA microspheres (18,19). We previously proposed the covalent conjugation of PEG to peptide for inhibiting peptide acylation and increasing the pharmacokinetic property (9,20–22).
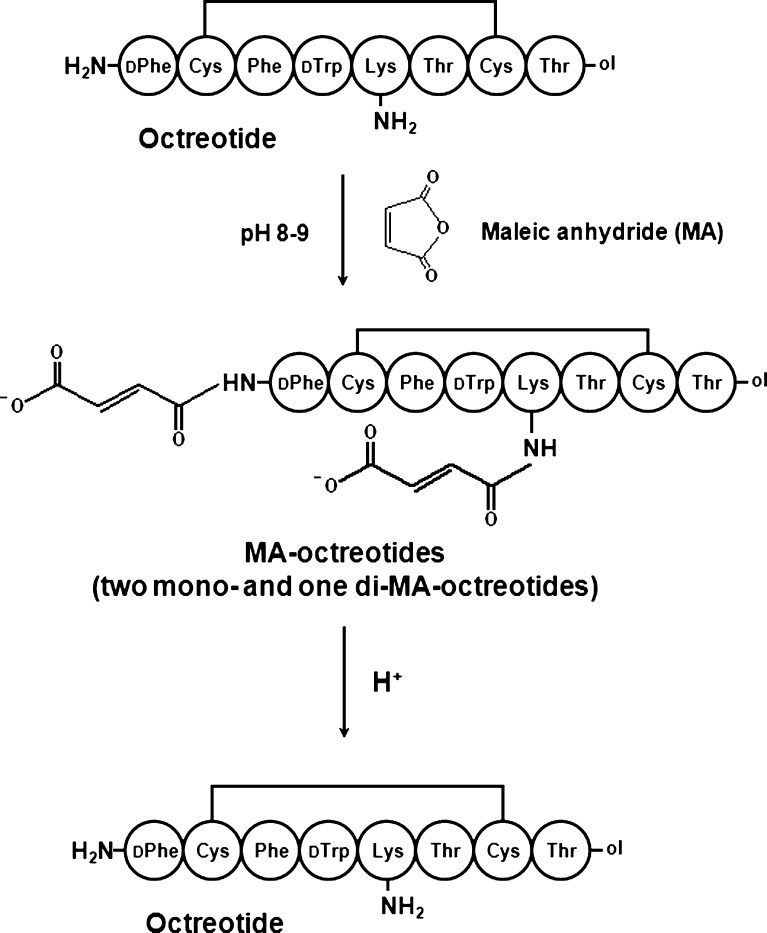
This study proposes the reversible blocking of peptide’s amino groups using maleic anhydride (MA), which is reversible amine-protective reagent (23). As the formation of acylated peptides involves an initial electrostatic adsorption between the amino groups of peptide and carboxyl end group of PLGA prior to acylation reaction, the MA conjugation to amino groups of peptide would be an approach for blocking the initial interaction of peptide with PLGA. As PLGA hydrolyzes and subsequently the pH decreases in formulations, intact peptide will be released from MA conjugates (Fig. 1). In this study, octreotide was modified with MA and the stability of MA-octreotide conjugates against the interaction with PLGA and peptide acylation in PLGA formulations was investigated.
Fig. 1.
Reversible blocking of amino groups of octreotide by MA. At acidic pH, the MA conjugates readily hydrolyze and the amines can be unblocked to create the original structure
MATERIALS AND METHODS
Materials
Octreotide acetate (H2N-d-Phe-Cys-Phe-d-Trp-Lys-Thr-Cys-Thr-ol, molecular weight of 1,019.26) was obtained from Bachem (Torrance, CA, USA). PLGA with free carboxylic end-groups (molar ratio of d,l-lactide/glycolide = 50:50; viscosity, 0.16–0.24 dL/g: product name, RESOMER® RG502H) was supplied by Boehringer Ingelheim (Ingelheim, Germany). MA and trifluoroacetic acid (TFA) were purchased from Sigma (St. Louis, MO, USA). Acetonitrile and acetone (both in HPLC grade) were obtained from J.T. Baker (Philipsburg, NJ, USA) and Merck (Darmstadt, Germany), respectively. All other chemicals were of analytical grade and were used as obtained commercially.
Conjugation of MA to Octreotide
A solution of octreotide (10 mg/mL in water) was added to a ten-time molar excess of MA in 0.1 M phosphate buffer at pH 8.0, 8.5, or 9.0. The reaction mixtures were incubated at room temperature for 60 min and loaded onto HPLC system (Dionex Ultimate-3000 VWD model, Sunnyvale, CA, USA) consisting of a quaternary gradient pump with an on-line vacuum degasser (Model LPG-3400A), an automated sample injector (Model WPS-3000SL), thermostatted column compartment (Model TCC-3000), and UV–vis detector (Model VWD-3100). Separation was carried out on an Acclaim C-18 column (4.6 × 250 mm, 5 μm, Dionex, Sunnyvale, CA, USA). A linear gradient elution was performed from 30% to 50% (v/v) acetonitrile in water containing 0.1% (v/v) TFA for 20 min. Total run time was 30 min. The flow rate was 1 mL/min, and UV absorbance was monitored at 215 nm. After the reaction condition was optimized by changing reaction pH, each MA-octreotide conjugates corresponding to two mono-MA-octreotides (mono-MA-Phe1-octreotide and mono-MA-Lys5-octreotide) and one di-MA-octreotide were isolated from reaction mixture. Each MA-octreotide conjugate was freeze-dried following evaporation of organic solvent under nitrogen. The molecular weights of the separated MA-octreotides were determined by liquid chromatography–mass spectrometry (LC-MS).
LC-MS Analysis
LC-MS analysis was performed using an Agilent Technologies Series 1100 LC/MSD VL system (Palo Alto, CA, USA). The column, mobile phase, and gradient elution conditions were the same as those used in HPLC method described above. The mass spectrometer was operated in the positive ion mode using the following conditions: drying gas (N2) flow of 10 L/min, drying gas at 350°C, nebulizer pressure of 45 psi, and capillary voltage of 3 kV. The fragmentor voltage was 100 V. Ions were detected by scan mode and mass range was set from m/z 400 to 1,400.
Polymer Interaction Study
The formation of acylated octreotides by interaction with PLGA was investigated by adding 10 mg of PLGA polymer to 1 mL of octreotide or MA-octreotide conjugates (peptide concentration of 200 μg/mL) in 0.1 M phosphate buffer (pH 7.4) at 37°C (n = 3 per sample). Samples were collected at 7, 14, 21, 28, 35, 42, 49, and 56 days. At each sampling time, the pH value in the medium was measured by pH strip. The supernatants after centrifuge were analyzed by HPLC. To analyze the interaction of octreotide with PLGA, a linear gradient of 20–50% (v/v) acetonitrile in water containing 0.1% (v/v) TFA for 20 min was used. The other conditions were the same as the HPLC method for the separation of MA-octreotides described above.
Film Preparation and Peptide Stability in PLGA Films
PLGA films were prepared as described in the previous publications (17,24,25) by dissolving 500 μg of octreotide or di-MA-octreotide with 10 mg of PLGA in 0.2 mL of acetone and sonicating for 30 min. The solution was then pipetted into eppendorf tubes and allowed to dry for 2 days. The dried PLGA films containing octreotide or di-MA-octreotide were placed into eppendorf tube containing 1 mL of 0.1 M phosphate buffer (pH 7.4) and incubated at 37°C. Samples were collected at specific time after vortex for 1 min and centrifuged for 10 min at 5,000 rpm. All samples were analyzed by HPLC.
RESULTS
Preparation and Characterization of MA-Conjugated Octreotides
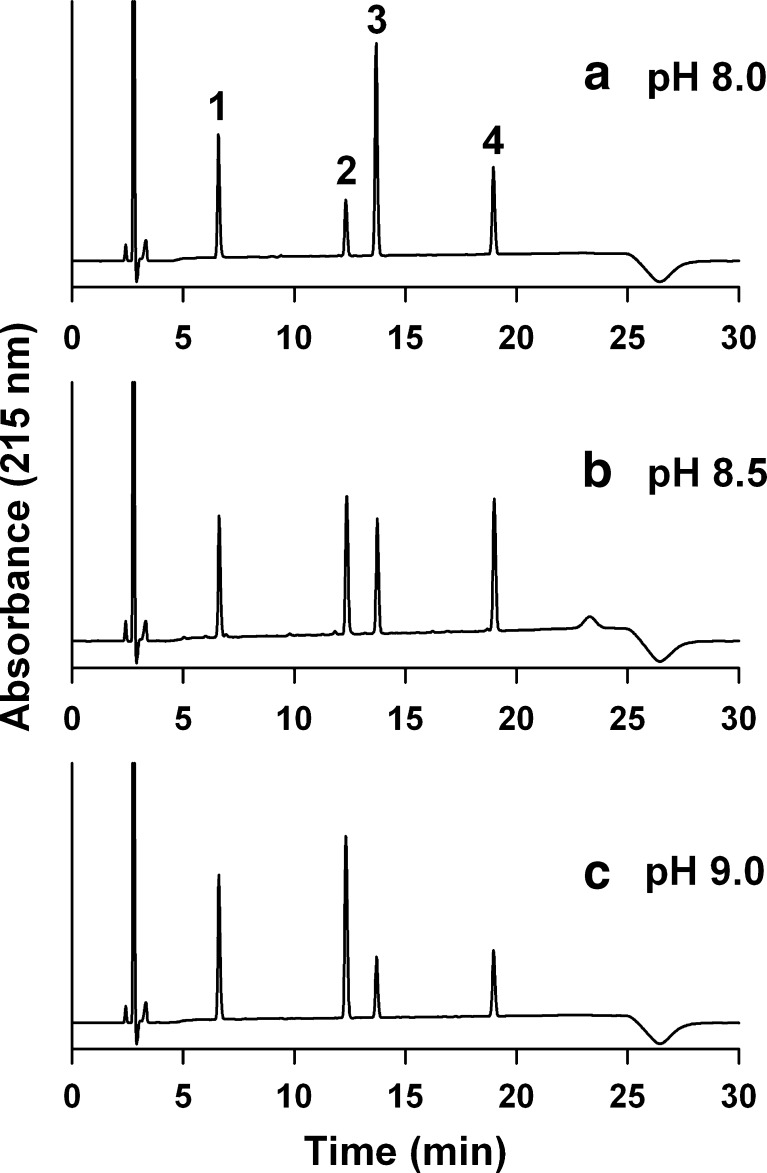
As octreotide has two reactive sites of N terminus (Phe1) and Lys5, three species of MA-octreotides can be produced; that is, two mono- and one di-MA-octreotide (Fig. 1). The three MA-octreotides were well separated into three peaks by HPLC (Fig. 2). Each peak was isolated and their molecular masses were determined by LC-MS (Table I). In Fig. 2, the first peak (retention time, 6.5 min) was native octreotide with a molecular mass of m/z 510.3. The molecular masses of the second (retention time, 12.3 min) and third peaks (retention time, 13.6 min) were the same as m/z 559.3, which corresponds to the mono-MA-octreotide. The fourth peak (retention time, 18.9 min) was identified as di-MA-octreotide with a molecular mass of m/z 608.3. To identify the MA-conjugation sites of the two mono-MA-octreotides, the difference of MA-conjugation reactivity between the α-amine (N terminus) and ε-amine (Lys) was assessed as a function of the medium pH. In general, the pKa value of the α-amino group is 7.6–7.8, whereas that of the ε-amino group is 10.0–10.2 (26). Therefore, the α-amino group of the N terminus would be more reactive than that of the Lys residue at low pH, whereas the ε-amino group of the Lys residue would be more susceptible to the MA-conjugation reaction at high pH. This phenomenon was demonstrated in the previous studies (9). As shown in Fig. 2, as medium pH increases, peak 2 became larger, whereas peak 3 became smaller. This indicates that peak 2 was mono-MA-Lys5-octreotide and peak 3 was mono-MA-Phe1-octreotide.
Fig. 2.
HPLC chromatograms of the MA-octreotides produced at pH 8.0 (a), 8.5 (b), and 9.0 (c). Peaks 1, octreotide; 2, mono-MA-Lys5-octreotide; 3, mono-MA-Phe1-octreotide; and 4, di-MA-octreotide
Table I.
LC-MS of MA-Octreotides Separated by HPLC
| Peptides | Retention time in HPLC (min) | Measured [M+2H]2+ | Expected [M+2H]2+ |
|---|---|---|---|
| Unmodified octreotide | 6.5 | 510.3 | 510.5 |
| Mono-MA-Lys5-octreotide | 12.3 | 559.3 | 559.5 |
| Mono-MA-Phe1-octreotide | 13.6 | 559.3 | 559.5 |
| Di-MA-octreotide | 18.9 | 608.3 | 608.5 |
Interaction of Octreotide with PLGA
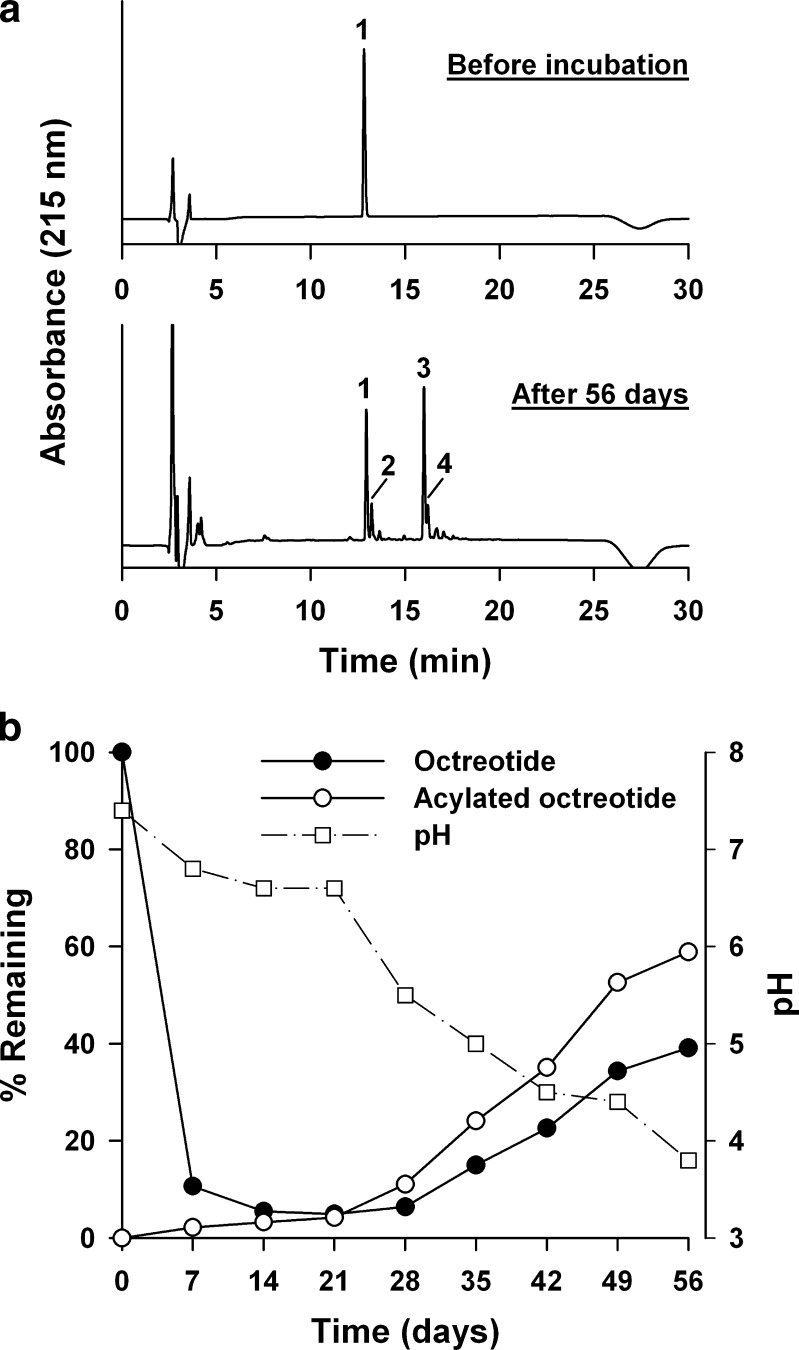
As demonstrated in previous studies (9,18,19,25), the interaction of octreotide with PLGA polymers involved an initial adsorption of the peptide to the polymer followed by an acylation reaction and subsequent release of intact and acylated octreotides. As shown in Fig. 3a, various acylated octreotides were found during incubation with PLGA in 0.1 M phosphate buffer (pH 7.4) at 37°C. Among the acylated octreotides, three distinct peaks (peaks 2, 3, and 4) were observed and they were identified based on the previous study (9,15,25) as follows: peak 2, mono-glycoyl-conjugated octreotide at Lys residue; peak 3, mono-glycoyl-conjugated octreotide at N terminus; peak 4, di-glycoyl-conjugated octreotide at the N terminus and Lys residue. The most prevalent acylation product was the N-terminally glycoyl-conjugated octreotide (peak 3). This means the greater nucleophilic activity of the N terminus over the lysine residue and it might be attributed to the lower pKa value of N-terminal amine than lysine’s amine. Figure 3b shows the profile of formation of acylated octreotides during incubation of octreotide with PLGA in 0.1 M phosphate buffer (pH 7.4) at 37°C. After incubation for 56 days, the intact octreotide was 39.1% in the supernatant, whereas the acylated octreotides were formed to be 58.9%.
Fig. 3.
Polymer interaction study of octreotide with PLGA in 0.1 M phosphate buffer (pH 7.4) at 37°C. a HPLC chromatograms of octreotides before and after incubation with PLGA for 56 days. Peaks 1, octreotide and 2–4, acylated octreotides. b Adsorption of octreotide to PLGA and the formation of acylated octreotides
Interaction of MA-Octreotide with PLGA
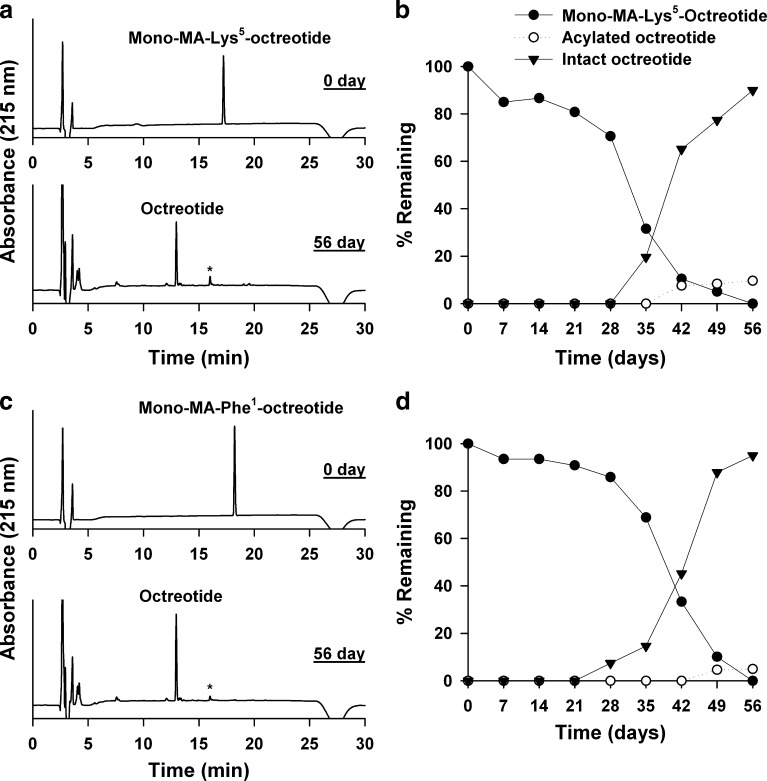
The conjugation of MA to octreotide substantially inhibited the formation of acylated peptides during incubation with PLGA. Figure 4 shows the HPLC chromatograms and polymer interaction profiles of two mono-MA-octreotides. The MA conjugation of octreotide significantly inhibited the initial adsorption of peptide to PLGA. Subsequently, each MA-octreotide turned into native octreotide owing to pH drop caused by accumulation of acidic products produced by PLGA hydrolysis. As shown in Fig. 4b, the initial adsorption of mono-MA-Lys5-octreotide was 15% through 7 days, and then the amount gradually decreased. At day 56, it totally disappeared. On the other hand, native octreotide and a small quantity of acylation products were observed from days 35 and 42, respectively. By day 56, the amount of native octreotide recovered to 90% and acylated octreotides was 10%. The interaction profile of mono-MA-Phe1-octreotide was similar to that of mono-MA-Lys5-octreotide (Fig. 4d). However, the inhibition effect of mono-MA-Phe1-octreotide on initial adsorption to PLGA and the acylated peptide formation was greater than mono-MA-Lys5-octreotide. The initial absorption of mono-MA-Phe1-octreotide was 7% through 7 days, and the amount of acylation product was only 5% after incubation of 56 days. The recovered octreotide reached 95%.
Fig. 4.
Polymer interaction study of mono-MA-Lys5-octreotide (a, b) and mono-MA-Phe1-octreotide (c, d) with PLGA. In HPLC chromatograms, asterisk represents acylation products
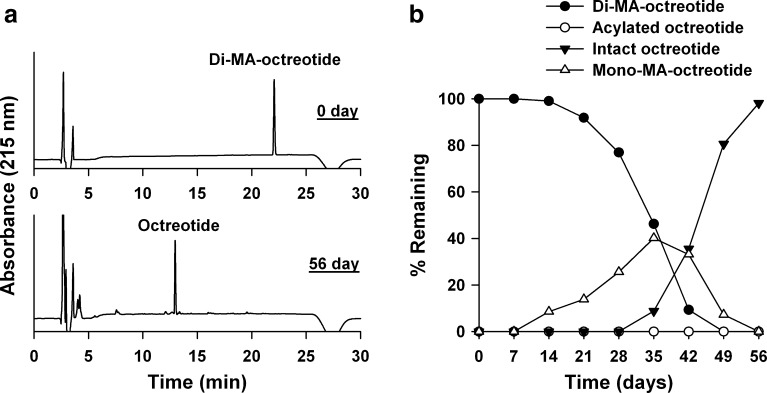
Figure 5 shows the HPLC chromatograms and the polymer interaction profile of di-MA-octreotide. The MA conjugation to two amine sites of octreotide strongly inhibited the initial adsorption to PLGA and acylated peptide formation. From day 14 through day 49, mono-MA-octreotide was observed as intermediate compound between di-MA-octreotide and native octreotide. After 56 days, native octreotide was recovered to 98%. In the HPLC chromatogram, the trace amounts of acylated or degraded peptides were detected but they were below quantitation limit.
Fig. 5.
Polymer interaction study of di-MA-octreotide with PLGA. a HPLC chromatograms of di-MA-octreotide before and after incubation with PLGA for 56 days. b Release of intact octreotide and mono-MA-octreotide from di-MA-octreotide during incubation with PLGA in 0.1 M phosphate buffer (pH 7.4) at 37°C
Stability of MA-Octreotide in PLGA Film
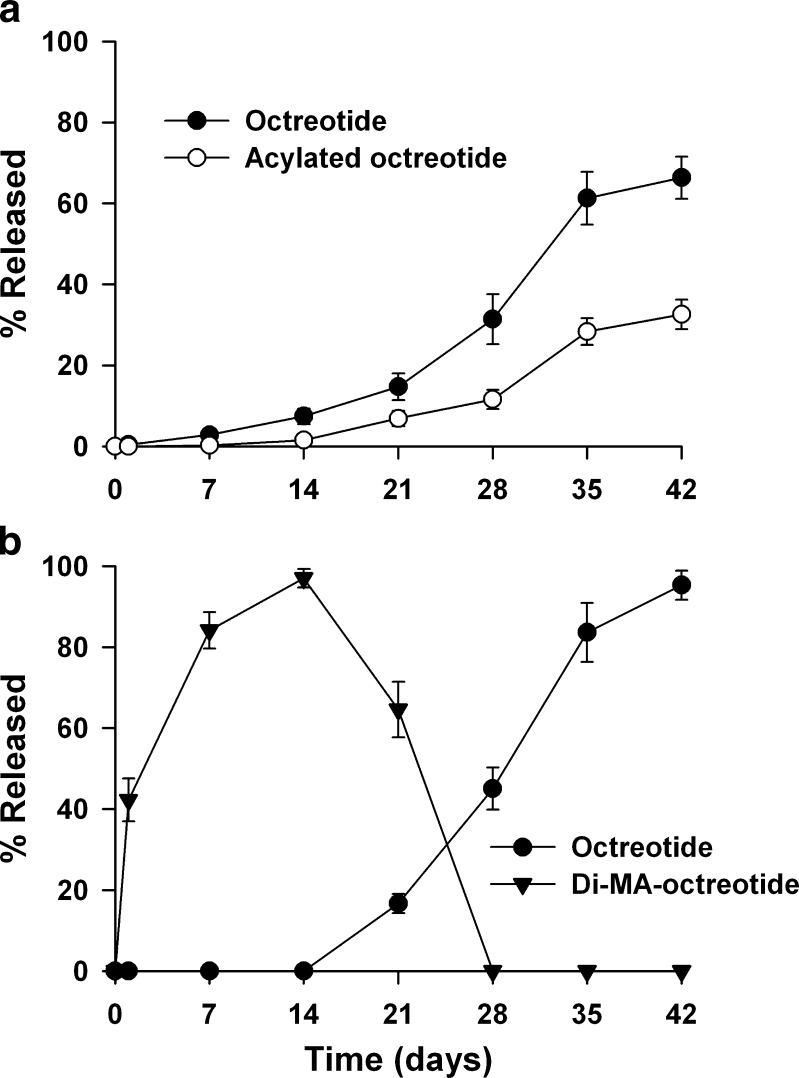
The stability of octreotide and di-MA-octreotide in PLGA films was investigated by incubating in 0.1 M phosphate buffer (pH 7.4) at 37°C for 42 days (Fig. 6). In the PLGA film containing octreotide, the acylated octreotides were found from day 14 and the amount reached to 32.7% based on the loaded octreotide amount (Fig. 6a). The PLGA film containing di-MA-octreotide showed initial burst (42.3%) and faster peptide release (Fig. 6b). The initial burst and faster release rate might be attributed to lower interaction property of di-MA-octreotide to PLGA polymer. Intact octreotide was found from day 21 and the release amount increased through 42 days. At day 42, 95.3% of intact octreotide was observed and the acylated octreotides were not found, which means complete inhibition of peptide acylation and successful release of intact peptide from MA conjugate.
Fig. 6.
Stability and release study of peptide from PLGA films containing octreotide (a) and di-MA-octreotide (b)
DISCUSSION
The stability of therapeutic peptides in the PLGA matrix remains as one of the major obstacles in the development of sustained-release depot formulations (4–6). Since the formation of acylated peptide impurities in PLGA delivery systems was found by mass spectrometric techniques, several strategies for inhibition of peptide acylation have been proposed (9,16–19,21,22). As the acylated peptide impurities can affect the pharmacological effect and toxicity of products, the prevention and evaluation are very important for securing the efficacy and safety of drug products (12).
Previously we have shown that the ionic interaction of cationic peptides with the carboxylic acid end-groups of PLGA is an important step for peptide acylation (9). The interaction of octreotide with PLGA was attenuated when the end-capped PLGA was used (9) and at low pH medium (27). Based on these findings, the incorporation of divalent cations, such as Ca2+ and Mn2+, into PLGA microspheres was proposed for blocking the carboxylic acid end-groups of PLGA (18,19). Contrary to this approach which blocks cationic groups of PLGA polymers, we previously investigated the PEGylation of octreotide as an approach for blocking the anionic groups of peptide (9,20–22). PEGylation is an attractive strategy for endowing additional benefits, such as, physical stability, improved proteolytic stability, reduced immunogenicity, and increased half-life, to therapeutic molecules (28). However, the PEGylated peptides produced by covalent bonding between peptide and PEG may need to be evaluated as a new drug entity. This can be another barrier because of financial concerns with gaining approval. With regard to this point, the reversible blocking of peptide with MA can be good alternative to PEGylation approach.
In the polymer interaction study, every MA-octreotides showed favorable results with inhibiting peptide acylation and conversion to intact octreotide. As di-MA-octreotide showed complete inhibition while two mono-MA-octreotides showed 5–10% acylated products, the di-MA-octreotide was selected as candidate for PLGA formulation. In addition, the conversion rates to native octreotide between mono- and di-MA-octreotides were significantly not different (Figs. 4 and 5). In the release study of PLGA films, di-MA-octreotide was released prior to conversion to intact octreotide inside films (Fig. 6b). This phenomenon is not favorable to demonstrate the usefulness of the reversible amine-blocking approach using MA. Although the complete conversion was observed after 42 days and any acylated peptides were not produced in release medium, the release of di-MA-octreotide in initial stage indicates that the MA-conjugate is not suitable to PLGA films for sustained release. Thus, alternative formulation approach to retard the release of di-MA-octreotide from PLGA matrix is necessary for further demonstrating the usefulness of MA conjugation to octreotide.
Concerning the toxicity potential of MA-octreotides, SMANCS, approved in Japan for the treatment of liver cancer, may be reference because this product was prepared by conjugating two chains of poly(styrene-co-maleic acid/maleic anhydride/partial half-n-butyl ester) (SMA) to neocarzinostatin (NCS) (29). Although SMA is polymer unlike maleic anhydride, the bond structure between SMA and NCS in SMANCS is similar to that of MA-octreotides. Nonetheless, the safety of MA-octreotides needs to be justified for toxicity evaluation.
CONCLUSIONS
This study investigated the effectiveness of MA conjugation on the inhibition of peptide acylation in PLGA matrix. Two isomers of mono-MA-octreotide and one di-MA-octreotide were successfully prepared and their stability against peptide acylation by PLGA was investigated. Although mono-MA-octreotide modified at N-terminal amine showed substantial inhibition of peptide acylation in PLGA interaction study, the inhibition effect was not complete. Therefore, di-MA-octreotide was chosen as candidate and it showed sufficient inhibition effect on peptide acylation in PLGA matrix. However, di-MA-octreotide showed fast release prior to conversion to intact octreotide inside PLGA films. The formulation for retarding release of di-MA-octreotide from PLGA matrix and the toxicity evaluation of MA-octreotides are required to be studied further.
Acknowledgments
This work was financially supported by the Ministry of Knowledge Economy (MKE) and Korea Institute for Advancement in Technology (KIAT) through the Workforce Development Program in Strategic Technology.
References
- 1.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/S0169-409X(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 2.Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995;6:332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 3.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 4.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/A:1026498209874. [DOI] [PubMed] [Google Scholar]
- 5.Bilati U, Allemann E, Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur J Pharm Biopharm. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Houchin ML, Topp EM. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J Pharm Sci. 2008;97:2395–2404. doi: 10.1002/jps.21176. [DOI] [PubMed] [Google Scholar]
- 7.Lucke A, Kiermaier J, Gopferich A. Peptide acylation by poly(alpha-hydroxy esters) Pharm Res. 2002;19:175–181. doi: 10.1023/A:1014272816454. [DOI] [PubMed] [Google Scholar]
- 8.Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J Control Release. 2003;92:291–299. doi: 10.1016/S0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 9.Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by poly(d,l-lactide-co-glycolide) Pharm Res. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- 10.Murty SB, Thanoo BC, Wei Q, DeLuca PP. Impurity formation studies with peptide-loaded polymeric microspheres: part I. In vivo evaluation. Int J Pharm. 2005;297:50–61. doi: 10.1016/j.ijpharm.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Murty SB, Na DH, Thanoo BC, DeLuca PP. Impurity formation studies with peptide-loaded polymeric microspheres: part II. In vitro evaluation. Int J Pharm. 2005;297:62–72. doi: 10.1016/j.ijpharm.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Na DH, Lee JE, Jang SW, Lee KC. Formation of acylated growth hormone-releasing peptide-6 by poly(lactide-co-glycolide) and its biological activity. AAPS PharmSciTech. 2007;8(2):E105–E109. doi: 10.1208/pt0802043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, O’Dorisio MS, Hermann G, Hayes J, Malarkey WB, O’Dorisio TM. Mechanisms of action of long-acting analogs of somatostatin. Regul Pept. 1993;44:285–295. doi: 10.1016/0167-0115(93)90138-X. [DOI] [PubMed] [Google Scholar]
- 14.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. J N Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 15.Murty SB, Goodman J, Thanoo BC, DeLuca PP. Identification of chemically modified peptide from poly(d,l-lactide-co-glycolide) microspheres under in vitro release conditions. AAPS PharmSciTech. 2003;4:E50. doi: 10.1208/pt040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucke A, Fustella E, Tessmar J, Gazzaniga A, Göpferich A. The effect of poly(ethylene glycol)-poly(d,l-lactic acid) diblock copolymers on peptide acylation. J Control Release. 2002;80:157–168. doi: 10.1016/S0168-3659(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 17.Houchin ML, Neuenswander SA, Topp EM. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J Control Release. 2007;117:413–420. doi: 10.1016/j.jconrel.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. J Control Release. 2009;137:179–184. doi: 10.1016/j.jconrel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Sophocleous AM, Schwendeman SP. Inhibition of peptide acylation in PLGA microspheres with water-soluble divalent cationic salts. Pharm Res. 2009;26:1986–1994. doi: 10.1007/s11095-009-9914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na DH, Murty SB, Lee KC, Thanoo BC, DeLuca PP. Preparation and stability of poly(ethylene glycol) (PEG)ylated octreotide for application to microsphere delivery. AAPS PharmSciTech. 2003;4:E72. doi: 10.1208/pt040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na DH, Lee KC, DeLuca PP. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharm Res. 2005;22:743–749. doi: 10.1007/s11095-005-2590-y. [DOI] [PubMed] [Google Scholar]
- 22.Park EJ, Tak TH, Na DH, Lee KC. Effect of PEGylation on stability of peptide in poly(lactide-co-glycolide) microspheres. Arch Pharm Res. 2010;33:1111–1116. doi: 10.1007/s12272-010-0718-z. [DOI] [PubMed] [Google Scholar]
- 23.Hermanson GT. Bioconjugate techniques. 2. London: Academic; 2008. [Google Scholar]
- 24.Houchin ML, Heppert K, Topp EM. Deamidation, acylation and proteolysis of a model peptide in PLGA films. J Control Release. 2006;112:111–119. doi: 10.1016/j.jconrel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Ryu KW, Na DH. Stability of octreotide acetate in aqueous solutions and PLGA films. J Kor Pharm Sci. 2009;39:353–357. [Google Scholar]
- 26.Wong SS. Chemistry of protein conjugation and crosslinking. Boston: CRC Press; 1991. [Google Scholar]
- 27.Na DH. Effect of pH on the formation of acylated octreotides by poly(lactide-co-glycolide) J Pharm Invest. 2010;40:251–254. doi: 10.4333/KPS.2010.40.4.251. [DOI] [Google Scholar]
- 28.Kang JS, Deluca PP, Lee KC. Emerging PEGylated drugs. Expert Opin Emerg Drugs. 2009;14:363–380. doi: 10.1517/14728210902907847. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]