Abstract
This paper developed solvent-free drug-eluting implants for metronidazole delivery for the treatment of periodontal disease and investigated the characteristics of the drug’s release from the implants, both in vitro and in vivo, using an HPLC assay. The metronidazole exhibited a two-stage release behavior in vitro with an initial burst release followed by a diffusion-controlled release and then a secondary burst release. The accumulated drug release reached 100% on the 18th day, and the drug-eluting implant was totally dissolved on the same day. Additionally, the drug-eluting disks were implanted within the sub-gingival space of both lower incisors of six rabbits. The curve of in vivo drug release was smoother and showed a predominantly diffusion-controlled release. The implants were totally dissolved at 2 weeks after implantation. The concentration of metronidazole remained above the MIC90 during the entire investigation.
KEY WORDS: drug-eluting implant, metronidazole, periodontal disease, PLGA
INTRODUCTION
Periodontal disease is a chronic inflammation of the periodontium resulting from bacterial infection. The opportunistic microflora present in the periodontal pocket provides an ideal environment for the growth and proliferation of bacteria (1). The pathogenic microflora are complex in nature and are mainly anaerobic Gram-negative bacteria (2). However, these bacteria not only erode periodontal tissue but can also create a special barrier—dental plaque biofilm.
Conventional periodontal treatment includes mechanical scaling and root planing to remove bacterial deposits, calculus, and cementum which are contaminated by bacteria and endotoxins. However, because of the complicated contour of the roots and defects, conventional therapy alone is insufficiently effective especially where dental plaque biofilm is formed. In this case, antibiotic treatments or chemotherapeutics are prescribed for patients with periodontal disease.
Systemic antibiotic therapy is unable to achieve a sufficiently high concentration in the gingival crevicular fluid due to dispersal of the drug over the whole body; only a small portion of the drug actually reaches the sub-gingival microflora in the periodontal pocket (3). Long-term systemic antibiotic therapy may give rise to adverse drug reactions (potential liver and kidney damage) or increase the risk of drug resistance (4). Disagreeable effects may decrease patients’ compliance (5).
Local delivery antimicrobial agents (LDAs) are available for use as adjuncts to scaling and root planing in the treatment of periodontitis. The controlled LDAs can deposit a high level of active agent in the periodontal pocket, and the carrier facilitates prolonged drug delivery (6–8). Despite the approved product such as Atridox® may deliver doxycycline (9) to the periodontal cavity for a period of 21 days, it requires an intensive mixing of liquid delivery system and drug powder by pushing the mixture back and forth between two syringes for 100 times before application. Mechanical oral hygiene procedures (i.e., tooth brushing, flossing) should also be avoided on any treated areas for 7 days. On the other hand, most of the other commercially available agents need direct injections into the periodontal pocket once per week for two to four consecutive weeks in order to reach a bacterial inhibitory concentration. All these delivery systems are inconvenient in the clinical setting.
We adopt a novel solvent-free compression sintering method (10–12) to manufacture biodegradable drug-eluting implants for drug delivery with the goals of improving the efficiency of administration, decreasing the inconvenience of patients, and reducing the waste of medical resources. Our drug-eluting device is an implantable biodegradable carrier of antibiotics against periodontal bacteria. The aim of our study is to achieve prolonged drug release that would suppress periodontal bacterial growth and reduce the bioburden. In addition, we hope that the device may effectively prevent complications following periodontal surgery by acting as an antimicrobial agent against periodontal bacteria.
MATERIALS AND METHODS
Fabrication of Drug-Eluting Implants
Commercially available 50:50 poly(d,l)-lactide-co-glycolide (PLGA) was used as our carrier material (Resomer RG 503, Boehringer, Germany). The PLGA had an intrinsic viscosity of 0.4. All polymers were available in powder form and were pre-mixed with commercial grade antibiotics (metronidazole powder, Sigma–Aldrich, St. Louis, MO). To manufacture the implants, polylactide–polyglycolide copolymers were pre-mixed with the drugs with a drug/polymer ratio of 1:10 (a total weight of 2.5 mg) using a dry mixer. The mixture was compressed and sintered (12) in an oven for 30 min to form a disk with a diameter of 3.0 mm and a thickness of 1/3 mm (Fig. 1).
Fig. 1.
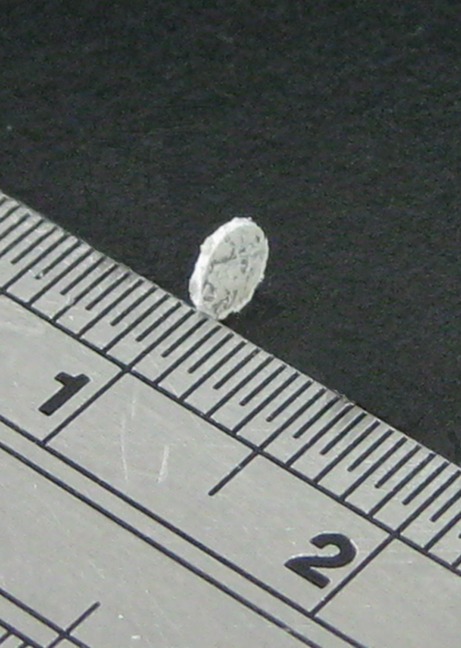
Photograph of drug-eluting implant
In Vitro Degradation Analysis
A 0.15 mol/L (pH 7.4) phosphate buffer was used as the dissolution medium for the in vitro degradation analysis. The disk implant was placed in a test tube containing 1 ml of phosphate buffer at 37°C. The dissolution medium was entirely collected for subsequent analyses at each 24-h interval. Fresh phosphate buffer (1 ml) was then added, and the analysis was repeated every 24 h until the disk was fully dissolved. The clinical appearance was measured using a scanning electron microscope (SEM) on days 1, 7, and 14. Prior to examination, the surfaces were covered with a thin layer (60 Å in thickness) of gold to make them conductive. The voltage used was 10 kV.
Characterization of Released Antibiotics
The antibiotic concentration in buffer was determined using a high-performance liquid chromatography (HPLC) assay (10,11,13). The HPLC analyses were conducted using a Hitachi L-2200 Multisolvent Delivery System. All samples were assayed in triplicate, and sample dilutions were performed to bring the unknown concentrations into the range of the assay standard curve. A calibration curve was made for each set of the measurements (correlation coefficient >0.99). The elution product was identified and quantified with high sensitivity using the HPLC system.
The activity test of metronidazole relative to Escherichia coli (ATCC25922) was determined using an antibiotic disk diffusion method in nutrient broth (beef extract, peptone, Difco Laboratories, Detroit, MI). The eluent of the implant was tested for up to 28 days. A daily buffer sample of 8 μL was collected and pipetted onto 6-mm absorption disks. The disks were placed on nutrient agar plates that were seeded with a layer of E. coli; the inoculum size used was 450,000 cfu/ml (14). The zones of inhibition were measured with a micrometer after 16–18 h of incubation at 35°C. The relative activity of the released antibiotics was defined as:
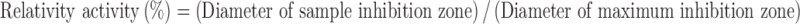 |
1 |
where the diameters of sample and maximum inhibition zones are those of eluted antibiotics and as-received antibiotics, respectively.
Animal Study
Six New Zealand white rabbits (age, 8–12 weeks; average weight, 1.5 kg) were used for the animal study. All surgical procedures and aftercare protocols were performed according to our facility’s guidelines for the care and use of laboratory animals.
Each rabbit was sedated with 2% xylazine-HCl (5 mg/kg body weight; Sanofi-Aventis, Frankfurt, Germany) and ketamine-HCl (Ketasol, 30 mg/kg body weight, administered intramuscularly; Dr. E. Graub, Bern, Switzerland). The operative area was cleaned and sterilized. The drug-eluting implant (disk with a diameter of 3.0 mm and thickness of 1/3 mm) was placed in the gingival sulculus of each rabbit’s lower incisors. All animal procedures received institutional approval, and all studied animals were cared for in accordance with the regulations of the National Institute of Health of the Republic of China (Taiwan) under the supervision of a licensed veterinarian.
The gingival crevicular fluid of the lower incisors was then extracted using #30 standardized sterile paper point (DiaDent, Korea) on days 1, 2, 4, 7, 10, and 14. The paper point was inserted 1 mm into the gingival crevice and was left in situ for 30 s (15) during each procedure. Immediately after collection, the paper point was eluted with 0.1 ml phosphate buffer solution and then stored at −20°C until it was analyzed. The HPLC analysis was performed at each time period using the same procedure as the in vitro study.
RESULTS
In Vitro Degradation
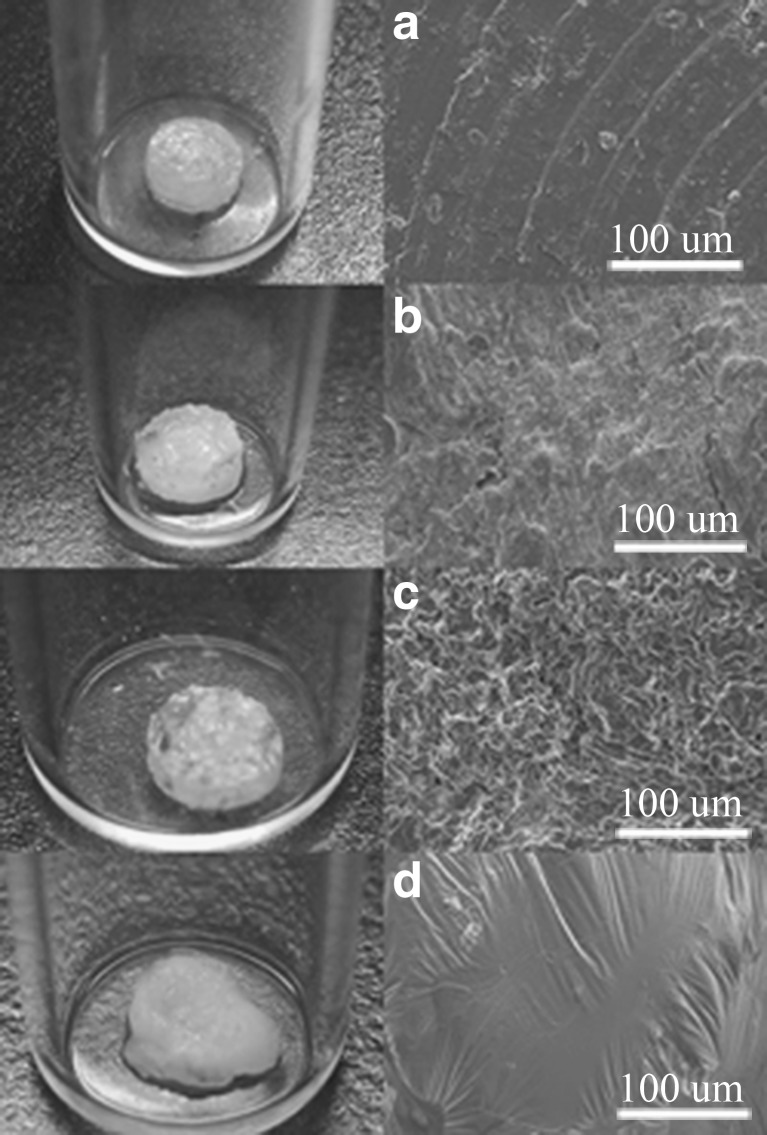
Figure 2 shows the results of the in vitro degradation analysis. The gross appearance of the drug-eluting implant shows similar changes as noted on the SEM photographs. One day after the elution, tiny pores on the surface of the implants could be observed (Fig. 2b), which indicated channel diffusion of the drug from the implants. After 7 days of elution, due to the osmotic pressure of the solution, the implants swelled and exhibited sponge-like surfaces (Fig. 2c). At day 14th, the drug-eluting implant degraded slowly over time and showed smooth surfaces (Fig. 2d).
Fig. 2.
The gross and SEM photographs of the drug-eluting implants with a diameter of 3.0 mm and a thickness of 1/3 mm. a as fabricated, b the first day, c the 7th day, d the 14th day after being eluted in phosphate buffer at 37°C (magnification of SEM, ×300; the voltage used was 10 kV)
Activities of Released Antibiotics
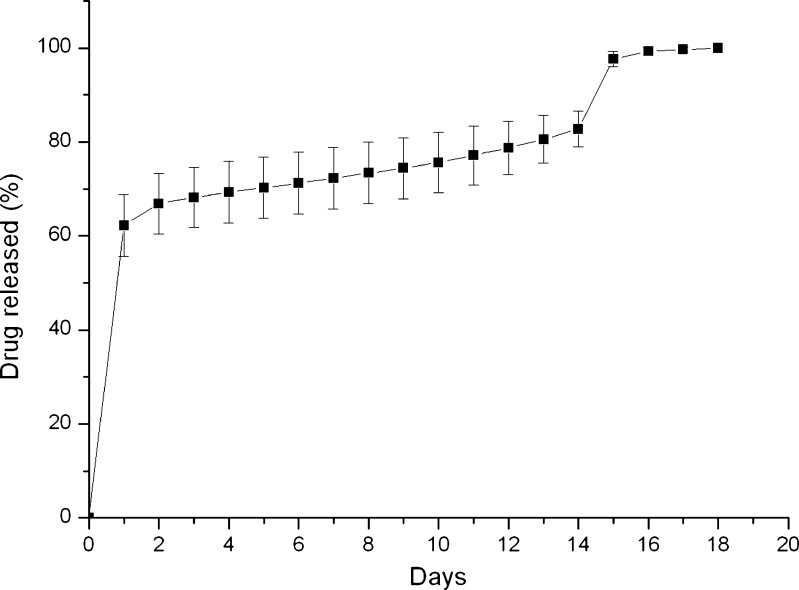
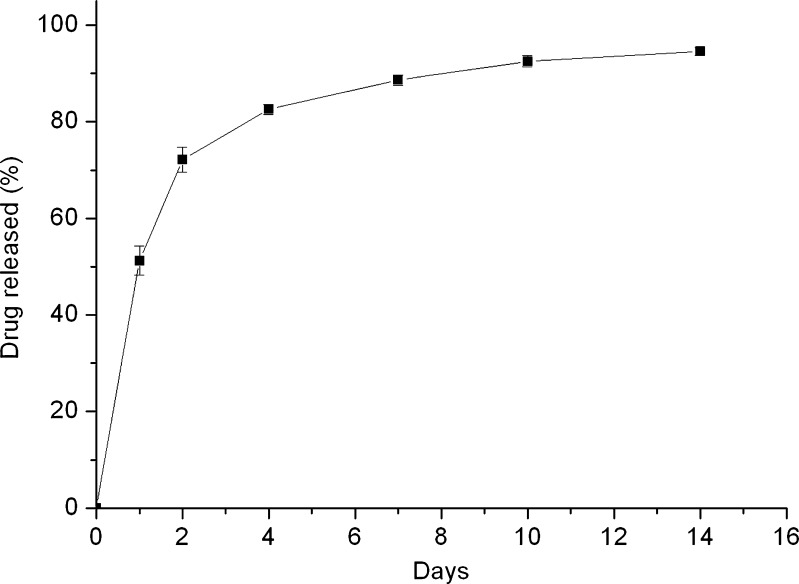
The result of the accumulated release of metronidazole is shown in Fig. 3. The metronidazole exhibited a two-stage release behavior with an initial burst release followed by a diffusion-controlled release and then a secondary burst release. The accumulated drug release reached 100% on the 18th day, and the drug-eluting implant was totally dissolved on the same day.
Fig. 3.
In vitro accumulated release curves of metronidazole from drug-eluting implant
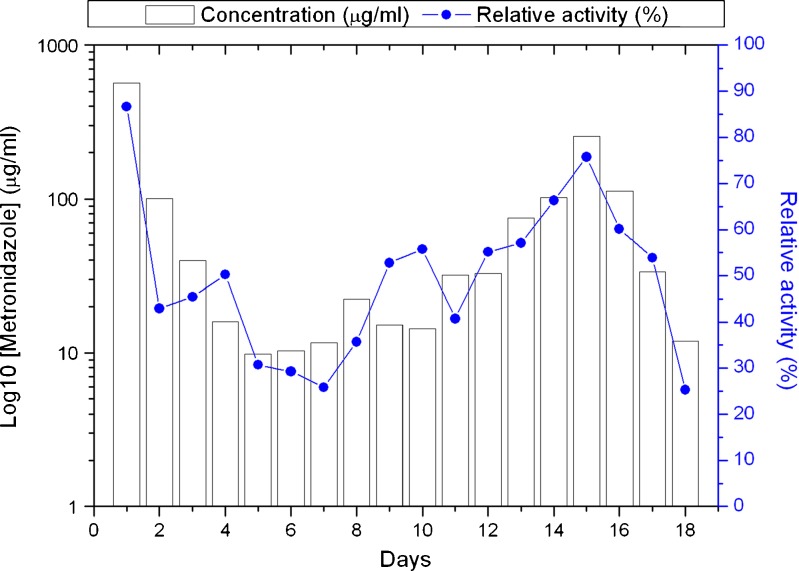
Figure 4 shows the bioactivity of the drug-eluting implant. The in vitro HPLC analysis and relative activity measurements demonstrated that the current drug-eluting implant can release an effective amount of metronidazole over approximately 3 weeks. The minimum inhibitory concentration, 90% (MIC90) of metronidazole (16) against E. coli was determined to be 0.4 μg/ml.
Fig. 4.
In vitro HPLC concentration and relative activity curve from drug-eluting implant
In Vivo Results
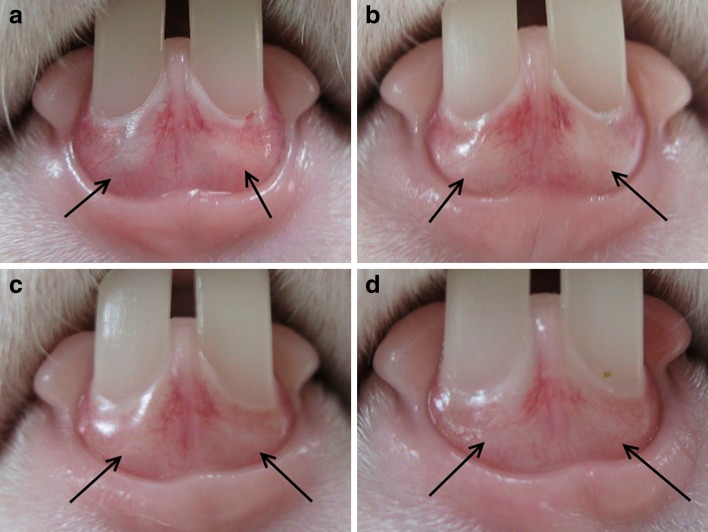
The drug-eluting disks were implanted within the sub-gingival space of both lower incisors of six rabbits (Fig. 5). The drug-eluting disks were totally dissolved at 2 weeks after implantation.
Fig. 5.
Photograph showing the drug-eluting disks implanted within the sub-gingival of both lower incisors of a rabbit. The arrow indicates the location of the drug-eluting implant. a before operation, b the first day, c the 7th day, d the 14th day of the experiment
The result of in vivo accumulated release of metronidazole is shown in Fig. 6. There was an approximately 10% drug diminution at the end of the investigation. Instead of the two-stage release behavior as noted in the in vitro results, the curve of in vivo drug release was smoother and showed a predominantly diffusion-controlled release.
Fig. 6.
The curve of accumulated in vivo metronidazole release from drug-eluting implant
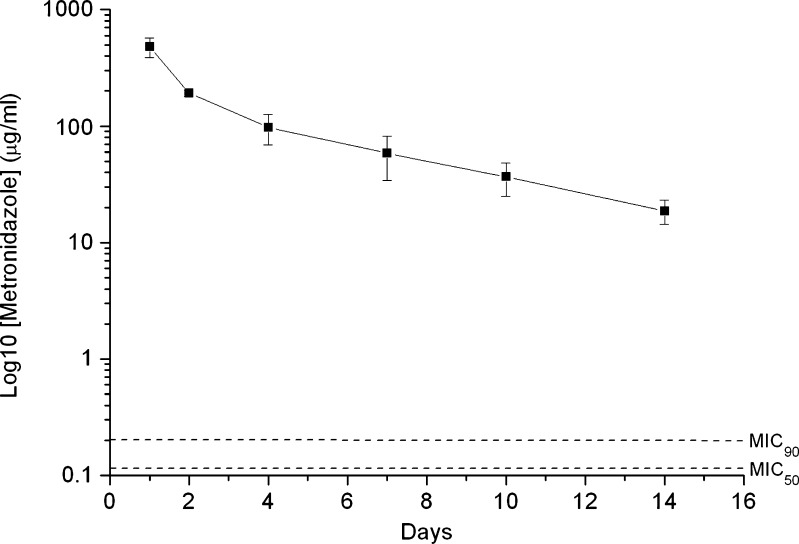
Figure 7 shows the in vivo HPLC concentration curve from the drug-eluting implant. The concentration of metronidazole remained above the MIC90 during the entire investigation.
Fig. 7.
In vivo HPLC concentration curve from drug-eluting implant
DISCUSSION
Periodontal disease is caused by the local colonization of pathogenic microflora in the ginigival crevice and periodontal pocket. Pharmacological agents which are applied locally for the treatment of periodontitis target to these areas. However, the highly organized accumulations of the adherent bacteria (dental plaque biofilm) may impair the diffusion or the activities of the pharmacologic agents. In addition, the gingival crevicular fluid has been estimated to be replaced about 40 times per hour in the periodontal pocket (17). To overcome the high rate of clearance which is due to the effect of the crevicular flow, we developed a sub-gingival drug delivery system to counteract the drug loss and facilitate prolonged drug delivery.
Since the introduction of the drug delivery system in the 1970s, several materials have been adopted as drug carriers to treat periodontal disease. These carriers are composed of degradable macromolecules. With the development of biodegradable materials, an increasing number of safer and more stable local drug delivery carriers have been designed. These various carriers, including fibers, gels, microspheres, and films, have been developed into periodontal implants using different manufacturing methods (18).
In 1995, Roskos et al. produced a tetracycline ointment containing Mg(OH)2, which prolonged the drug release period (19). One year later, Litch et al. applied the Actisite® periodontal fiber containing tetracycline hydrochloride to 13 subjects. One week after implantation, the researchers observed persistent high drug concentrations (20). In their 2001 study, Bromberg et al. treated periodontal disease with PLGA films enforced with penicillin G and tetracycline hydrochloride and performed drug release tests using two medicated films (21). However, during the production of antibiotics carriers, organic solvents were used for polymerization, raising concerns regarding the effect of the potential residual solvents on both the carrier and the drug (22). In our study, a solvent-free compression sintering method (12) was employed to manufacture drug-eluting implants that released antibiotics over a sustained time period. Since no solvents were used during the manufacturing process, we avoided the problems associated with their use.
Many antibacterial agents can be delivered into periodontal sites through local drug delivery systems (6). However, locally delivered metronidazole requires lower concentrations to achieve complete elimination of sub-gingival flora compared to other drugs (23). In order to treat periodontal disease, some investigators have administered systemic antibiotics to patients during the first 2 to 4 weeks after surgical intervention. However, postoperative wound infections or abscess formations have been reported (24). Local antibiotic application would be preferable if a sufficiently high concentration could be attained at the surgical site at a much lower dosage than needed in systemic treatment. The use of the drug-eluting implant in our study showed effective drug delivery for at least 2 weeks, in both in vivo and in vitro studies. The implant could also be inserted into the periodontal cavity directly. This provides advantages in terms of sustained release of effective antibiotic and easy deployment of the implants. Furthermore, we believe that increasing the thickness of the implant may extend the drug delivery period even further.
It is generally believed that the release mechanisms are controlled by channel diffusion, osmotic pressure, and polymer degradation (25). In this study, the drug is initially released by the effect of channel diffusion (as shown in Fig. 2b), which has a higher release rate and causes the initial burst release of metronidazole noted in both the in vitro and in vivo experiments (Figs. 3 and 6). The drug release is then controlled by the effect of osmotic pressure (as can be seen on the sponge-like surface of the implants in Fig. 2c) which exhibits a smoother curve, and another burst release of drug occurs when the polymer is dissolved or degraded (as shown in Fig. 2d). The in vitro drug release curve conformed to regular steps, but the curve of the in vivo experiment did not. Compared to that of in vitro release, the curve of in vivo drug release was smoother and no initial burst was observed. This can be explained by the fact that the disk in the periodontal was protected by the sub-gingival tissue from the saliva. The release rate of antibiotics was thus reduced and exhibited a diffusion-controlled release. On the other hand, the shape of the current drug-eluting implant was an extremely thin disk which provided advantages of easy implantation in the sub-gingival area. Nevertheless, the disk may have slipped out from the sub-gingival area due to the gradual degradation of the PLGA (Fig. 2). This might explain why the total period of in vivo drug release was somewhat shorter than that of in vitro.
Our study had several limitations. Although the solvent-free technique of compression sintering, used during the fabrication of the drug-eluting implant, eliminated any toxic reaction, the whole procedure required temperatures as high as 65°C and pressures up to 278.23 MPa. The drug efficacy of the antibiotics may have been changed by the high temperature and pressure. An additional in vitro study of thermal and pressure stability may be required before our technique can be applied to further animal studies. However, in the current study, the bioactivity of metronidazole was shown to be effective during the in vitro qualitative analysis.
In addition, after implantation of the drug-eluting disk in the sub-gingival area, the disk was not fixed in position by any means. The drug-eluting disk could have dislodged if the gingiva were not re-attached in time. Fortunately, the course of our in vivo experiment was smooth, and we recorded complete data from all six study animals, although the in vivo drug release curve differed from the in vitro result.
CONCLUSION
We have developed a solvent-free method for processing biodegradable polymers as antibiotic implants for a long-term drug release in periodontal pockets. The in vitro quantitative and qualitative analysis proved that the drug-eluting implant provided at least 3 weeks of stable and effective antibiotic delivery. In the animal study, although the pattern of drug release was slightly different from the in vitro result, the concentration of the antibiotic was effective for over 2 weeks.
Our drug delivery implant using a solvent-free method of manufacture is promising for adaptation to animals because it is toxin-free. Further studies, however, are required to perfect the process before it can be used in humans for the treatment of various periodontal diseases.
REFERENCES
- 1.Offenbacher S. Periodontal diseases: pathogenesis. Annal Periodontol. 1996;1(1):821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodson JM. Antimicrobial strategies for treatment of periodontal diseases. Periodontology 2000. 1994;5:142–168. doi: 10.1111/j.1600-0757.1994.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Walker CB. Selected antimicrobial agents: mechanisms of action, side effects and drug interactions. Periodontology 2000. 1996;10:12–28. doi: 10.1111/j.1600-0757.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 5.Loesche WJ, Grossman N, Giordano J. Metronidazole in periodontitis (IV). The effect of patient compliance on treatment parameters. J Clin Periodontol. 1993;20(2):96–104. doi: 10.1111/j.1600-051X.1993.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998;69(5):507–520. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 7.Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol. 2005;76(8):1227–1236. doi: 10.1902/jop.2005.76.8.1227. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein G, Tonetti M. The role of controlled drug delivery for periodontitis. J Periodontol. 2000;71(1):125–140. doi: 10.1902/jop.2000.71.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Stoller NH, Johnson LR, Trapnell S, Harrold CQ, Garrett S. The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol. 1998;69(10):1085–1091. doi: 10.1902/jop.1998.69.10.1085. [DOI] [PubMed] [Google Scholar]
- 10.Lin SS, Ueng SW, Liu SJ, Chan EC, Chao EK, Tsai CH, et al. Development of a biodegradable antibiotic delivery system. Clin Orthop Rel Res. 1999;362:240–250. [PubMed] [Google Scholar]
- 11.Liu SJ, Ueng SW, Chan EC, Lin SS, Tsai CH, Wei FC, et al. In vitro elution of vancomycin from biodegradable beads. J Biom Mater Res. 1999;48(5):613–620. doi: 10.1002/(SICI)1097-4636(1999)48:5<613::AID-JBM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Liu SJ, Tsai YE, Ueng SWN, Chan EC. A novel solvent-free method for the manufacture of biodegradable antibiotic-capsules for a long-term drug release using compression sintering and ultrasonic welding techniques. Biomaterials. 2005;26(22):4662–4669. doi: 10.1016/j.biomaterials.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 13.Milojevic Z, Agbaba D, Eric S, Boberic-Borojevic D, Ristic P, Solujic M. High-performance liquid chromatographic method for the assay of dexamethasone and xylometazoline in nasal drops containing methyl p-hydroxybenzoate. J Chromatogra. 2002;A. 949(1–2):79–82. doi: 10.1016/S0021-9673(01)01510-2. [DOI] [PubMed] [Google Scholar]
- 14.Morrissey I, George JT. The effect of the icoculum size on bactericidal activity. J Antimicrob Chemother. 1999;43:423–424. doi: 10.1093/jac/43.3.423a. [DOI] [PubMed] [Google Scholar]
- 15.Perinetti GSG. The use of ISO endodontic paper points in determining small fluid volumes. J Appl Sci Clin Dent. 2004;1:7–11. [Google Scholar]
- 16.Wu H, Shi XD, Wang HT, Liu JX. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxycillin. J Antimicro Chemother. 2000;46:121–123. doi: 10.1093/jac/46.1.121. [DOI] [PubMed] [Google Scholar]
- 17.Goodson JM. Pharmacokinetic principles controlling efficacy of oral therapy. J Dent Res. 1989;68:1625–1632. [Google Scholar]
- 18.Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ, et al. Recent approaches for the treatment of periodontitis. Drug Disc Today. 2008;13(21–22):932–943. doi: 10.1016/j.drudis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Roskos KV, Fritzinger BK, Rao SS, Armitage GC, Heller J. Development of a drug delivery system for the treatment of periodontal disease based on bioerodible poly(ortho esters) Biomaterials. 1995;16(4):313–317. doi: 10.1016/0142-9612(95)93259-G. [DOI] [PubMed] [Google Scholar]
- 20.Litch JM, Encarnacion M, Chen S, Leonard J, Burkoth TL. Use of the polymeric matrix as internal standard for quantitation of in vivo delivery of tetracycline HCl from Actisite tetracycline fiber during periodontal treatment. J Periodont Res. 1996;31(8):540–544. doi: 10.1111/j.1600-0765.1996.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 21.Bromberg LE, Buxton DK, Friden PM. Novel periodontal drug delivery system for treatment of periodontitis. J Contr Release. 2001;71(3):251–259. doi: 10.1016/S0168-3659(01)00226-7. [DOI] [PubMed] [Google Scholar]
- 22.Freier T, Kunze C, Schmitz KP. Solvent removal from solution-cast films of biodegradable polymers. J Mater Sci Lett. 2001;20:1929–1931. doi: 10.1023/A:1013174400236. [DOI] [Google Scholar]
- 23.Somayaji BV, Jariwala U, Jayachandran P, Vidyalakshmi K, Dudhani RV. Evaluation of antimicrobial efficacy and release pattern of tetracycline and metronidazole using a local delivery system. J Periodontol. 1998;69(4):409–413. doi: 10.1902/jop.1998.69.4.409. [DOI] [PubMed] [Google Scholar]
- 24.Selvig KA, Nilveus RE, Fitzmorris L, Kersten B, Khorsandi SS. Scanning electron microscopic observations of cell populations and bacterial contamination of membranes used for guided periodontal tissue regeneration in humans. J Periodontol. 1990;61(8):515–520. doi: 10.1902/jop.1990.61.8.515. [DOI] [PubMed] [Google Scholar]
- 25.Seigel RA, Langer R. Mechanistic studies of macromolecular drug release from macroporous polymers. II. Models for the kinetics of drug release. J Contr Release. 1990;14:153–167. doi: 10.1016/0168-3659(90)90152-J. [DOI] [Google Scholar]